Abstract
Purpose of Review
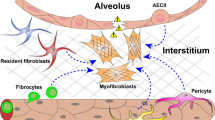
Idiopathic pulmonary fibrosis (IPF) is the most common form of interstitial lung disease of unknown etiopathogenesis with mean survival of 3–5 years and limited therapeutics. IPF is characterized by a loss of alveolar type II epithelial cells and aberrant activation of stromal cells, leading to a considerable effort to characterize the origin and activation mechanisms of fibroblasts and myofibroblasts in IPF lungs. In this review, the origin and contribution of fibroblast and myofibroblasts in lung fibrosis will be summarized.
Recent Findings
Lineage tracing experiments suggested that interstitial lung fibroblasts and lipofibroblasts, pericytes, and mesothelial cells differentiate into myofibroblasts. However, epithelial- and bone marrow-derived cells may give rise to collagen expressing cells but may not contribute to the pool of myofibroblasts.
Summary
There is great heterogeneity in fibroblasts and myofibroblasts in fibrotic lungs. Further, there is evidence for the expansion of pericyte-derived myofibroblasts and loss of lipofibroblasts and lipofibroblast-derived myofibroblasts in IPF.

Similar content being viewed by others
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Wolters PJ, Collard HR, Jones KD (2014) Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9:157–179. doi:10.1146/annurev-pathol-012513-104706
du Bois RM (2010) Strategies for treating idiopathic pulmonary fibrosis. Nat Rev Drug Discov 9(2):129–140. doi:10.1038/nrd2958
King TE Jr (2005) Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 172(3):268–279. doi:10.1164/rccm.200503-483OE
King TE Jr, Pardo A, Selman M (2011) Idiopathic pulmonary fibrosis. Lancet 378(9807):1949–1961. doi:10.1016/S0140-6736(11)60052-4
Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE Jr et al (2007) Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 176(7):636–643. doi:10.1164/rccm.200703-463PP
Hogan BL (1999) Morphogenesis Cell 96(2):225–233
Arora R, Metzger RJ, Papaioannou VE (2012) Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet 8(8):e1002866. doi:10.1371/journal.pgen.1002866
• Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W et al (2016) Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest 126(8):3063–3079. doi:10.1172/JCI85328 This study nicely shows the importance of TBX4 as a pulmonary mesodermal transcription factor, where TBX4 lineage-traced cells give rise to multiple stromal lineages in the lung.
Li C, Li M, Li S, Xing Y, Yang CY, Li A et al (2015) Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 33(3):999–1012. doi:10.1002/stem.1911
Pepicelli CV, Lewis PM, McMahon AP (1998) Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 8(19):1083–1086
Caprioli A, Villasenor A, Wylie LA, Braitsch C, Marty-Santos L, Barry D et al (2015) Wnt4 is essential to normal mammalian lung development. Dev Biol 406(2):222–234. doi:10.1016/j.ydbio.2015.08.017
De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y et al (2008) Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One 3(1):e1516. doi:10.1371/journal.pone.0001516
Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA et al (2013) Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188(7):820–830. doi:10.1164/rccm.201212-2297OC
Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J et al (2011) Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 108(52):E1475–E1483. doi:10.1073/pnas.1117988108
Levéen P, Törnell J, Betsholtz C, Pekna M, Pekny M, Lindahl P et al (1996) PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85(6):863–873
Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK et al (1997) Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124(20):3943–3953
•• Green J, Endale M, Auer H, Perl AK (2016) Diversity of interstitial lung fibroblasts is regulated by platelet-derived growth factor receptor alpha kinase activity. Am J Respir Cell Mol Biol 54(4):532–545. doi:10.1165/rcmb.2015-0095OC Utilizing a PDGFRα lineage tracer, this study nicely shows all of the PDGFRα-derived myofibroblasts in naïve and injured murine lungs.
Kim N, Vu TH (2006) Parabronchial smooth muscle cells and alveolar myofibroblasts in lung development. Birth Defects Res C Embryo Today 78(1):80–89. doi:10.1002/bdrc.20062
Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM et al (2007) Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 307(2):237–247. doi:10.1016/j.ydbio.2007.04.033
Yi L, Domyan ET, Lewandoski M, Sun X (2009) Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn 238(1):123–137. doi:10.1002/dvdy.21831
Dixit R, Ai X, Fine A (2013) Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development 140(21):4398–4406. doi:10.1242/dev.098079
von Gise A, Stevens SM, Honor LB, Oh JH, Gao C, Zhou B et al (2016) Contribution of fetal, but not adult, pulmonary mesothelium to mesenchymal lineages in lung homeostasis and fibrosis. Am J Respir Cell Mol Biol 54(2):222–230. doi:10.1165/rcmb.2014-0461OC
Torday JS, Torres E, Rehan VK (2003) The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med 22(3):189–207
Al Alam D, El Agha E, Sakurai R, Kheirollahi V, Moiseenko A, Danopoulos S et al (2015) Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development 142(23):4139–4150. doi:10.1242/dev.109173
Varisco BM, Ambalavanan N, Whitsett JA, Hagood JS (2012) Thy-1 signals through PPARgamma to promote lipofibroblast differentiation in the developing lung. Am J Respir Cell Mol Biol 46(6):765–772. doi:10.1165/rcmb.2011-0316OC
Li A, Ma S, Smith SM, Lee MK, Fischer A, Borok Z et al (2016) Mesodermal ALK5 controls lung myofibroblast versus lipofibroblast cell fate. BMC Biol 14:19. doi:10.1186/s12915-016-0242-9
McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K et al (2009) Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells 27(3):623–633. doi:10.1634/stemcells.2008-0866
Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD et al (2015) Characterization of the platelet-derived growth factor receptor-alpha-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol 309(9):L942–L958. doi:10.1152/ajplung.00272.2014
•• El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G et al (2016) Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. doi:10.1016/j.stem.2016.10.004 This study nicely shows the role of lipofibroblasts in myofibroblast generation in injured murine lungs and the importance of myofibroblast to lipofibroblast transdifferentiation in lung regeneration.
• Barron L, Gharib SA, Duffield JS (2016) Lung pericytes and resident fibroblasts: busy multitaskers. Am J Pathol 186(10):2519–2531. doi:10.1016/j.ajpath.2016.07.004 This review nicely highlights the role(s) of pericytes and resident fibroblasts in lung fibrosis.
•• Marriott S, Baskir RS, Gaskill C, Menon S, Carrier EJ, Williams J et al (2014) ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am J Physiol Cell Physiol 307(8):C684–C698. doi:10.1152/ajpcell.00114.2014 This study nicely shows the role of ABCG2+ pericytes in murine and human lung fibrosis, where these pericytes can give rise to ACTA2 and collagen 1-expressing myofibroblasts.
Lama VN, Phan SH (2006) The extrapulmonary origin of fibroblasts: stem/progenitor cells and beyond. Proc Am Thorac Soc 3(4):373–376. doi:10.1513/pats.200512-133TK
Phan SH (2012) Genesis of the myofibroblast in lung injury and fibrosis. Proc Am Thorac Soc 9(3):148–152. doi:10.1513/pats.201201-011AW
Gomperts BN, Strieter RM (2007) Fibrocytes in lung disease. J Leukoc Biol 82(3):449–456. doi:10.1189/jlb.0906587
Maharaj S, Shimbori C, Kolb M (2013) Fibrocytes in pulmonary fibrosis: a brief synopsis. Eur Respir Rev 22(130):552–557. doi:10.1183/09059180.00007713
Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH (2004) Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 113(2):243–252. doi:10.1172/JCI18847
Epperly MW, Guo H, Gretton JE, Greenberger JS (2003) Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 29(2):213–224. doi:10.1165/rcmb.2002-0069OC
• Sontake V, Shanmukhappa SK, DiPasquale BA, Reddy GB, Medvedovic M, Hardie WD et al (2015) Fibrocytes regulate Wilms tumor 1-positive cell accumulation in severe fibrotic lung disease. J Immunol 195(8):3978–3991. doi:10.4049/jimmunol.1500963 This study nicely shows a novel role for fibrocytes, where these cells contribute to the activation of Wilms tumor 1+ mesothelial cells in remodeled murine lungs.
Karki S, Surolia R, Hock TD, Guroji P, Zolak JS, Duggal R et al (2014) Wilms’ tumor 1 (Wt1) regulates pleural mesothelial cell plasticity and transition into myofibroblasts in idiopathic pulmonary fibrosis. FASEB J 28(3):1122–1131. doi:10.1096/fj.13-236828
Zolak JS, Jagirdar R, Surolia R, Karki S, Oliva O, Hock T et al (2013) Pleural mesothelial cell differentiation and invasion in fibrogenic lung injury. Am J Pathol 182(4):1239–1247. doi:10.1016/j.ajpath.2012.12.030
Mubarak KK, Montes-Worboys A, Regev D, Nasreen N, Mohammed KA, Faruqi I et al (2012) Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur Respir J 39(1):133–140. doi:10.1183/09031936.00141010
• Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J et al (2016) Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1(20):e90558. doi:10.1172/jci.insight.90558 Utilizing single-cell RNA sequencing, this study nicely shows the presence of basal-like cells in IPF lungs co-expressing mesenchymal markers. This is the first study to provide strong evidence for mesenchymal transcript expression in basal cells from IPF but not normal lung explants.
Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W et al (2009) Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 180(7):657–665. doi:10.1164/rccm.200903-0322OC
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN et al (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103(35):13180–13185. doi:10.1073/pnas.0605669103
Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB et al (2010) Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 299(4):L442–L452. doi:10.1152/ajplung.00026.2010
Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X et al (2007) Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an alpha-smooth muscle actin-Cre transgenic mouse. Respir Res 8:1. doi:10.1186/1465-9921-8-1
Liang J, Zhang Y, Xie T, Liu N, Chen H, Geng Y et al (2016) Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat Med 22(11):1285–1293. doi:10.1038/nm.4192
Bartis D, Crowley LE, D'Souza VK, Borthwick L, Fisher AJ, Croft AP et al (2016) Role of CD248 as a potential severity marker in idiopathic pulmonary fibrosis. BMC Pulm Med 16(1):51. doi:10.1186/s12890-016-0211-7
Naylor AJ, Azzam E, Smith S, Croft A, Poyser C, Duffield JS et al (2012) The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice. Arthritis Rheum 64(10):3334–3343. doi:10.1002/art.34556
Smith SW, Croft AP, Morris HL, Naylor AJ, Huso DL, Isacke CM et al (2015) Genetic deletion of the stromal cell marker CD248 (endosialin) protects against the development of renal fibrosis. Nephron 131(4):265–277. doi:10.1159/000438754
Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD et al (2015) PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29(11):1106–1119. doi:10.1101/gad.260554.115
Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK (2012) Dynamic regulation of platelet-derived growth factor receptor alpha expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol 47(4):517–527. doi:10.1165/rcmb.2012-0030OC
Chen LJ, Ye H, Zhang Q, Li FZ, Song LJ, Yang J et al (2015) Bleomycin induced epithelial-mesenchymal transition (EMT) in pleural mesothelial cells. Toxicol Appl Pharmacol 283(2):75–82. doi:10.1016/j.taap.2015.01.004
Herzog EL, Bucala R (2010) Fibrocytes in health and disease. Exp Hematol 38(7):548–556. doi:10.1016/j.exphem.2010.03.004
Habiel DM, Hogaboam C (2014) Heterogeneity in fibroblast proliferation and survival in idiopathic pulmonary fibrosis. Front Pharmacol 5:2. doi:10.3389/fphar.2014.00002
Srour N, Thebaud B (2015) Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: a systematic review. Stem Cells Transl Med 4(12):1500–1510. doi:10.5966/sctm.2015-0121
Chambers DC, Enever D, Ilic N, Sparks L, Whitelaw K, Ayres J et al (2014) A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 19(7):1013–1018. doi:10.1111/resp.12343
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1(1):71–81
Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S (2003) Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 171(1):380–389
Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY et al (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114(3):438–446. doi:10.1172/JCI20997
Shi Y, Ou L, Han S, Li M, Pena MM, Pena EA et al (2014) Deficiency of Kruppel-like factor KLF4 in myeloid-derived suppressor cells inhibits tumor pulmonary metastasis in mice accompanied by decreased fibrocytes. Oncogene 3:e129. doi:10.1038/oncsis.2014.44
Ou L, Shi Y, Dong W, Liu C, Schmidt TJ, Nagarkatti P et al (2015) Kruppel-like factor KLF4 facilitates cutaneous wound healing by promoting fibrocyte generation from myeloid-derived suppressor cells. J Invest Dermatol 135(5):1425–1434. doi:10.1038/jid.2015.3
Fernandez IE, Greiffo FR, Frankenberger M, Bandres J, Heinzelmann K, Neurohr C et al (2016) Peripheral blood myeloid-derived suppressor cells reflect disease status in idiopathic pulmonary fibrosis. Eur Respir J 48(4):1171–1183. doi:10.1183/13993003.01826-2015
Trujillo G, Hartigan AJ, Hogaboam CM (2010) T regulatory cells and attenuated bleomycin-induced fibrosis in lungs of CCR7−/− mice. Fibrogenesis Tissue Repair 3:18. doi:10.1186/1755-1536-3-18
Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM (2007) Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun 353(1):104–108. doi:10.1016/j.bbrc.2006.11.149
Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A et al (2008) Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 40(10):2129–2140. doi:10.1016/j.biocel.2008.02.012
Hogaboam CM, Murray L, Martinez FJ (2012) Epigenetic mechanisms through which toll-like receptor-9 drives idiopathic pulmonary fibrosis progression. Proc Am Thorac Soc 9(3):172–176. doi:10.1513/pats.201201-002AW
Huang SK, Scruggs AM, McEachin RC, White ES, Peters-Golden M (2014) Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS One 9(9):e107055. doi:10.1371/journal.pone.0107055
Sanders YY, Liu H, Scruggs AM, Duncan SR, Huang SK, Thannickal VJ (2017) Epigenetic regulation of caveolin-1 gene expression in lung fibroblasts. Am J Respir Cell Mol Biol 56(1):50–61. doi:10.1165/rcmb.2016-0034OC
Xiao X, Senavirathna LK, Gou X, Huang C, Liang Y, Liu L (2016) EZH2 enhances the differentiation of fibroblasts into myofibroblasts in idiopathic pulmonary fibrosis. Physiol Rep 4(17):e12915. doi:10.14814/phy2.12915
Korfei M, Skwarna S, Henneke I, MacKenzie B, Klymenko O, Saito S et al (2015) Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis. Thorax 70(11):1022–1032. doi:10.1136/thoraxjnl-2014-206411
Sanders YY, Liu H, Liu G, Thannickal VJ (2015) Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic Biol Med 79:197–205. doi:10.1016/j.freeradbiomed.2014.12.008
O'Dwyer DN, Armstrong ME, Trujillo G, Cooke G, Keane MP, Fallon PG et al (2013) The toll-like receptor 3 L412F polymorphism and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 188(12):1442–1450. doi:10.1164/rccm.201304-0760OC
Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T et al (2014) Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 6(231):231ra47. doi:10.1126/scitranslmed.3008182
Im J, Kim K, Hergert P, Nho RS (2016) Idiopathic pulmonary fibrosis fibroblasts become resistant to Fas ligand-dependent apoptosis via the alteration of decoy receptor 3. J Pathol 240(1):25–37. doi:10.1002/path.4749
Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ et al (2003) Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol 29(4):490–498. doi:10.1165/rcmb.2002-0262OC
Scaffidi AK, Mutsaers SE, Moodley YP, McAnulty RJ, Laurent GJ, Thompson PJ et al (2002) Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br J Pharmacol 136(5):793–801. doi:10.1038/sj.bjp.0704769
Ebener S, Barnowski S, Wotzkow C, Marti TM, Lopez-Rodriguez E, Crestani B et al Toll-like receptor 4 (TLR4) activation attenuates pro-fibrotic response in control lung fibroblasts but not in fibroblasts from IPF patients. Am J Physiol Lung Cell Mol Physiol. doi:10.1152/ajplung.00119.2016
Meneghin A, Choi ES, Evanoff HL, Kunkel SL, Martinez FJ, Flaherty KR et al (2008) TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem Cell Biol 130(5):979–992. doi:10.1007/s00418-008-0466-z
Trujillo G, Meneghin A, Flaherty KR, Sholl LM, Myers JL, Kazerooni EA et al (2010) TLR9 differentiates rapidly from slowly progressing forms of idiopathic pulmonary fibrosis. Sci Transl Med 2(57):57ra82. doi:10.1126/scitranslmed.3001510
Chilosi M, Zamo A, Doglioni C, Reghellin D, Lestani M, Montagna L et al (2006) Migratory marker expression in fibroblast foci of idiopathic pulmonary fibrosis. Respir Res 7:95. doi:10.1186/1465-9921-7-95
Kirillov V, Siler JT, Ramadass M, Ge L, Davis J, Grant G et al (2015) Sustained activation of toll-like receptor 9 induces an invasive phenotype in lung fibroblasts: possible implications in idiopathic pulmonary fibrosis. Am J Pathol 185(4):943–957. doi:10.1016/j.ajpath.2014.12.011
Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R et al (2011) Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208(7):1459–1471. doi:10.1084/jem.20102510
Ahluwalia N, Grasberger PE, Mugo BM, Feghali-Bostwick C, Pardo A, Selman M et al (2016) Fibrogenic lung injury induces non-cell-autonomous fibroblast invasion. Am J Respir Cell Mol Biol 54(6):831–842. doi:10.1165/rcmb.2015-0040OC
Cai GQ, Zheng A, Tang Q, White ES, Chou CF, Gladson CL et al (2010) Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res 316(9):1600–1609. doi:10.1016/j.yexcr.2010.01.021
Suganuma H, Sato A, Tamura R, Chida K (1995) Enhanced migration of fibroblasts derived from lungs with fibrotic lesions. Thorax 50(9):984–989
Shea BS, Tager AM (2012) Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc 9(3):102–110. doi:10.1513/pats.201201-005AW
Jun D, Garat C, West J, Thorn N, Chow K, Cleaver T et al (2011) The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells 29(4):725–735. doi:10.1002/stem.604
Leaf IA, Nakagawa S, Johnson BG, Cha JJ, Mittelsteadt K, Guckian KM et al (2016) Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury. J Clin Invest. doi:10.1172/JCI87532
Chanda D, Kurundkar A, Rangarajan S, Locy M, Bernard K, Sharma NS et al (2016) Developmental reprogramming in mesenchymal stromal cells of human subjects with idiopathic pulmonary fibrosis. Sci Rep 6:37445. doi:10.1038/srep37445
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David Habiel and Cory Hogaboam declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Activated Myofibroblasts and Fibrosis in Various Organs
Rights and permissions
About this article
Cite this article
Habiel, D.M., Hogaboam, C.M. Heterogeneity of Fibroblasts and Myofibroblasts in Pulmonary Fibrosis. Curr Pathobiol Rep 5, 101–110 (2017). https://doi.org/10.1007/s40139-017-0134-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-017-0134-x