Abstract
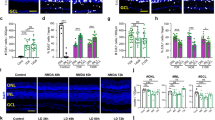
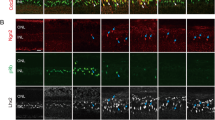
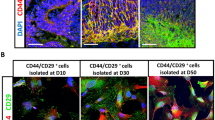
Unlike mammals, teleost fish mount a robust regenerative response to retinal injury that culminates in restoration of visual function1,2. This regenerative response relies on dedifferentiation of Müller glia into a cycling population of progenitor cells. However, the mechanism underlying this dedifferentiation is unknown. Here, we report that genes encoding pluripotency factors are induced following retinal injury. Interestingly, the proneural transcription factor, Ascl1a, and the pluripotency factor, Lin-28, are induced in Müller glia within 6 h following retinal injury and are necessary for Müller glia dedifferentiation. We demonstrate that Ascl1a is necessary for lin-28 expression and that Lin-28 suppresses let-7 microRNA (miRNA) expression. Furthermore, we demonstrate that let-7 represses expression of regeneration-associated genes such as, ascl1a, hspd1, lin-28, oct4, pax6b and c-myc. hspd1, oct4 and c-myca exhibit basal expression in the uninjured retina and let-7 may inhibit this expression to prevent premature Müller glia dedifferentiation. The opposing actions of Lin-28 and let-7 miRNAs on Müller glia differentiation and dedifferentiation are similar to that of embryonic stem cells3 and suggest novel targets for stimulating Müller glia dedifferentiation and retinal regeneration in mammals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
25 February 2015
In the version of this Letter originally published the first lin-28 targeting morpholino oligonucleotide should have read: 5′-GGGCATCTTTATGATTTAGCCTTCT-3′. This has been corrected in all online versions of the Letter.
References
Sherpa, T. et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev. Neurobiol. 68, 166–181 (2008).
Mensinger, A. F. & Powers, M. K. Visual function in regenerating teleost retina following cytotoxic lesioning. Vis. Neurosci. 16, 241–251 (1999).
Melton, C., Judson, R. L. & Blelloch, R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463, 621–626 (2010).
Fausett, B. V. & Goldman, D. A role for α1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 26, 6303–6313 (2006).
Bernardos, R. L., Barthel, L. K., Meyers, J. R. & Raymond, P. A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 27, 7028–7040 (2007).
Fimbel, S. M., Montgomery, J. E., Burket, C. T. & Hyde, D. R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 27, 1712–1724 (2007).
Thummel, R., Kassen, S. C., Montgomery, J. E., Enright, J. M. & Hyde, D. R. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 68, 392–408 (2008).
Ooto, S. [Potential for neural regeneration in the adult mammalian retina]. Nippon Ganka Gakkai Zasshi 110, 864–871 (2006).
Wan, J. et al. Preferential regeneration of photoreceptor from Muller glia after retinal degeneration in adult rat. Vision Res. 48, 223–234 (2008).
Karl, M. O. et al. Stimulation of neural regeneration in the mouse retina. Proc. Natl Acad. Sci. USA 105, 19508–19513 (2008).
Takeda, M. et al. α-Aminoadipate induces progenitor cell properties of Muller glia in adult mice. Invest. Ophthalmol. Vis. Sci. 49, 1142–1150 (2008).
Fausett, B. V., Gumerson, J. D. & Goldman, D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 28, 1109–1117 (2008).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Hochedlinger, K. & Plath, K. Epigenetic reprogramming and induced pluripotency. Development 136, 509–523 (2009).
Zupanc, G. K. & Horschke, I. Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J. Comp. Neurol. 353, 213–233 (1995).
Nimmo, R. A. & Slack, F. J. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma 118, 405–418 (2009).
Eisen, J. S. & Smith, J. C. Controlling morpholino experiments: don't stop making antisense. Development 135, 1735–1743 (2008).
Thummel, R. et al. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev. Dyn. 235, 336–346 (2006).
Bill, B. R., Petzold, A. M., Clark, K. J., Schimmenti, L. A. & Ekker, S. C. A primer for morpholino use in zebrafish. Zebrafish 6, 69–77 (2009).
Li, J. et al. Identification and analysis of the mouse basic/Helix-Loop-Helix transcription factor family. Biochem. Biophys. Res. Commun. 350, 648–656 (2006).
Bertrand, N., Castro, D. S. & Guillemot, F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517–530 (2002).
Rybak, A. et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 10, 987–993 (2008).
Viswanathan, S. R., Daley, G. Q. & Gregory, R. I. Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008).
Heo, I. et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 (2009).
Blackshaw, S. et al. Genomic analysis of mouse retinal development. PLoS Biol. 2, E247 (2004).
Jadhav, A. P., Roesch, K. & Cepko, C. L. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog. Retin. Eye Res. 28, 249–262 (2009).
Roesch, K. et al. The transcriptome of retinal Muller glial cells. J. Comp. Neurol. 509, 225–238 (2008).
Trimarchi, J. M., Stadler, M. B. & Cepko, C. L. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One 3, e1588 (2008).
Kumar, M. S., Lu, J., Mercer, K. L., Golub, T. R. & Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 39, 673–677 (2007).
Qin, Z., Barthel, L. K. & Raymond, P. A. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc. Natl Acad. Sci. USA 106, 9310–9315 (2009).
Thummel, R. et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp. Eye Res. 90, 572–582 (2010).
Miranda, K. C. et al. A pattern-based method for the identification of microRNA-binding sites and their corresponding heteroduplexes. Cell 126, 1203–1217 (2006).
Thummel, R. et al. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 87, 433–444 (2008).
Xu, B., Zhang, K. & Huang, Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA 15, 357–361 (2009).
Kassen, S. C. et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev. Neurobiol. 67, 1009–1031 (2007).
Chung, K. H. et al. Polycistronic RNA polymerase II expression vectors for RNA interference based on BIC/miR-155. Nucleic Acids Res. 34, e53 (2006).
Lindeman, L. C., Vogt-Kielland, L. T., Alestrom, P. & Collas, P. Fish'n ChIPs: chromatin immunoprecipitation in the zebrafish embryo. Methods Mol. Biol. 567, 75–86 (2009).
Senut, M. C., Gulati-Leekha, A. & Goldman, D. An element in the β1-tubulin promoter is necessary for retinal expression during optic nerve regeneration but not after eye injury in the adult zebrafish. J. Neurosci. 24, 7663–7673 (2004).
Jowett, T. Double in situ hybridization techniques in zebrafish. Methods 23, 345–358 (2001).
Wang, W. X. et al. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 28, 1213–1223 (2008).
Acknowledgements
We thank D. Hyde for sharing gfap:gfp transgenic fish, R. Thompson, D. Turner and M. Uhler for sharing expression vectors and reagents, J. Beals for help with confocal microscopy, the UM Flow Cytometry Core for help purifying GFP-labelled Müller glia, V. Kapuria for help with western blots; B. R. Wilfred and D. Turner for advice on miRNAs, P. Macpherson for help with statistics, T. Melendez for expert care of fish and D. Turner, J. Parent and members of the Goldman lab for their support and comments on this work. This work was supported by funds from the NIH NEI RO1 EY018132 (to D.G.) and NIH NICHD T32HD007507 (to R.R.).
Author information
Authors and Affiliations
Contributions
D.G. and R.R. designed and analyzed the research and wrote the paper. D.G. generated the 1016 tuba1a:gfp transgenic fish. R.R. performed all experiments except for Fig. 4a, e where B.V.F. assayed let-7a and let-7f miRNA levels by RT–PCR.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1419 kb)
Rights and permissions
About this article
Cite this article
Ramachandran, R., Fausett, B. & Goldman, D. Ascl1a regulates Müller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol 12, 1101–1107 (2010). https://doi.org/10.1038/ncb2115
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2115
This article is cited by
-
Integrative analysis of transcriptomic profile reveals potential roles of miRNAs in regulating development of Marsupenaeus japonicas
Journal of Oceanology and Limnology (2024)
-
Cell cycle-dependent activation of proneural transcription factor expression and reactive gliosis in rat Müller glia
Scientific Reports (2023)
-
Hippo-Yap/Taz signalling in zebrafish regeneration
npj Regenerative Medicine (2022)
-
Disruption of miR-18a Alters Proliferation, Photoreceptor Replacement Kinetics, Inflammatory Signaling, and Microglia/Macrophage Numbers During Retinal Regeneration in Zebrafish
Molecular Neurobiology (2022)
-
Context-dependent effects of inflammation on retina regeneration
Molecular Neurobiology (2022)