Abstract
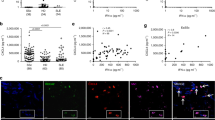
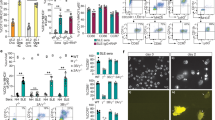
Antibodies are critical for defense against a variety of microbes, but they may also be pathogenic in some autoimmune diseases. Many effector functions of antibodies are mediated by Fcγ receptors (FcγRs), which are found on most immune cells, including dendritic cells (DCs)—important antigen-presenting cells that play a central role in inducing antigen-specific tolerance or immunity1,2. Following antigen acquisition in peripheral tissues, DCs migrate to draining lymph nodes via the lymphatics to present antigen to T cells. Here we demonstrate that FcγR engagement by IgG immune complexes (ICs) stimulates DC migration from peripheral tissues to the paracortex of draining lymph nodes. In vitro, IC-stimulated mouse and human DCs showed greater directional migration in a chemokine (C-C) ligand 19 (CCL19) gradient and increased chemokine (C-C) receptor 7 (CCR7) expression. Using intravital two-photon microscopy, we observed that local administration of IC resulted in dermal DC mobilization. We confirmed that dermal DC migration to lymph nodes depended on CCR7 and increased in the absence of the inhibitory receptor FcγRIIB. These observations have relevance to autoimmunity because autoantibody-containing serum from humans with systemic lupus erythematosus (SLE) and from a mouse model of SLE also increased dermal DC migration in vivo, suggesting that this process may occur in lupus, potentially driving the inappropriate localization of autoantigen-bearing DCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Steinman, R.M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Krause, R.M., Dimmock, N.J. & Morens, D.M. Summary of antibody workshop: the role of humoral immunity in the treatment and prevention of emerging and extant infectious diseases. J. Infect. Dis. 176, 549–559 (1997).
Joller, N., Weber, S.S. & Oxenius, A. Antibody-Fc receptor interactions in protection against intracellular pathogens. Eur. J. Immunol. 41, 889–897 (2011).
Ravetch, J.V. & Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 19, 275–290 (2001).
Itano, A.A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Roake, J.A. et al. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 181, 2237–2247 (1995).
Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 6, 476–483 (2006).
Boruchov, A.M. et al. Activating and inhibitory IgG Fc receptors on human dendritic cells mediate opposing functions. J. Clin. Invest. 115, 2914–2923 (2005).
Dhodapkar, K.M. et al. Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl. Acad. Sci. USA 102, 2910–2915 (2005).
Dhodapkar, K.M. et al. Selective blockade of the inhibitory Fcγ receptor (FcγRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J. Exp. Med. 204, 1359–1369 (2007).
Kalergis, A.M. & Ravetch, J.V. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J. Exp. Med. 195, 1653–1659 (2002).
van Montfoort, N. et al. Fcγ receptor IIb strongly regulates fcγ receptor-facilitated T cell activation by dendritic cells. J. Immunol. 189, 92–101 (2012).
Pricop, L. et al. Differential modulation of stimulatory and inhibitory Fcγ receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 166, 531–537 (2001).
Smith, K.G.C. & Clatworthy, M.R. FcγRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat. Rev. Immunol. 10, 328–343 (2010).
Bolland, S. & Ravetch, J.V. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity 13, 277–285 (2000).
Brownlie, R.J. et al. Distinct cell-specific control of autoimmunity and infection by FcγRIIB. J. Exp. Med. 205, 883–895 (2008).
Boross, P. et al. The inhibiting Fc receptor for IgG, FcγRIIB, is a modifier of autoimmune susceptibility. J. Immunol. 187, 1304–1313 (2011).
Floto, R.A. et al. Loss of function of a lupus-associated FcγRIIB polymorphism through exclusion from lipid rafts. Nat. Med. 11, 1056–1058 (2005).
Kono, H. et al. FcγRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum. Mol. Genet. 14, 2881–2892 (2005).
Randolph, G.J., Angeli, V. & Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Cyster, J.G. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J. Exp. Med. 189, 447–450 (1999).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Sallusto, F. et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 (1998).
Ratzinger, G. et al. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J. Immunol. 168, 4361–4371 (2002).
Yen, J.H., Khayrullina, T. & Ganea, D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood 111, 260–270 (2008).
Lebre, M.C., Kalinski, P., Das, P.K. & Everts, V. Inhibition of contact sensitizer-induced migration of human Langerhans cells by matrix metalloproteinase inhibitors. Arch. Dermatol. Res. 291, 447–452 (1999).
Kobayashi, Y., Matsumoto, M., Kotani, M. & Makino, T. Possible involvement of matrix metalloproteinase-9 in Langerhans cell migration and maturation. J. Immunol. 163, 5989–5993 (1999).
Ichiyasu, H. et al. Matrix metalloproteinase-9-deficient dendritic cells have impaired migration through tracheal epithelial tight junctions. Am. J. Respir. Cell Mol. Biol. 30, 761–770 (2004).
Rahman, A. & Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 358, 929–939 (2008).
Blanco, P., Palucka, A.K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Decker, P., Kotter, I., Klein, R., Berner, B. & Rammensee, H.G. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 45, 1087–1095 (2006).
Ding, D., Mehta, H., McCune, W.J. & Kaplan, M.J. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J. Immunol. 177, 5878–5889 (2006).
Crispín, J.C., Vargas-Rojas, M.I., Monsivais-Urenda, A. & Alcocer-Varela, J. Phenotype and function of dendritic cells of patients with systemic lupus erythematosus. Clin. Immunol. 143, 45–50 (2012).
Willcocks, L.C. et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA 107, 7881–7885 (2010).
Schaefer, B.C., Schaefer, M.L., Kappler, J.W., Marrack, P. & Kedl, R.M. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell. Immunol. 214, 110–122 (2001).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Lämmermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Bowden, E.T., Coopman, P.J. & Mueller, S.C. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 63, 613–627 (2001).
Haessler, U., Pisano, M., Wu, M. & Swartz, M.A. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc. Natl. Acad. Sci. USA 108, 5614–5619 (2011).
Tal, O. et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 208, 2141–2153 (2011).
Zinselmeyer, B.H., Lynch, J.N., Zhang, X., Aoshi, T. & Miller, M.J. Video-rate two-photon imaging of mouse footpad—a promising model for studying leukocyte recruitment dynamics during inflammation. Inflamm. Res. 57, 93–96 (2008).
Robbiani, D.F. et al. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3β, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 103, 757–768 (2000).
Acknowledgements
This work was supported by a Wellcome Trust Intermediate Fellowship (WT081020) to M.R.C. and by the National Institute for Health Research Cambridge Biomedical Research Centre and the Intramural Research Program of the US National Institute of Allergy and Infectious Diseases, part of the US National Institutes of Health. We thank J. Yoon for technical advice and M. Espeli for helpful discussions.
Author information
Authors and Affiliations
Contributions
M.R.C. conceived of the project, carried out experiments, designed and supervised experiments, analyzed data and wrote the manuscript. C.E.P.A. and R.J.M. carried out experiments and analyzed data. N.Y.M. provided technical advice to C.E.P.A. for the in vitro DC chemotaxis assay. K.G.C.S. provided mentorship for M.R.C. and scientific advice. R.N.G. provided advice on experimental design, assisted with manuscript writing and editing and provided mentorship for M.R.C. and C.E.P.A.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Supplementary Table 1 (PDF 2077 kb)
Migration of WT BMDCs from the skin through the interfollicular zone of the draining lymph node.
Representative movie of WT BMDC migration (green) in vivo in the popliteal lymph node, 20 hours following their administration subcutaneously into the footpad. Lymph node imaged over 1 hour and DCs are observed moving deeper into the lymph node paracortex. (MOV 310 kb)
Chemotaxis of OVA-stimulated WT DCs in a CCL19 gradient in vitro
Representative movie of cell migration observed for WT BMDCs (green) placed in a soluble rising CCL19 gradient following 24 hour incubation with ovalbumin prior to transfer into a 3D collagen matrix. The CCL19 gradient develops from left (source concentration) to right (base medium). Cell tracks (multi-color) demonstrate migration paths taken by motile cells exposed to soluble gradient over 1 hour. (MOV 436 kb)
Chemotaxis of immune complex-stimulated WT DCs in a CCL19 gradient in vitro
Representative movie of cell migration observed for WT BMDCs (green) placed in a soluble rising CCL19 gradient following 24 hour incubation with IgG-opsonised ovalbumin prior to transfer into a 3D collagen matrix. The CCL19 gradient develops from left (source concentration) to right (base medium). Cell tracks (multi-color) demonstrate migration paths taken by motile cells exposed to soluble gradient over 1 hour. (MOV 773 kb)
Chemotaxis of OVA-stimulated Fcgr2b–/– DCs in a CCL19 gradient in vitro
Representative movie of cell migration observed for Fcgr2b–/– BMDCs (green) placed in a soluble rising CCL19 gradient following 24 hour incubation with ovalbumin prior to transfer into a 3D collagen matrix. The CCL19 gradient develops from left (source concentration) to right (base medium). Cell tracks (multi-color) demonstrate migration paths taken by motile cells exposed to soluble gradient over 1 hour. (MOV 992 kb)
Chemotaxis of immune complex-stimulated Fcgr2b–/– DCs in a CCL19 gradient in vitro
Representative movie of cell migration observed for Fcgr2b–/– BMDCs (green) placed in a soluble rising CCL19 gradient following 24 hour incubation with IgG-opsonised ovalbumin prior to transfer into a 3D collagen matrix. The CCL19 gradient develops from left (source concentration) to right (base medium). Cell tracks (multi-color) demonstrate migration paths taken by motile cells exposed to soluble gradient over 1 hour. (MOV 1210 kb)
Chemotaxis of OVA-stimulated human monocyte-derived DCs in a CCL19 gradient in vitro
Representative movie of cell migration observed for human monocyte-derived DCs placed in a soluble rising CCL19 gradient following 24 hour incubation with ovalbumin prior to transfer into a 3D collagen matrix. The CCL19 gradient develops from left (source concentration) to right (base medium). Cell tracks (multi-color) demonstrate migration paths taken by motile cells exposed to soluble gradient over 1 hour. (MOV 9849 kb)
Chemotaxis of immune complex-stimulated human monocyte-derived DCs in a CCL19 gradient in vitro
Representative movie of cell migration observed for human monocyte-derived DCs placed in a soluble rising CCL19 gradient following 24 hour incubation with IgG-opsonised ovalbumin prior to transfer into a 3D collagen matrix. The CCL19 gradient runs from left (source concentration) to right (base medium). Cell tracks (multi-color) demonstrate migration paths taken by motile cells exposed to soluble gradient over 1 hour. (MOV 9310 kb)
Footpad dermal DC migration following administration of ovalbumin
Representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of ovalbumin. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with 655 Qdot tracer (red). (MP4 2412 kb)
Footpad dermal DC migration following administration of immune complexes
Representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of IgG-opsonised ovalbumin. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MP4 1966 kb)
Footpad dermal DC migration into lymphatics following administration of immune complexes
Right, representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of IgG-opsonised ovalbumin. Dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Lymphatic vessels delineated with an anti-LYVE-1 antibody (red) administered intra-dermally. Left, movie rotated and viewed in perpendicular plane to that viewed at right; confirms that the DC is moving within the lymphatic vessel rather than adjacent to it. (MOV 1360 kb)
Footpad dermal DC migration in Fcgr2b–/– mice following administration of ovalbumin
Representative movie of endogenous dermal DC migration in Fcgr2b–/– CD11cEYFP mice 16 hours following the administration (footpad injection) of ovalbumin. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MOV 1783 kb)
Footpad dermal DC migration in Fcgr2b–/– mice following administration of immune complexes
Representative movie of endogenous dermal DC migration in Fcgr2b–/– CD11cEYFP mice 16 hours following the administration (footpad injection) of IgG-opsonised ovalbumin. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MOV 1774 kb)
Footpad dermal DC migration following administration of heat-inactivated serum from an NZB/W F1 mouse
Representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of heat-inactivated serum obtained from an NZB/W F1 mouse with anti-nuclear antibodies and nephritis. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MOV 1448 kb)
Footpad dermal DC migration following administration of heat-inactivated serum from an aged WT mouse
Representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of heat-inactivated serum obtained from an aged WT mouse. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MOV 1509 kb)
Footpad dermal DC migration following administration of heat-inactivated serum from a patient with SLE
Representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of heat-inactivated serum obtained from a patient with systemic lupus erythematosus. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MOV 1167 kb)
Footpad dermal DC migration following administration of heat-inactivated serum from a healthy control
Representative movie of endogenous dermal DC migration in WT CD11cEYFP mice 16 hours following the administration (footpad injection) of heat-inactivated serum obtained from a healthy control subject. Individual movies of dermal DCs (green) imaged over 1.5 hours with migration tracks shown in white. Blood vessels delineated with a 655 Qdot tracer (red). (MOV 1657 kb)
Rights and permissions
About this article
Cite this article
Clatworthy, M., Aronin, C., Mathews, R. et al. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nat Med 20, 1458–1463 (2014). https://doi.org/10.1038/nm.3709
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3709
This article is cited by
-
Recycling of memory B cells between germinal center and lymph node subcapsular sinus supports affinity maturation to antigenic drift
Nature Communications (2022)
-
Immunogenicity Challenges Associated with Subcutaneous Delivery of Therapeutic Proteins
BioDrugs (2021)
-
The Therapeutic Strategies for SLE by Targeting Anti-dsDNA Antibodies
Clinical Reviews in Allergy & Immunology (2021)
-
Noninvasive Photoacoustic Imaging of Dendritic Cell Stimulated with Tumor Cell-Derived Exosome
Molecular Imaging and Biology (2020)
-
Light Guided In-vivo Activation of Innate Immune Cells with Photocaged TLR 2/6 Agonist
Scientific Reports (2017)