Abstract
Protostemonine (PSN) is the main anti-inflammatory alkaloid extracted from the roots of Stemona sessilifolia (known as “Baibu” in traditional Chinese medicine). Here, we reported the inhibitory effects of PSN on lipopolysaccharide (LPS)-induced macrophage activation in vitro and LPS-induced acute lung injury in mice. Macrophage cell line RAW264.7 cells and mouse bone marrow-derived macrophages (BMDMs) were treated with PSN (1, 3, 10, 30 and 100 μmol/L) for 0.5 h and then challenged with LPS (0.1 μg/mL) for 24 h. Pretreatment with PSN significantly inhibited LPS-induced phosphorylation of MAPKs and AKT, iNOS expression and NO production in the macrophages. C57BL/6 mice were intratracheally injected with LPS (5 mg/kg) to induce acute lung injury (ALI). The mice were subsequently treated with PSN (10 mg/kg, ip) at 4 and 24 h after LPS challenge. PSN administration significantly attenuated LPS-induced inflammatory cell infiltration, reduced pro-inflammatory cytokine (TNF-α, IL-1β and IL-6) production and eliminated LPS-mediated lung edema. Furthermore, PSN administration significantly inhibited LPS-induced pulmonary MPO activity. Meanwhile, LPS-induced phosphorylation of p38 MAPK, iNOS expression and NO production in the lungs were also suppressed. The results demonstrate that PSN effectively attenuates LPS-induced inflammatory responses in vitro and in vivo; the beneficial effects are associated with the decreased phosphorylation of MAPK and AKT and the reduced expression of pro-inflammatory mediators, such as iNOS, NO and cytokines. These data suggest that PSN may be a potential therapeutic agent in the treatment of ALI.
Similar content being viewed by others
Introduction
Inflammation is a defensive reaction that protects the host from harmful stimuli, such as pathologic microbes, cell debris, irritants, or tissue injury, with a primary aim of initiating repair of damaged tissue1. Various cells participate in and prompt the process of inflammation. In particular, macrophages play an important role in inflammatory responses through the production of pro-inflammatory mediators, including reactive oxygen species, nitric oxide (NO), growth factors, chemokines and cytokines such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-62,3,4. Lipopolysaccharide (LPS), an endotoxin derived from gram-positive bacterial cell walls, has been defined as one of the most potent activators of macrophages. LPS-activated macrophages generate cytokines and chemokines that subsequently lead to a transient immune activation. This breaks the balance of the intracellular reduction-oxidation state, leads to oxidative stress and induces an inflammatory response5,6,7.
MAP kinases are a group of serine/threonine protein kinases comprising three subfamilies: extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 MAPKs. These kinases have been well characterized as an important superfamily that plays the pivotal role in cell proliferation, inflammation, and apoptosis8 by inducing phosphorylation of intracellular signaling molecules. AKT, also known as protein kinase B (PKB), is a serine/threonine-specific protein kinase and is a critical substrate of phosphoinositide-3-kinase (PI3K). The PI3k/AKT signaling pathway can be activated by LPS through Toll-like receptor 49.
The inducible nitric oxide synthase (iNOS) produces NO, which is essential for host innate immune responses to pathogens such as viruses, bacteria, fungi, and parasites10. Sustained production of NO endows macrophages with cytostatic or cytotoxic activity against pathogens (viruses, bacteria, fungi, protozoa) and tumor cells11. However, excessive production of NO is related to the development of many diseases, especially inflammatory conditions, such as septic shock, rheumatoid arthritis (RA), and other autoimmune disorders12. Thus, the excessive expression of iNOS is one of the features of inflammation.
Acute lung injury (ALI) is a life-threatening condition characterized by diffuse pulmonary interstitial and alveolar edema that leads to respiratory failure and death. Severe ALI may trigger acute respiratory distress syndrome (ARDS), the most severe form of ALI, and result in multiple organ failure with high mortality (approximately 30%-50%)13,14,15. Unfortunately, there are no effective approaches to treat these acute inflammatory diseases and the pharmacotherapy for ALI is extremely limited. Therefore, it is vital to find effective therapies and drugs for ALI.
Chinese herbal medicine has a long history and is widely used for treating diseases16. Stemona sessilifolia, known as “Baibu” in traditional Chinese medicine, belongs to the Stemonaceae family and is widely distributed in the East Asia17,18. The root of S sessilifolia has been recognized by the Pharmacopoeia of China (hereafter referred to as China Pharmacopoeia) as authentic sources of the herb Radix Stemonae since 200519. The extract of S sessilifolia roots was identified to have antitussive, antitumor and antibacterial activity. It has been used as the indigenous system of medicine for treating respiratory disorders, including pulmonary tuberculosis and bronchitis. Recently, it has also been used as an insecticide19,20,21,22,23. The active components of S sessilifolia are a group of alkaloids, including oxymatrine, tetrandrine, palmatine, and protostemonine (PSN). These alkaloids are found to be protective in lipopolysaccharide/D-galactosamine-induced liver injury in mice24,25,26. However, the precise components and effects in the acute lung injury have not yet been defined. In the present study, we determined the role of PSN (Figure 1A), one of the S sessilifolia-derived alkaloids, in LPS-induced inflammatory responses and ALI both in vitro and in vivo. LPS-induced MAPK and AKT signaling pathways and iNOS expression were detected in response to PSN treatment. Meanwhile, a LPS-induced ALI model was established and the effect of PSN on ALI was analyzed, including inflammatory cell infiltration, tissue injury and cytokines expression. Collectively, our results not only demonstrated that PSN can attenuate LPS-induced ALI but also identified a potential compound for treating ARDS.
PSN reduced phosphorylation of MAPKs and AKT in LPS-challenged RAW264.7 cells. (A) The chemical structure of PSN. (B and G) Western blot analysis of LPS-induced p-p38, p-ERK, p-JNK and p-AKT. RAW264.7 cells were pretreated with 30 μmol/L PSN or 0.1% DMSO for 30 min before stimulation with LPS (100 ng/mL) for indicated time. (H) The p-p38 and p-AKT were measured in RAW264.7 cells that were pretreated with PSN at 0, 1, 10 and 100 μmol/L for 30 min and then challenged with LPS (100 ng/mL) for 45 min. Whole cell extracts were prepared and the protein levels of p-p38, p-ERK, p-JNK and p-AKT were detected. β-Actin was used as loading control. Densitometric analysis of the ratios of p-p38/T-p38 (C, I), p-ERK/ERK (D), p-JNK/JNK (E), p-AKT/β-actin (G) and p-AKT/AKT (J) was performed using ImageJ software. Statistical analysis was performed using Student's t-test. n=3. *P<0.05, **P<0.01.
Materials and methods
Material and reagents
PSN (protostemonine, (5Z)-4-methoxy-3-methyl-5{(1S,3aR, 8S,10aS,10bR)-1-methyl-8-[(2S,4S)-4-methyl-5-oxotetrahydrofuran-2-yl]decahydro-2H-furo[3,2-c]pyrrolo[1,2-a]azepin-2-ylidene}furan-2(5H)-one, C23H31NO6; MW, 417.50; purity >98%) was purchased from Baoji Herbest Bio-Tech Co, Ltd (Baoji, China). A stock solution of PSN (for in vitro study) was prepared at a concentration of 100 mmol/L in dimethylsulfoxide (DMSO) (Sigma, St Louis, MO, USA) and stored at -20 °C. A bicinchoninic acid (BCA) protein assay kit was obtained from Pierce Biotechnology, Inc (Rockford, IL, USA). The ELISA kits for TNF-α and IL-6 were purchased from R&D Systems (Minneapolis, MN, USA). A Nitric Oxide Assay Kit was purchased from Beyotime Biotechnology (Shanghai, China).
The primers used in this study were synthesized by HuaGene Biotech Co, Ltd (Shanghai, China). The primary antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA), including antibodies against p-p38, p38 total, p-ERK, ERK, p-JNK, JNK, p-AKT (S-473), AKT, iNOS and β-actin. Other chemical reagents without special indication were obtained from Sigma (St Louis, MO, USA).
Cell culture and treatment
RAW264.7 cells (ATCC, Manassas, VA, USA) were cultured in RPMI-1640 media supplemented with antibiotics (100 units/mL of penicillin and 100 μg/mL streptomycin) and 10% FBS (37 °C, 5% CO2). BMDMs were isolated from male C57BL/6 mice, and cultured in DMEM media supplemented with antibiotics (100 units/mL of penicillin and 100 μg/mL streptomycin), 10% FBS and 10% culture supernatant of L929 cell for 7 d (37 °C, 5% CO2). Cells were treated with indicated concentrations of PSN according to experiment requirements; 0.1% DMSO was added into culture medium as the solvent control.
Cell viability assay
Cell viability was evaluated by MTT assay. RAW264.7 cells were planted into 96-well plates at a density of 4000 per well in a 37 °C, 5% CO2 incubator overnight. Cells were then treated with different concentrations of PSN (1, 3, 10, 30, 100 μmol/L) for 48 h and 10 μL of MTT (5 mg/mL) was added to each well and incubated for 4 h. After 4 h, the supernatant was removed and the formation of formazan was resolved with 150 μL/well of DMSO. The optical density was measured at 570 nm on a microplate reader. Concentrations were determined for three wells of each sample, and each experiment was performed in triplicate.
Animals
Male C57BL/6 mice (6–10 weeks old, 20±3 g, specific pathogen free) were purchased from SLAC Laboratory Animal Corporation (Shanghai, China). Five mice were housed in one cage in a climate-controlled room (25 °C, 55% humidity and 12-h light/darkness cycle), fed a standard laboratory diet and acclimated for at least 2 weeks prior to the experiment. All experimental protocols described in this study were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University.
Mouse model building
Mice were randomly divided into 5 groups: control, LPS/vehicle (4 h and 24 h) groups, and LPS/PSN (4 h and 24 h) groups. Each group contained five mice. PSN was dissolved in a vehicle (polyoxyethylene hydrogenated castor oil:ethanol:H2O=1:1:8). Mice were administered an intratracheal injection of LPS (5 mg/kg) and subsequent intraperitoneal injection of vehicle or PSN (10 mg/kg). For mice treated with LPS for 24 h, another injection with solvent or PSN was administered (12 h after LPS challenge). At the indicated time points (4 h and 24 h), mice were euthanized and samples (bronchoalveolar lavage fluid and lung tissue samples) were collected.
Acquisition and analysis of BALF
The lungs were lavaged three times with 1 mL PBS and BAL fluid was centrifuged at 4 °C. The cell-free supernatant was harvested for total protein analysis using the BCA protein assay kit (Beyotime, Shanghai, China).
Histopathological Analysis
Lung tissues (left lobe) were fixed by 4% paraformaldehyde, embedded in paraffin and cut into 5-μm-thick sections in a microtome (RM2235, Leica Biosystems, Wetzlar, Germany). Sections were stained with hematoxylin and eosin, and images were captured by a microscope (RX51, Olympus Optical Co Ltd, Tokyo, Japan). The degree of lung injury was assessed using a semiquantitative scoring method, with lung injury graded from 0 (normal) to 4 (severe) in four categories: interstitial inflammation, inflammatory cell infiltration, congestion, and edema. As previously reported27, the total lung injury score was calculated by adding up the individual scores of each category.
Western blotting analysis
Macrophages were planted on 6-well plates (1.0×106 cells per well) and incubated overnight (37 °C, 5% CO2). Cells were then treated with different doses of PSN (0, 1, 3, 10, 30, and 100 μmol/L) for 0.5 h before being stimulated with LPS (0.1 μg/mL) for 24 h, or they were treated with 30 μmol/L PSN for 0–8 h. Cells were collected with loading buffer (175 mmol/L Tris-HCl, 100 mmol/L DTT, 4.0% SDS, 7.5% glycerine and 0.2% bromophenol blue in ddH2O). For in vivo analysis, lung tissues were collected and lysed in RIPA buffer (WEIBO BioTech Co Ltd, Shanghai, China) plus 1 mmol/L PMSF. Proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were washed and incubated in 5% skim milk at room temperature for 1 h. The samples were then probed at 4 °C overnight with primary antibodies (1:1000 dilution) as described above, followed by incubation with secondary antibodies (KPL, Gaithersburg, MD, USA) for 1 h at room temperature. Quantification of Western blots was analyzed with ImageJ software (National Institute of Mental Health, Bethesda, MD, USA).
Measurement of cytokine production
Frozen lungs (right upper lobe) were homogenized and the supernatant was collected to measure the expression of cytokines TNF-α and IL-6 using ELISA assay kits. These concentrations were interpolated from the standard curves for recombinant TNF-α and IL-6.
RNA isolation, reverse transcription and quantitative PCR
RAW264.7 cells (5×105) were incubated with PSN (0, 1, 3, 10, 30, and 100 μmol/L) and challenged with LPS for 4 h. The cells were collected, and total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For in vivo analysis, frozen lungs (right upper lobe) were homogenized and total RNA was isolated. cDNA was prepared by ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan) and amplified by real-time PCR on a StepOne Plus system (Thermo Fisher Scientific, Waltham, MA, USA) with primer sets for IL-6 (forward, 5′-CCACCAAGAACGATAGTCAA-3′; reverse, 5′-TTTCCACGATTTCCCAGA-3′), TNF-α (forward, 5′-TTCTCATTCCTGCTTGTGG-3′; reverse, 5′-ACTTGGTGGTTTGCTACG-3′), IL-1β (forward, 5′-CCAGCTTCAAATCTCACAGCAG-3′; reverse, 5′-CTTCTTTGGGTATTGCTTGGGATC-3′), and GAPDH (forward, 5′-TGCGACTTCAACAGCAACTC-3′; reverse, 5′-CTTGCTCAGTGTCCTTGCTG-3′).
MPO activity assay
The largest right lobes of lungs were collected and were homogenized in 50 mmol/L potassium phosphate buffer containing 0.5% hexadecyl trimethyl ammonium bromide (HTAB). After centrifugation, supernatant was discarded and pellets were resuspended in 0.5% HTAB. The pellet-HTAB was subjected to a freeze-thaw process three times. Supernatants were collected and were diluted in reaction solution containing 3, 3′, 5, 5′-tetramethylbenzidine and H2O2. The mixture was incubated for 5 min at 25 °C and the change of absorbance at 655 nm was measured by the microplate reader (FlexStation 3, Molecular Devices, CA, USA). Protein concentrations of the supernatants were determined as described above. The MPO activity value was defined as the absorbance change per min per gram protein as previously described28.
Quantitative determination of nitrite levels
To assess LPS-induced inflammation, NO2- levels were determined using Griess reagent. Macrophages were planted on 12-well plates (5×105 cells per well) and incubated overnight (37 °C, 5% CO2). Cells were then treated with different concentrations of PSN (0, 1, 3, 10, 30, and 100 μmol/L) for 0.5 h before being stimulated with LPS (0.1 μg/mL) for 24 h. Supernatants were collected and NO levels were measured using the Nitric Oxide Assay Kit (Beyotime, Shanghai, China), as previously described28.
Statistical analysis
Data are presented as mean±SD obtained from at least three independent tests. Student's t-test (paired comparison) was performed using Prism 5 (GraphPad, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
PSN attenuated the phosphorylated MAPKs and AKT induced by LPS in both RAW264.7 cells and BMDMs
To elucidate the effects of PSN on MAPK and AKT signaling pathways in macrophages, we measured the phosphorylation of p38 MAPK, ERK, JNK and AKT in the macrophage cell line RAW264.7 (Figure 1) and BMDMs (Figure 2). Macrophages were treated with 30 μmol/L PSN or 0.1% DMSO for 0.5 h, followed by LPS (0.1 μg/mL) challenge for 0–8 h. LPS markedly increased the levels of p-p38, p-ERK, p-JNK and p-AKT that were significantly inhibited by the PSN treatment (Figure 1B-1G, 2A-2E). RAW264.7 cells were treated with PSN (0, 1, 10, 100 μmol/L) for 0.5 h, followed by LPS (0.1 μg/mL) challenge for 45 min. LPS-induced expression of p-p38 and p-AKT was inhibited by PSN in a dose-dependent manner (Figure 1H-1J). These results demonstrate that PSN attenuates LPS-induced activation of MAPKs and AKT in macrophages.
PSN reduced the phosphorylation of MAPKs and AKT in LPS-challenged BMDMs. (A) Western blot analysis of LPS-induced p-p38, p-ERK, p-JNK and p-AKT expression. BMDMs were pretreated with 30 μmol/L PSN or 0.1% DMSO for 30 min before stimulation of LPS (100 ng/mL). The expressions of p-p38, p-ERK, p-JNK and p-AKT were detected as above. Total p38, ERK, JNK and AKT antibodies were used as loading controls, respectively. (B–E) Densitometric analysis of the ratios of p-p38/T-p38, p-ERK/ERK, p-JNK/JNK, and p-AKT/AKT was performed using ImageJ software. Statistical analysis was performed using Student's t-test. n=3. *P<0.05, **P<0.01.
PSN inhibited the LPS-induced expression of pro-inflammatory mediators in both RAW264.7 cells and BMDMs
The potential cytotoxicity of PSN on macrophages was measured using an MTT assay after incubating RAW264.7 cells with PSN for 48 h. PSN displayed no cellular toxicity against RAW264.7 cells (Figure 3A). Pretreatment with PSN (0, 1, 3, 10, 30, and 100 μmol/L) reduced the LPS-induced iNOS expression in a dose-dependent manner in both RAW264.7 cells (Figures 3B and 3C) and BMDMs (Figures 3D and 3E). Accordingly, the LPS-induced generation of nitric oxide (NO), the major product of iNOS, was dose-dependently inhibited by PSN (Figures 3F and 3G). Moreover, PSN pretreatment reduced the production of pro-inflammatory cytokines including IL-6, TNF-α and IL-1β (Figure 3H-3J) in LPS-induced RAW264.7 cells. These data suggest that PSN attenuates LPS-induced iNOS expression, NO secretion and pro-inflammatory cytokine production in macrophages.
PSN inhibited iNOS expression, NO and pro-inflammatory cytokine production in LPS-stimulated macrophages. (A) PSN had no effect on toxicity of macrophages. RAW264.7 cells were treated with PSN at 0, 1, 3, 10, 30 and 100 μmol/L for 48 h. Cell viability was determined by MTT assay. (B and D) PSN decreased LPS-induced iNOS expression in RAW264.7 cells and BMDMs. Macrophages were pretreated with PSN at 0, 1, 3, 10, 30 and 100 μmol/L for 30 min before stimulation with LPS (100 ng/mL). After 24 h incubation, cell lysates were collected for Western blot analysis and supernatant was collected for NO detection. β-Actin was used as loading control. (C and E) Densitometric analysis of the ratios of iNOS and β-actin was performed using ImageJ software. (F and G) PSN reduced the NO production in LPS-induced RAW264.7 cells and BMDMs. (H–J) PSN inhibited LPS-induced mRNA expression of pro-inflammatory cytokines. RAW264.7 cells were treated with PSN at indicated concentrations before LPS challenge for 4 h. Total RNAs were then isolated, and the mRNA levels of IL-6 (H), TNF-α (I) and IL-1β (J) were quantified by quantitative real-time PCR. Statistical analysis was performed using Student's t-test. n=3. *P<0.05 **P<0.01, ***P<0.001.
PSN inhibited the lung edema and inflammatory cell infiltration in LPS-induced ALI
To determine whether PSN can attenuate endotoxin-induced pneumonia, we established the mouse model of LPS-induced ALI, in which intratracheal injection of LPS (5 mg/kg in PBS) into mice was followed by intraperitoneal administration of solvent (polyoxyethylene hydrogenated castor oil:ethanol:H2O=1:1:8) or 10 mg/kg PSN (Figure 4A). A schematic illustration of the experimental design is shown in Figure 4A. The total protein concentration in BALF was increased approximately 10-fold after treatment with LPS for both 4 h (Figure 4B) and 24 h (Figure 4C). In response to PSN treatment for 4 h and 24 h, the protein concentration in BALF was reduced by approximately 58% and 32%, respectively (Figure 4B and 4C, P<0.05).
PSN attenuated inflammatory cell accumulation in lung tissues of ALI mice. (A) The schematic timeline of LPS-induced acute lung injury model. Mice were subjected to an intratracheal injection of LPS (5 mg/kg) and subsequent intraperitoneal injection of solvent or PSN (10 mg/kg). An additional injection of solvent or PSN was administered (12 h after LPS challenge) to mice that underwent a 24 h LPS treatment. (B, C) The total protein concentrations in BALF after LPS challenge for 4 h (B) and 24 h (C) were detected. (D, E) Myeloperoxidase (MPO) activity was determined according to the manufacturer's instructions 4 h (D) and 24 h (E) after LPS challenge. Values represent mean±SD. n=5 mice each group. *P<0.05, **P<0.01, ***P<0.001.
MPO activity of lung tissue is a specific marker of neutrophil infiltration and indicates the pulmonary inflammatory injury. Compared with increased MPO activity in LPS-induced lung tissue, the MPO activity was relieved with a decrease of approximately 46% and 63% by treatment with PSN for 4 h and 24 h, respectively (Figure 4D and 4E).
PSN ameliorated lung histopathological alteration in LPS-induced ALI
To assess the effects of PSN on LPS-induced acute lung injury, we detected the histological changes of lung tissue. As shown in Figures 5A-5E, mice treated with 5 mg/kg of LPS displayed severe ALI. In comparison, treatment with 10 mg/kg of PSN for 4 h (Figures 5B, 5C) and 24 h (Figures 5D, 5E) significantly attenuated LPS-induced lung injury, including decreased infiltration of inflammatory cells and thickening of the alveolar walls. The degree of lung injury was further determined by a semiquantitative scoring method27 (Figure 5F). These data indicate that PSN protects against LPS-induced lung injury.
PSN ameliorated pathological changes in ALI mice. The right lobes were fixed and processed for histological evaluation. (A–E) After H&E staining, the representative changes of the lungs from mice of different groups were shown with original magnification (100×) and partial enlarged detail (400×). (F) The degree of lung injury was quantitatively evaluated according to the histological analysis. Values are mean±SD. n=5. *P<0.05, **P<0.01.
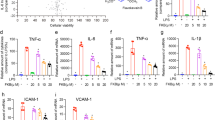
PSN reduced pro-inflammatory mediator production and inhibited p38 MAPK activation in LPS-induced ALI
To further demonstrate the anti-inflammatory effect of PSN, we measured pro-inflammatory cytokine production in the lungs of ALI mice that were treated with LPS for 4 h. The analysis for mRNA expression showed that 10 mg/kg of PSN significantly inhibited the LPS-induced enhancement of IL-6, TNF-α and IL-1β (Figures 6A-6C, P<0.05). The protein levels of IL-6 and TNF-α were also reduced by PSN treatment by approximately 48% and 57% (Figures 6D, 6E, P<0.05), respectively, by ELISA assay. In addition to pro-inflammatory cytokines, the LPS-induced expression of iNOS (Figures 7A and 7C) and NO release (Figure 7D) were also decreased by PSN treatment. In addition, PSN also inhibited the phosphorylation of p38 without affecting total p38 expression (Figures 7A and 7B). These in vivo data suggest that PSN alleviates LPS-induced inflammatory responses and inhibits the activation of p38 MAPK.
PSN decreased expression of IL-6, TNF-α and IL-1β in mice treated with LPS for 4 h. After treatment with LPS and LPS/PSN for 4 h, mice were euthanized and the lung tissues were collected for homogenization. The total RNAs were isolated from lung homogenates, and the supernatant was stored. The mRNA levels of IL-6 (A), TNF-α (B), IL-1β (C) and protein production of IL-6 (D) and TNF-α (E) were then quantified by real-time PCR and ELISA assays, respectively. Values represent mean±SD. n=3. *P<0.05.
PSN decreased p38 phosphorylation, iNOS expression and NO production in the lungs of ALI mice. Sepsis-induced ALI were made by intratracheal injection of LPS (5 mg/kg) with or without PSN (10 mg/kg) administration for 4 h. Mice were then euthanized, and the lung tissues were collected. (A) Western blot analysis of p-p38 and iNOS protein expression in the lungs of control mice, ALI mice and PSN treated mice. (B and C) The ratio of p-p38/p38 and iNOS/β-actin were measured by densitometry. (D) The lung tissues were homogenized, and the supernatants were collected to detect the amounts of secreted NO. Data represent mean±SD. n=3. *P<0.05.
Discussion
Sepsis-induced ALI is a leading cause of mortality and morbidity worldwide29,30. ALI is part of a systemic inflammatory process, particularly systemic sepsis, which is closely associated with the excessive release of inflammatory mediators, extravasation of protein-rich fluid, and excessive inflammatory cell infiltration31,32,33. It is well known that inflammatory responses promote lung injury following an endotoxin challenge. Once LPS, the bacteria-derived endotoxin, enters the bloodstream, it can elicit systemic inflammation that mimics many of the initial clinical features of ALI34. In recent years, the mouse model of LPS-induced ALI has been widely used to study the pathogenesis of ALI35,36,37.
Suppression of pro-inflammatory cytokine production provides a possibility of regulating inflammatory responses, while inhibiting TNF-α, IL-1β and IL-6 production can alleviate LPS-induced inflammation38,39,40,41,42,43. In recent decades, several commercially successful anti-cytokine therapeutics have been developed, including adalimumab and etanercept, which target TNF-α signaling44. However, these medicines also have the drawbacks of difficult production, high costs, and side effects. To date, there are no clinically available medicines or efficient treatments for ALI.
Herbal medicines have been widely used for treating various human diseases. However, traditional Chinese medicine treatment has been criticized for its unclear composition and toxicology. With the development of pharmacology research, numerous therapeutic components and/or analogs derived from herbal extracts have been identified, such as Taxol, camptothecin and flavoxate29. Increasing attention has been aimed at the re-evaluation and development of traditional Chinese medicines (TCMs). Alkaloids are major active components isolated from the roots of S sessilifolia, which is often used in prescription inflammatory therapy as a TCM and as a Korean herbal formula16. PSN, an alkaloid, can significantly inhibit the production of pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, and attenuate acute liver injury by reduction of boosted malondialdehyde (MDA) and reactive oxygen species (ROS) levels45. In this study, both in vivo and in vitro data showed that PSN inhibited pro-inflammatory cytokine expression and protected against LPS-induced ALI and inflammatory responses. During the early stage of lung injury, inflammatory cells, especially neutrophils and macrophages, are recruited into lungs and contribute to LPS-induced inflammatory responses46,47,48, including the production of nitric oxide (NO) and inflammatory cytokines (eg, TNF-α, IL-1β, and IL-6)2,3. The expression of iNOS and NO are hallmarks of classically activated macrophages. LPS-induced NO release is of importance in host defense and invader-killing at inflammatory sites49. However, excessive amounts of NO can promote cytokine and matrix metalloproteinase production, mitochondrial dysfunction and cell apoptosis, leading to the aggravation of inflammation and tissue injury50. NO is generally produced by iNOS in macrophage activation51. The iNOS-deficient mice are more resistant to LPS-induced ALI than wild-type mice52. In the present study, PSN treatment significantly reduced the LPS-induced expression of iNOS in a murine macrophage cell line, RAW264.7 cells and primary BMDMs. These results suggest that PSN inhibits LPS-stimulated inflammatory responses by suppressing the expression of iNOS in macrophages.
Our data indicate that PSN can reduce the MAPKs and PI3K/AKT signaling pathways (Figures 1, 2, 3). Both pathways were triggered by TLR4, a recognition receptor of TLR4 implicated in cytokine expression5,53,54,55,56. Accumulating evidence demonstrates that TLR4 plays a critical role in innate immune and inflammatory responses, in which MAPK and AKT signals are important for mediating inflammatory processes in response to infection57,58,59,60,61,62. Compounds that can down-regulate MAPKs and AKT signaling are expected to be useful in protecting against inflammatory diseases. Because MAPKs and AKT-mediated signals also modulate cell proliferation and differentiation, most compounds against these two signals display severe cytotoxic effects and inhibit cell proliferation. However, our data and those obtained in other studies demonstrated that PSN had no side effects in vitro or in vivo45. Based on our data, PSN could protect against LPS-induced lung injury and inflammatory responses both in vivo and in vitro by inhibiting MAPK pathways and AKT signaling. Collectively, our study suggests that PSN might be a promising agent in the treatment of acute inflammatory diseases.
Conclusions
PSN, the main active alkaloid component of a traditional Chinese herb S sessilifolia, has anti-inflammatory activities against LPS-induced inflammatory responses both in vitro and in vivo. Treatment with PSN in a LPS-induced murine ALI model resulted in reduction of inflammatory cell infiltration, decreased expression of pro-inflammatory cytokines and alleviated pathologic changes in lung tissue. In vitro analysis demonstrated that PSN inhibited the expression of iNOS in both RAW264.7 and BMDM cells challenged with LPS. The results also demonstrated that PSN can modulate inflammatory processes by inhibiting MAPKs and PI3K/AKT signaling pathways. Furthermore, PSN had no cytotoxic effects in vitro or in vivo. Therefore, our results suggested that PSN protected against LPS-induced ALI by inhibiting iNOS expression and suppressing MAPK and PI3K/AKT signaling transduction in macrophages. PSN may serve as a potential agent in the treatment of sepsis-induced ALI.
Author contribution
Feng QIAN and Ya-xian WU conceived of the study and drafted the manuscript; Hui-qiong HE participated in the design, established animal models and helped draft the manuscript; Yun-juan NIE performed the experiments and data analysis; Yun-he DING participated in the animal experiments; and Lei SUN was involved in discussion of the experiments. All authors read and approved the final manuscript.
References
Gao HM, Hong JS . Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol 2008; 29: 357–65.
Molloy RG, Mannick JA, Rodrick ML . Cytokines, sepsis and immunomodulation. Br J Surg 1993; 80: 289–97.
Mosser DM, Edwards JP . Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–69.
Medina EA, Morris IR, Berton MT . Phosphatidylinositol 3-kinase activation attenuates the TLR2-mediated macrophage proinflammatory cytokine response to francisella tularensis live vaccine strain. J Immunol 2010; 185: 7562–72.
Goodman RB, Pugin J, Lee JS, Matthay MA . Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 2003; 14: 523–35.
Jeyaseelan S, Chu HW, Young SK, Worthen GS . Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 2004; 72: 7247–56.
Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR . Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol 2005; 289: F1324–32.
Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, et al. P38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotectiveautocrine signaling pathway in a cardiac myocyte model system. J Biol Chem 2000; 275: 23814–24.
Ci XX, Chu X, Wei MM, Yang XF, Cai QR, Deng XM . Different effects of farrerol on an OVA-induced allergic asthma and LPS-induced acute lung injury. PLoS One 2012; 7: e34634.
MacMicking J, Xie QW, Nathan C . Nitric oxide and macrophage function. Annu Rev Immunol 1997; 15: 323–50.
Nathan C, Shiloh MU . Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 2000; 97: 8841–8.
O'Shea JJ, Ma A, Lipsky P . Cytokines and autoimmunity. Nat Rev Immunol 2002; 2: 37–45.
Ware LB, Matthay MA . The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–49.
Fu YH, Liu B, Feng XS, Li FY, Liang DJ, Liu ZC, et al. The effect of magnolol on the toll-like receptor 4/nuclear factor κB signaling pathway in lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 2012; 689: 255–61.
Zambon M, Vincent JL . Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008; 133: 1120–7 .
Jung KH, Choi HL, Park S, Lee G, Kim M, Min JK, et al. The effects of the standardized herbal formula PM014 on pulmonary inflammation and airway responsiveness in a murine model of cockroach allergen-induced asthma. J Ethnopharmacol 2014; 155: 113–22.
Jiangsu New Medical College (Ed), The Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Press, Shanghai, 1986, p 860.
Tsi ZH, Duyfjes BEE . Stemonaceae. In Flora of China, Wu ZY, Raven P (Eds), Science Press, Beijing, 2000, p 70–2.
Chinese Pharmacopoeia Committee, “Pharmacopoeia of the People's Republic of China,” 2005 ed, Part I, Chemical Industry Press, Beijing, China, 2005, p 88.
Lin LG, Zhong QX, Cheng TY, Tang CP, Ke CQ, Lin G, et al. Stemoninines from the roots of Stemona tuberosa. J Nat Prod 2006; 69: 1051–4.
Chung HS, Hon PM, Lin G, But PP, Dong H . Antitussive activity of Stemona alkaloids from Stemona tuberosa. Planta Med 2003; 69: 914–20.
Jiang RW, Hon PM, Zhou Y, Chan YM, Xu YT, Xu HX, et al. Alkaloids and chemical diversity of Stemona tuberosa. J Nat Prod 2006; 69: 749–54.
Cong XD, Xu GJ . Species systematization and quality evaluation of commonly used Chinese traditional drugs: (Xu GJ, Xu GS, Eds.). Fuzhou Science and Technology Press, Fujian, China, 1994, p 97–105.
Xiang X, Wang G, Cai X, Li Y . Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chin Med J 2002; 115: 593–6.
Gong X, Luo FL, Zhang L, Li HZ, Wu MJ, Li XH, et al. Tetrandrine attenuates lipopolysaccharide-induced fulminant hepatic failure in D-galactosamine-sensitized mice. Int Immunopharmacol 2010; 10; 357–63.
Lee WC, Kim JK, Kang JW, Oh WY, Jung JY, Kim YS, et al. Palmatine attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure inmice. Food Chem Toxicol 2010; 48: 222–8.
Shi X, Sun H, Zhou D, Xi H, Shan L . Arctigenin attenuates lipopolysaccharide-induced acute lung injury in rats. Inflammarion 2015; 38: 623–31.
He HQ, Wu YX, Nie YJ, Wang J, Ge M, Qian F . LYRM03, an ubnimex derivative, attenuates LPS-induced acute lung injury in mice by suppressing the TLR4 signaling pathway. Acta Pharmacol Sin 2017; 38: 342–50.
Martin GS, Mannino DM, Eaton S, Moss M . The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546–54.
Angus DC, Wax RS . Epidemiology of sepsis: an update. Crit Care Med 2001; 29: S109–16.
Zhong WT, Jiang LX, Wei JY, Qiao AN, Wei MM, Soromou LW, et al. Protective effect of esculentoside A on lipopolysaccharide-induced acute lung injury in mice. J Surg Res 2013; 185: 364–72.
Yoshida T, Nagai K, Inomata T, Ito Y, Betsuyaku T, Nishimura M . Relationship between neutrophil influx and oxidative stress in alveolar space in lipopolysaccharide-induced lung injury. Respir Physiol Neurobiol 2014; 191: 75–83.
Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 2003; 167: 1027–35.
Glauser MP, Zanetti G, Baumgartner JD, Cohen J . Septic shock: pathogenesis. Lancet 1991; 338: 732–6.
Joh EH, Gu W, Kim DH . Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-kappa B and MAPK pathways. Biochem Pharmacol 2012; 84: 331–40.
Wu J, Zhang YY . Guo L, Li H, Chen DF . Bupleurum polysaccharides attenuates lipopolysaccharide-induced inflammation via modulating Toll-like receptor 4 signaling. PLoS One 2013; 8: e78051.
Luo Y, Zhang B, Xu DQ, Liu Y, Dong MQ, Zhao PT, et al. Protective effect of on lipopolysaccharide-induced acute lung injury in mice. Pulm Pharmacol Ther 2011; 24: 240–6.
Shim DW, Shin HJ, Han JW, Ji YE, Jang CH, Koppula S, et al. A novel synthetic derivative of melatonin, 5-hydroxy-2-isobutyl-streptochlorin (HIS), inhibits inflammatory responses via regulation of TRIF-dependent signaling and inflammasome activation. Toxicol Appl Pharmacol 2015; 284: 227–35.
Li C, Yang D, Cao X, Wang F, Jiang H, Guo H, et al. LFG-500, a newly synthesized flavonoid, attenuates lipopolysaccharideinduced acute lung injury and inflammation in mice. Biochem Pharmacol 2016; 113: 57–69.
Ma C, Zhu L, Wang J, He H, Chang X, Gao J, et al. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-inducedinflammation in acute lung injury by suppressing PI3K/AKT/mTOR signaling pathway. J Ethnopharmacol 2015; 168: 349–55.
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu C, et al. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Int Immunopharmacol 2016; 35: 315–22.
Hsieh YH, Deng JS, Pan HP, Liao JC, Huang SS, Huang GJ . Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int Immunopharmacol 2017; 44: 16–25.
Chu X, Ci X, Wei M, Yang X, Cao Q, Guan M, et al. Licochalcone A inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo. J Agric Food Chem 2012; 60: 3947–54.
Fiore M, Forli S, Manetti F . Targeting mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2, MK2): medicinal chemistry efforts to lead small molecule inhibitors to clinical trials. J Med Chem 2016; 59: 3609–34.
Cheng Z, Yue L, Zhao W, Yang X, Shu G . Protective effects of protostemonine on LPS/GalN-induced acute liver failure: Roles of increased hepatic expression of heme oxygenase-1. Int Immunopharmacol 2015; 29: 798–807.
Letsiou E, Sammani S, Wang H, Belvitch P, Dudek SM . Parkin regulates lipopolysaccharide-induced pro-inflammatory responses in acute lung injury. Transl Res 2017; 181: 71–82.
Zeng L, Yang XT, Li HS . The cellular kinetics of lung alveolar epithelial cells and its relationship with lung tissue repair after acute lung injury. Respir Res 2016; 17: 164.
Jiang Z, Zhou Q, Gu C, Li D, Zhu L, et al. Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-inducedacute lung injury. Am J Physiol Lung Cell Mol Physiol 2017; 312: L231–42.
Korhonen R, Lahti A, Kankaanranta H, Moilanen E . Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 2005; 4: 471–9.
Jin M, Suh SJ, Yang JH, Lu Y, Kim SJ, Kwon S, et al. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-κB and ERK1/2 inactivation. Food Chem Toxicol 2010; 48: 3073–9.
Chakraborty PD, Bhattacharyya D, Pal S, Ali N . In vitro induction of nitric oxide by mouse peritoneal macrophages treated with human placental extract. Int Immunopharmacol 2006; 6: 100–7.
Weimann J, Bloch KD, Takata M, Steudel W, Zapol WM . Congenital NOS2 deficiency protects mice from LPS-induced hyporesponsiveness to inhaled nitric oxide. Anesthesiology 1999; 91: 1744–53.
McCubrey JA, Lahair MM, Franklin RA . Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 2006; 8: 1775–89.
Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M . Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol 2003; 33: 597–605.
Laird MH, Rhee SH, Perkins DJ, Medvedev AE, Piao W, Fenton MJ, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol 2009; 85: 96677.
Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M . Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol 2003; 33: 597–605.
Erridge C . Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 2010; 87: 989–99.
Lee CY, Yang JJ, Lee SS, Chen CJ, Huang YC, Huang KH, et al. Protective effect of Ginkgo biloba leaves extract, EGb761, on endotoxin-induced acute lung injury via a JNK- and AKT-dependent NF-κB pathway. J Agric Food Chem 2014; 62: 6337–44.
He R, Feng J, Xun Q, Qin Q, Hu C . High-intensity training induces EIB in rats through neuron transdifferentiation of adrenal medulla chromaffin cells. Am J Physiol Lung Cell Mol Physiol 2013; 304: L602–12.
Chen CC, Lin MW, Liang CJ, Wang SH . The anti-inflammatory effects and mechanisms of eupafolin in lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages. PLos One 2016; 11: e0158662.
Hudlikar RR, Venkadakrishnan VB, Kumar R, Thorat RA, Kannan S, Ingle AD, et al. Polymeric black tea polyphenols (PBPs) inhibit benzo(a) pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung carcinogenesis potentially through down-regulation of p38 and AKT phosphorylation in A/J mice. Mol Carcinog 2017; 56: 625–40.
Liu B, Huang J, Zhang B . Nobiletin protects against murine L-arginine-induced acute pancreatitis in association with downregulating p38MAPK and AKT. Biomed Pharmacother 2016; 81: 104–10.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81373424, 81401302, and 81573438 and 81773741) as well as the Specialized Research Fund for the Doctoral Program of Higher Education of China (20130073120108).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Yx., He, Hq., Nie, Yj. et al. Protostemonine effectively attenuates lipopolysaccharide-induced acute lung injury in mice. Acta Pharmacol Sin 39, 85–96 (2018). https://doi.org/10.1038/aps.2017.131
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.131
Keywords
This article is cited by
-
The structure and bioactivities of Stemona alkaloids and alkaloids with [1,2-α] azepine nucleus (2009–2021)
Phytochemistry Reviews (2023)
-
Ruscogenin attenuates particulate matter-induced acute lung injury in mice via protecting pulmonary endothelial barrier and inhibiting TLR4 signaling pathway
Acta Pharmacologica Sinica (2021)
-
Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway
Acta Pharmacologica Sinica (2021)
-
CXCR4 knockdown prevents inflammatory cytokine expression in macrophages by suppressing activation of MAPK and NF-κB signaling pathways
Cell & Bioscience (2019)
-
Isoalantolactone suppresses LPS-induced inflammation by inhibiting TRAF6 ubiquitination and alleviates acute lung injury
Acta Pharmacologica Sinica (2019)