Abstract
Energy homeostasis, food intake, and body weight are regulated by specific brain circuits. Here we introduce an unexpected neuron, the tyrosine hydroxylase (TH) neuron of the arcuate nucleus (ARC), that we show makes an orexigenic contribution. Optogenetic stimulation of mouse ARC TH neurons increased food intake; attenuating transmitter release reduced body weight. Optogenetic stimulation of ARC TH cells inhibited pro-opiomelanocortin (POMC) neurons through synaptic mechanisms. ARC TH cells project to the hypothalamic paraventricular nucleus; optogenetic stimulation of ARC TH axons inhibited paraventricular nucleus neurons by dopamine and GABA co-release. Dopamine excited orexigenic neurons that synthesize agouti-related peptide and neuropeptide Y but inhibited anorexigenic neurons that synthesize POMC, as determined by whole cell recording. Food deprivation increased c-fos expression and spike frequency in ARC TH neurons. The gut peptide ghrelin evoked direct excitatory effects, suggesting these neurons monitor metabolic cues. Together these data support the view that ARC TH cells play an unrecognized and influential positive role in energy homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Flier, J.S. Obesity wars: molecular progress confronts an expanding epidemic. Cell 116, 337–350 (2004).
Schwartz, M.W., Woods, S.C., Porte, D. Jr., Seeley, R.J. & Baskin, D.G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Grill, H.J., Ginsberg, A.B., Seeley, R.J. & Kaplan, J.M. Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J. Neurosci. 18, 10128–10135 (1998).
Saper, C.B., Chou, T.C. & Elmquist, J.K. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211 (2002).
Atasoy, D., Betley, J.N., Su, H.H. & Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Shah, B.P. et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci. USA 111, 13193–13198 (2014).
Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Tong, Q., Ye, C.P., Jones, J.E., Elmquist, J.K. & Lowell, B.B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 11, 998–1000 (2008).
Aponte, Y., Atasoy, D. & Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355 (2011).
Luquet, S., Perez, F.A., Hnasko, T.S. & Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310, 683–685 (2005).
Baldo, B.A. & Kelley, A.E. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology (Berl.) 191, 439–459 (2007).
Palmiter, R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 30, 375–381 (2007).
Wise, R.A. Role of brain dopamine in food reward and reinforcement. Phil. Trans. R. Soc. Lond. B 361, 1149–1158 (2006).
Zhang, X. & van den Pol, A.N. Dopamine/tyrosine hydroxylase neurons of the hypothalamic arcuate nucleus release GABA, communicate with dopaminergic and other arcuate neurons, and respond to dynorphin, met-enkephalin, and oxytocin. J. Neurosci. 35, 14966–14982 (2015).
Fenno, L.E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Lin, J.Y., Lin, M.Z., Steinbach, P. & Tsien, R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Xu, W. & Südhof, T.C. A neural circuit for memory specificity and generalization. Science 339, 1290–1295 (2013).
Zoli, M., Agnati, L.F., Tinner, B., Steinbusch, H.W. & Fuxe, K. Distribution of dopamine-immunoreactive neurons and their relationships to transmitter and hypothalamic hormone-immunoreactive neuronal systems in the rat mediobasal hypothalamus. A morphometric and microdensitometric analysis. J. Chem. Neuroanat. 6, 293–310 (1993).
Everitt, B.J., Hökfelt, T., Wu, J.Y. & Goldstein, M. Coexistence of tyrosine hydroxylase-like and gamma-aminobutyric acid-like immunoreactivities in neurons of the arcuate nucleus. Neuroendocrinology 39, 189–191 (1984).
Goto, Y., Otani, S. & Grace, A.A. The yin and yang of dopamine release: a new perspective. Neuropharmacology 53, 583–587 (2007).
Gonon, F.G. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience 24, 19–28 (1988).
Taylor, I.M., Ilitchev, A.I. & Michael, A.C. Restricted diffusion of dopamine in the rat dorsal striatum. ACS Chem. Neurosci. 4, 870–878 (2013).
Fitzgerald, P. & Dinan, T.G. Prolactin and dopamine: what is the connection? A review article. J. Psychopharmacol. 22 (Suppl.): 12–19 (2008).
van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012).
Scott, N., Prigge, M., Yizhar, O. & Kimchi, T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519–522 (2015).
Belousov, A.B. & van den Pol, A.N. Dopamine inhibition: enhancement of GABA activity and potassium channel activation in hypothalamic and arcuate nucleus neurons. J. Neurophysiol. 78, 674–688 (1997).
Romero-Fernandez, W. et al. Dopamine D1 and D2 receptor immunoreactivities in the arcuate-median eminence complex and their link to the tubero-infundibular dopamine neurons. Eur. J. Histochem. 58, 2400 (2014).
Liu, T. et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron 73, 511–522 (2012).
Yan, C. et al. Apolipoprotein A-IV inhibits AgRP/NPY neurons and activates POMC neurons in the arcuate nucleus. Neuroendocrinology http://dx.doi.org/10.1159/000439436 (2016).
Groessl, F., Jeong, J.H., Talmage, D.A., Role, L.W. & Jo, Y.H. Overnight fasting regulates inhibitory tone to cholinergic neurons of the dorsomedial nucleus of the hypothalamus. PLoS One 8, e60828 (2013).
Kojima, M. et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402, 656–660 (1999).
Tschöp, M., Smiley, D.L. & Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Lyons, D.J., Horjales-Araujo, E. & Broberger, C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: switch to tonic discharge by thyrotropin-releasing hormone. Neuron 65, 217–229 (2010).
Krashes, M.J. et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507, 238–242 (2014).
van den Pol, A.N. & Cassidy, J.R. The hypothalamic arcuate nucleus of rat—a quantitative Golgi analysis. J. Comp. Neurol. 204, 65–98 (1982).
Matera, C. & Wardlaw, S.L. Dopamine and sex steroid regulation of POMC gene expression in the hypothalamus. Neuroendocrinology 58, 493–500 (1993).
Kobayashi, M. et al. Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc. Natl. Acad. Sci. USA 101, 11465–11470 (2004).
Broadwell, R.D. & Brightman, M.W. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J. Comp. Neurol. 166, 257–283 (1976).
Abizaid, A. et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 116, 3229–3239 (2006).
Naleid, A.M., Grace, M.K., Cummings, D.E. & Levine, A.S. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26, 2274–2279 (2005).
Everitt, B.J. et al. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res. 396, 97–155 (1986).
Meister, B. et al. Coexistence of tyrosine hydroxylase and growth hormone-releasing factor in a subpopulation of tubero-infundibular neurons of the rat. Neuroendocrinology 42, 237–247 (1986).
Zhan, C. et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 33, 3624–3632 (2013).
Savitt, J.M., Jang, S.S., Mu, W., Dawson, V.L. & Dawson, T.M. Bcl-x is required for proper development of the mouse substantia nigra. J. Neurosci. 25, 6721–6728 (2005).
van den Pol, A.N. et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J. Neurosci. 29, 4622–4639 (2009).
van den Pol, A.N., Herbst, R.S. & Powell, J.F. Tyrosine hydroxylase-immunoreactive neurons of the hypothalamus: a light and electron microscopic study. Neuroscience 13, 1117–1156 (1984).
Acknowledgements
We thank D. Spergel, J. Paglino and J. Davis for manuscript suggestions; I. Araujo and W. Han for suggestions on behavioral optogenetics; Y. Yang for technical assistance; R. Greene (UT Southwestern) and J. Zigman, T. Dawson, J. Savitt (Johns Hopkins University) for TH-Cre mice; M. Low (University Michigan) for POMC-GFP mice; and B. Lowell (Harvard University) for NPY-GFP mice. We thank H. Steinbusch (Maastricht University) for the dopamine antibody. Support provided by NIH DK084052, DK103176, and NS79274 (A.N.v.d.P.).
Author information
Authors and Affiliations
Contributions
X.Z. and A.N.v.d.P. designed the experiments. X.Z. performed experiments and analyzed the data. X.Z. and A.N.v.d.P. cowrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
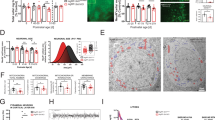
Supplementary Figure 1 Arcuate ChIEF-tdTomato neurons expressed TH
These micrographs show that TH-Cre-ChIEF-tdTomato (magenta), expressed by AAV in a TH-Cre mouse, were immunostained with TH (green) in the arcuate nucleus. Scale: 20 μm.
Supplementary Figure 2 Micrographs show sections from a transgenic mouse with red TH neurons and green NPY/AgRP neurons
No immunostaining was used. TH-tdTomato transgenic mice were crossed with NPY-GFP transgenic mice. Arcuate TH neurons are red, NPY/AgRP neurons are green. No cells showed both green and red fluorescence, indicating completely different populations of neurons. Bar, 30 μm. In corroborative immunocytochemical tests, we found that almost all red tdTomato reporter expression under control of the TH promoter was colocalized with immunostaining using TH antisera. In our experiments we focused on those areas of the ARC where TH neurons also contain dopamine; we recently showed that 94% of the TH neurons in the dorsomedial ARC expressed dopamine immunoreactivity, whereas in the ventrolateral ARC the number of TH neurons that contained dopamine were only a minority, as previously shown in rats.
Supplementary Figure 3 No colocalization of TH and POMC neurons
a. Neurons from a POMC-GFP mouse are green. b. TH immunostaining with a red fluorophore shows TH immunopositive neurons with red color. c. Merge: No coexpression of POMC and TH is found. Scale: 20 μm. d, Enlarged image c to show the absence of co-expression. Scale: 20 μm.
Supplementary Figure 4 Optogenetic activation of an arcuate TH neuron
a, Representative traces show that repeated optogenetic stimulation (6 sets of stimuli) using 10 Hz (above) and 20 Hz (bottom) light pulses of 2 s followed by 3 s interval activates a bursting TH neuron in the dorsomedial ARC. b, Representative trace showing optogenetic activation of a nonbursting TH neuron in the same area by repeated stimulation using 10 Hz light pulses of 2 s followed by a 3 s non-stimulation interval.
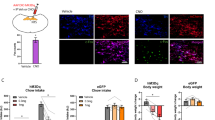
Supplementary Figure 6 Arcuate TeNT-GFP neurons express TH
left, Green image shows TH-Cre-TeNT-GFP neurons expressed by AAV in a TH-Cre mouse, middle, Orange image shows neurons immunostained for TH in the arcuate nucleus. right, Merged photo shows colocalization of TeNT-GFP and TH immunostaining. Scale: 20 μm.
Supplementary Figure 7 Photostimulation-evoked inhibitory postsynaptic current in TH neurons
a, Representative traces showing inhibitory postsynaptic currents (IPSCs) before, during and after TH neuron photostimulation (10 Hz) as recorded in a POMC neuron. IPSCs are evoked by each photostimulation. b, representative traces showing IPSCs before, during and after photostimulation (10 Hz) in PVN neuron.
Supplementary Figure 8 (AAV)dj-ChIEF-tdTomato-mediated ChIEF-tdTomato expression in ARC and PVN
a. High density of axons was found in PVN after the AAV was microinjected into the ARC. Arrows indicate some examples where PVN neurons are surrounded by positive axons arising from ARC TH neurons. PVN cell bodies did not express ChIEF-tdTomato. Bar, 10 μm. b. Low-resolution photo shows ChIEF-tdTomato fluorescence in ARC, the ventral part of the hypothalamic ventromedial nucleus (VMH-VL) and the dorsomedial nucleus (DMN) of same slice. Bar: 50 μm. c, High-resolution image showing ChIEF-tdTomato expression in cell body and axons in ARC 21 days after AAVdj-CAG-DIO-ChIEF-tdTomato was injected into the ARC of the TH-cre mouse. Arrows point to neurons that express tdTomato-Chief with typical smooth plasma membrane expression. Bar, 9 μm. d-e, Labeled axons from ARC TH cells were found in VMH-VL and DMN with relatively lower density. Bar, 10 μm. Outside the hypothalamus, AgRP neurons project to the parabrachial nucleus (PBN). We found no positive axons in the PBN after ARC labeling.
Supplementary Figure 9 Arcuate TH neuron inhibition in the PVN
a, Tonic outward current evoked by 20 Hz photostimulation for 30 s, showing control, bicuculline (Bic, 30 μM) and bicuculline (30 μM) + sulpiride (10 μM) in the PVN. b, Graph shows tonic outward current (n=6) in Ctrl and in the presence of Bic and Bic+sulpiride (BS), one-way repeated measure ANOVA, F(5,10)=21.6, P<0.0001; Bic versus Ctrl: ***, P=0.0003, BS versus Bic: *P=0.026,. Data are consistent with release of GABA and dopamine from arcuate TH axons in the PVN.
Supplementary Figure 10 Dopamine evokes GIRK current through direct activation of D2 receptors in POMC neurons
a, Representative traces show dopamine (30 μM) evoked outward current in control and in the presence of the D2 antagonist sulpiride (10 μM). Holding potential, -60 mV. b, Dopamine activates outward current in the presence of TTX (0.5 μM), AP5 (50 μM), CNQX (10 μM) and Bic (30 μM). Holding potential, -60 mV. c, A voltage ramp protocol from -140 to -20 mV was used to test GIRK current. d, Inward rectifying current with EReversal around -95 mV showing K+ current in control and in the presence of dopamine (black line)(30 μM). The red line shows the response of dopamine in the presence of barium (Ba2+). The blue line shows the response to dopamine (30 μM) in the presence of GDP-β-s. The recordings were done in the presence of TTX (0.5 μM), AP5 (50 μM), CNQX (10 μM) and Bic (30 μM).
Supplementary Figure 11 Excitatory postsynaptic effect of dopamine on NPY/AgRP neurons
a, Representative trace shows dopamine (30 μM) excites an NPY/AgRP neuron in the presence of AP5 (50 μM), CNQX (10 μM) and Bic (30 μM). b, Graph shows the mean resting membrane potential (n=15) before and in the presence of dopamine after blocking synaptic transmission. Paired t test, t(14)=2.313, *P=0.036. c, Graph shows firing rate (n=15) in control and in the presence of dopamine after blocking synaptic transmission. Paired t test, t(14)=3.149, *P=0.007. d, DA-antibody immunostained orange axons surround GFP-expressing NPY/AgRP cell. Arrows denote multiple contacts between DA- immunoreactive axons and NPY/AgRP neuron. Scale bar, 12 μm.
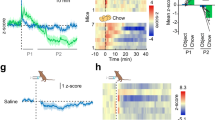
Supplementary Figure 12 Ghrelin excites arcuate TH neurons
a. Ghrelin (100 nM) depolarizes and excites a previously silent TH neuron to tonic firing in a hypothalamic brain slice from transgenic mice with the tdTomato reporter expressed selectively under control of the tyrosine hydroxylase promoter. b. Ghrelin increases the firing rate of an irregularly firing cell. c. Ghrelin induces a previously silent TH neuron to a burst firing state with a high frequency of spike bursts. d. Ghrelin increases burst rate of a bursting TH neuron, and reduces inter-burst intervals. All recorded cells were from the dorsomedial arcuate nucleus where dopamine has been found in almost all tyrosine hydroxylase positive neurons. e, Ghrelin increases burst rate of a bursting dopamine neuron in the presence of AP5 (50 μM), CNQX (10 μM) and Bic (30 μM).
Supplementary Figure 13 Schematic showing the hypothalamic projections of ARC TH neurons
Dopamine excites NPY/AgRP neuron; dopamine inhibits POMC neurons and also inhibits PVN neurons. Arcuate TH neurons inhibit PVN neurons by release of both GABA and dopamine; TH neurons inhibit POMC neurons by GABA release. Arrows indicate axonal excitation (up arrow) or inhibition (down arrow). Dopamine is released in enhanced amounts during burst firing, and dopamine can be released non-synaptically. Therefore hypothetically, TH axons on POMC neurons or near NPY/AgRP neurons may release dopamine to act by bulk flow mechanisms to increase the excitability of NPY/AgRP neurons. NPY/AgRP cells may receive a dopamine input from an unidentified source. This diagram only shows local projections of these cells, and does not include extrahypothalamic projections of TH, NPY/AgRP or POMC neurons or projections of PVN neurons.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 2066 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., van den Pol, A. Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci 19, 1341–1347 (2016). https://doi.org/10.1038/nn.4372
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4372
This article is cited by
-
A brainstem–hypothalamus neuronal circuit reduces feeding upon heat exposure
Nature (2024)
-
Advanced neurobiological tools to interrogate metabolism
Nature Reviews Endocrinology (2023)
-
A hypothalamic dopamine locus for psychostimulant-induced hyperlocomotion in mice
Nature Communications (2022)
-
Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding
Nature Metabolism (2022)
-
Brain circuits for promoting homeostatic and non-homeostatic appetites
Experimental & Molecular Medicine (2022)