Abstract
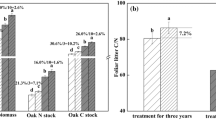
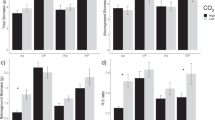
The effects of elevated CO2 on nutrient cycling and selected belowground processes in the closed-canopy sweetgum plantation were assessed as part of a free-air CO2 enrichment (FACE) experiment at Oak Ridge, Tennessee. We hypothesized that nitrogen (N) constraints to growth response to elevated CO2 would be mitigated primarily by reduced tissue concentrations (resulting in increased biomass production per unit uptake) rather than increased uptake. Conversely, we hypothesized that the constraints of other nutrients to growth response to elevated CO2 would be mitigated primarily by increased uptake because of adequate soil supplies. The first hypothesis was not supported: although elevated CO2 caused reduced foliar N concentrations, it also resulted in increased uptake and requirement of N, primarily because of greater root turnover. The additional N uptake with elevated CO2 constituted between 10 and 40% of the estimated soil mineralizeable N pool. The second hypothesis was largely supported: elevated CO2 had no significant effects on tissue concentrations of P, K, Ca, or Mg and caused significantly increased uptake and requirement of K, Ca, and Mg. Soil exchangeable pools of these nutrients are large and should pose no constraint to continued growth responses. Elevated CO2 also caused increased microbial biomass, reduced N leaching and increased P leaching from O horizons (measured by resin lysimeters), reduced soil solution NH +4 , SO 2−4 , and Ca2+ concentrations, and increased soil solution pH. There were no statistically significant treatment effects on soil nutrient availability as measured by resin capsules, resin stakes, or in situ incubations. Despite significantly lower litterfall N concentrations in the elevated CO2 treatment, there were no significant treatment effects on translocation or forest floor biomass or nutrient contents. There were also no significant treatment effects on the rate of decomposition of fine roots. In general, the effects of elevated CO2 on nutrient cycling in this study were not large; future constraints on growth responses imposed by N limitations will depend on changes in N demand, atmospheric N deposition, and soil mineralization rates.
Similar content being viewed by others
References
Allen A.A., Andrews J.A., Finzi A.C., Matamala R., Richter D.D. and Schlesinger W.A. 2000. Effects of free-air CO2 enrichment (FACE) on belowground processes in a Pinus taeda forest. Ecol. Appl. 10: 437–448.
Andrews J.A. and Schlesinger W.H. 2001. Soil CO2 dynamics, acidification, and chemical weathering in a temperate forest with experimental CO2 enrichment. Global Biogeochem. Cycles 15: 149–162.
Bernston G.M. and Bazzaz F.A. 1996. Belowground positive and negative feedbacks on CO2 and growth enhancement. Plant Soil 187: 119–133.
Cheng W., Sims D.A., Luo Y., Johnson D.W., Ball J.T. and Coleman J.S. 2000. Carbon budgeting in plant–soil mesocosms under elevated CO2: locally missing carbon? Global Change Biol. 6: 99–110.
Cole D.W. and Rapp M. 1981. Elemental cycling in forest ecosystems. In: Reichle D.E. (ed) Dynamic Properties of Forest Ecosystems (pp. 341–409 Cambridge University Press, London.
Curtis P.S., Vogel C.S., Wang X., Pregitzer K.S., Zak D.R., Lussenhop J., Kubiske M. and Teeri J. 2000. Gas exchange, leaf nitrogen, and growth efficiency of Populus tremloides in a CO2-enriched atmosphere. Ecol. Appl. 10: 3–17.
Dobermann A.H., Langner A.H., Mutscher H., Yang J.E., Skogley E.O., Adviento M.A. and Pampolino M.F. 1994. Nutrient adsorption kinetics of ion exchange resin capsules: a study with soils of international origin. Commun. Soil Sci. Plant. Anal. 25: 1329–1353.
Finzi A.C., DeLucia E.H., Hamilton J.G., Richter D.D. and Schlesinger W.H. 2002. The nitrogen budget of a pine forest under free air CO2 enrichment. Oecologia 132: 567–578.
Hamilton J.G., DeLucia E.H., George K., Naidu S.L., Finzi A.C. and Schlesinger W.H. 2001. Forest carbon balance under elevated CO2. Oecologia 131: 250–260.
Hendrey G.R., Ellsworth D.S., Lewin K.F. and Nagy J. 1999. A free-air system for exposing tall forest vegetation to elevated atmospheric CO2. Global Change Biol. 5: 293–309.
Johnson D.W. 1992. Nitrogen retention in forest soils. J. Environ. Qual. 21: 1–12.
Johnson D.W. and Ball J.T. 1996. Ch. 16. Interactions between CO2 and nitrogen in forests: can we extrapolate from the seedling to the stand level? In: Koch G. and Mooney H. (eds) Carbon Dioxide and Terrestrial Ecosystems Academic Press, San Diego (pp. 283–316).
Johnson D.W. and Lindberg S.E. 1992. Atmospheric Deposition and Forest Nutrient Cycling: A Synthesis of the Integrated Forest Study. Springer-Verlag, New York.
Johnson D.W. and Todd D.E. 1990. Nutrient cycling in forests of Walker Branch Watershed: roles of uptake and leaching in causing soil change. J. Environ. Qual. 19: 97–104.
Johnson D.W., Henderson G.S., Huff D.D., Lindberg S.E., Richter D.D., Shriner D.S. and Turner J. 1982. Cycling of organic and inorganic sulphur in a chestnut oak forest. Oecologia 54: 141–148.
Johnson D.W., Miegroet H.V., Lindberg S.E., Harrison R.B. and Todd D.E. 1991. Nutrient cycling in red spruce forests of the Great Smoky Mountains. Can. J. For. Res. 21: 769–787.
Johnson D.W., Ball J.T. and Walker R.F. 1997. Effects of CO2 and nitrogen fertilization on vegetation and soil nutrient content in juvenile ponderosa pine. Plant Soil 190: 29–40.
Johnson M.G., Tingey D.T., Phillips D.L. and Storm M.J. 2001. Advancing fine root research with minirhizotrons. Environ. Exp. Bot. 45: 263–289.
Johnson D.W., Cheng W. and Ball J.T. 2002a. Effects of [CO2] and nitrogen fertilization on soils planted with ponderosa pine. Plant Soil 224: 99–113.
Johnson D.W., Cheng W. and Ball J.T. 2002b. Effects of CO2 and N fertilization on decomposition and N immobilization in ponderosa pine litter. Plant Soil 224: 115–122.
Johnson D.W., Hungate B.A., Dijkstra P., Hymus G., Hinkle C.R. and Stiling P. 2003. The effects of elevated CO2 on nutrient distribution in a fire-adapted Scrub Oak Forest. Ecol. Appl.
Körner C. and Arnone J.A. 1992. Responses to elevated carbon dioxide in artificial tropical ecosystems. Science 257: 1672–1675.
McGuire A.D., Melillo J.M. and Joyce L.A. 1995. The role of nitrogen in the response of forest net primary production to elevated atmospheric carbon dioxide. Ann. Rev. Ecol. Syst. 26: 473–503.
Medlyn M.E., McMurtrie R.E., Dewar R.C. and Jeffreys M.P. 2000. Soil processes dominate the long-term response of forest net primary productivity to increased temperature and atmospheric CO2 concentration. Can. J. For. Res. 30: 873–888.
Miegroet H.V., Norby R.J. and Tschaplinski T.J. 1995. Nitrogen fertilization strategies in a short-rotation sycamore plantation. For. Ecol. Manage. 64: 13–24.
Nambiar E.K.S. and Fife D.N. 1991. Nutrient translocation in temperate conifers. Tree Physiol. 9: 185–208.
Norby R.J., O'Neill E.G., Hood W.G. and Luxmoore R.J. 1987. Carbon allocation, root exudation, and mycorrhizal colonization of Pinus echinata seedlings grown under CO2 enrichment. Tree Physiol. 3: 203–210.
Norby R.J., Wullschleger S.D., Gunderson C.A., Johnson D.W. and Ceulemans R. 1999. Tree responses to rising CO2: implications for the future forest. Plant Cell Environ. 22: 683–714.
Norby R.J., Cotrufo M.F., Ineson P., O'Neill E.G. and Canadell H.P. 2001a. Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127: 153–165.
Norby R.J., Todd D.E., Fults J. and Johnson D.W. 2001b. Allometric determination of tree growth in a CO2-enriched sweetgum stand. New Phytol. 150: 477–487.
Norby R.J., Hanson P.J., O'Neill E.G., Tschaplinski T.J., Weltzin J.F., Hansen R.T., Cheng W., Wullschleger S.D., Gunderson C.A., Edwards N.T. and Johnson D.W. 2002. Net primary productivity of a CO2-enriched deciduous forest and the implications for carbon storage. Ecol. Appl. 12: 1261–1266.
Oren R., Ellsworth D.S., Johnsen K.H., Phillips N., Ewers B.E., Maier C., Schäfer K.V.R., McCarthy H., Hendry G.E., McNulty S.G. and Katu C.G. 2001. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-rich atmosphere. Nature 411: 469–472.
Paul E.A. and Clark F.E. 1989. Soil Microbiology and Biochemistry. Academic Press, New York.
Pregitzer K.S., Zak D.R., Maziasaz J., Forest J.D., Curtis P.S. and Lussenhop J. 2000. Interactive effects of atmospheric CO2 and soil-N availability on fine roots of Populus tremloides.
Rogers H.H., Peterson C.M., McCrimmon J.N. and Cure J.D. 1992. Response of plant roots to elevated atmospheric carbon dioxide. Plant Cell Environ. 15: 749–752.
Rowland A.P. and Roberts J.D. 1994. Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods. Commun. Soil Sci. Plant Anal. 25: 269–277.
Strain B.R. 1985. Physiological and ecological controls on carbon sequestering in terrestrial ecosystems. Biogeochemistry 1: 219–232.
Susfalk R.B. and Johnson D.W. 2002. Ion exchange resin based soil solution lysimeters and snowmelt collectors. Comm. Soil Sci. Plant Anal. 33: 1261–1275.
Tingey D.T., Johnson M.G., Phillips D.R., Johnson D.W. and Ball J.T. 1996. Effects of elevated CO2 and nitrogen on the synchrony of shoot and root growth in ponderosa pine. Tree Physiol. 16: 905–914.
Torbert H.A., Prior S.A., Rogers H.H., Schlesinger W.H., Mullins G.L. and Runion R.B. 1996. Elevated atmospheric carbon dioxide in agroecosystems affects groundwater quality. J. Environ. Qual. 25: 720–726.
Vance E.D., Brookes P.C. and Jenkinson D.J. 1987. An extraction method for measuring soil microbial biomass C. Soil Bio. Biochem. 19: 703–707.
Velleman P. 1997. DataDesk Version 6.0 Statistics guide. Data Description, Inc., Ithaca, NY.
Wullschleger S.D. and Norby R.J. 2001. Sap velocity and canopy transpiration for a 12-year-old sweetgum stand exposed to free-air CO2 enrichment. New Phytol. 150: 489–498.
Yang J.E. and Skogley E.O. 1992. Diffusion kinetics of multinutrient accumulation by mixed-bed ion-exchange resin. Soil Sci. Soc. Amer. J. 56: 408–414.
Zak D.R. Pregitzer K.S. Curtis P.S. Teeri J.A. Fogel R. and Randlett D.L. 1993. Elevated atmospheric CO2 and feedback between carbon and nitrogen cycles. Plant Soil 151: 105–117.
Rights and permissions
About this article
Cite this article
Johnson, D.W., Cheng, W., Joslin, J.D. et al. Effects of elevated CO2 on nutrient cycling in a sweetgum plantation. Biogeochemistry 69, 379–403 (2004). https://doi.org/10.1023/B:BIOG.0000031054.19158.7c
Issue Date:
DOI: https://doi.org/10.1023/B:BIOG.0000031054.19158.7c