Abstract
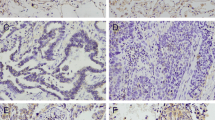
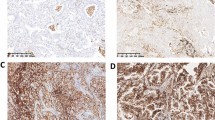
This study aimed to analyze the expression, clinical significance of B cell translocation gene 1 (BTG1) in nonsmall cell lung cancer (NSCLC) and the biological effect in its cell line by BTG1 overexpression. Immunohistochemistry and western blot were used to analyze BTG1 protein expression in 82 cases of NSCLC and 38 cases of normal tissues to study the relationship between BTG1 expression and clinical factors. Recombinant lentiviral vector was constructed to overexpress EMP-1 and then infect NSCLC H1299 cell line. Quantitative real-time RT-PCR and western blot were used to detect the mRNA level and protein of BTG1. 3-[4,5-dimethylthiazol -2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, cell apoptosis, cell cycles, and migration and invasion assays were also conducted as to the influence of the upregulated expression of BTG1 that might be found on H1299 cells biological effect. The level of BTG1 protein expression was found to be significantly lower in NSCLC tissue than normal tissues (P < 0.05). Decreased expression of BTG1 was significantly correlated with lymph node metastasis, clinic stage, and histological grade of patients with NSCLC (P < 0.05). Meanwhile, loss of BTG1 expression correlated significantly with poor overall survival time by Kaplan–Meier analysis (P < 0.05). The result of biological function show that H1299 cell transfected BTG1 had a lower survival fraction; higher percentage of the G0/G1 phases; higher cell apoptosis; significant decrease in migration and invasion; and lower CyclinD1, Bcl-2, and MMP-9 protein expression compared with H1299 cell untransfected BTG1 (P < 0.05). BTG1 expression decreased in NSCLC and correlated significantly with lymph node metastasis; clinical stage; histological grade; poor overall survival; cell proliferation; cell cycles; cell apoptosis; and migration and invasion in NSCLC cell by regulating CyclinD1, Bcl-2, and MMP-9 protein expression, suggesting that BTG1 may play important roles as a negative regulator to NSCLC cell.









Similar content being viewed by others
References
Okuyama T, Maehara Y, Kabashima A, Takahashi I, Kakeji Y, Sugimachi K. Combined evaluation of expressions of p53 and p21 proteins as prognostic factors for patients with gastric carcinoma. Oncology. 2002;63:353–61.
Cortes U, Moyret-Lalle C, Falette N, Duriez C, Ghissassi FE, Barnas C, et al. BTG gene expression in the p53-dependent and -independent cellular response to DNA damage. Mol Carcinog. 2000;27:57–64.
Winkler GS. The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol. 2010;222:66–72.
Rouault JP, Rimokh R, Tessa C, Paranhos G, Ffrench M, Duret L, et al. BTG1, a member of a new family of antiproliferative genes. EMBO J. 1992;11:1663–70.
Matsuda S, Rouault J, Magaud J, Berthet C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001;497:67–72.
Rouault JP, Falette N, Guéhenneux F, Guillot C, Rimokh R, Wang Q, et al. Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular responsepathway. Nat Genet. 1996;14:482–6.
Zhu R, Zou ST, Wan JM, Li W, Li XL, Zhu W. BTG1 inhibits breast cancer cell growth through induction of cell cycle arrest and apoptosis. Oncol Rep. 2013;30:2137–44.
Pramesh CS, Mistry RC, Jambhekar NA, Laskar SG. Does the TNM staging system for esophageal cancer need revision? J Am Coll Surg. 2006;202:855–6.
Martinez-Outschoorn UE, Pavlides S, Sotgia F, Lisanti MP. Mitochondrial biogenesis drives tumor cell proliferation. Am J Pathol. 2011;178:1949–52.
Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–28.
Kwon TK, Nordin AA. Overexpression of cyclin E and cyclin dependent kinase inhibitor p27kip1: effect on cell cycle regulation in Hela cell. Biochem Biophys Res Commun. 1997;238:534–8.
Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science. 2003;299:214–5.
Tirone F. The gene PC3(TIS21/BTG2), prototype member of the PC3/BTG/TOB family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol. 2001;187:155–65.
Corjay MH, Kearney MA, Munzer DA, Diamond SM, Stoltenborg JK. Antiproliferative gene BTG1 is highly expressed in apoptotic cells in macrophage-rich areas of advanced lesions in Watanabe heritable hyperlipidemic rabbit and human. Lab Invest. 1998;78:847–58.
Lee H, Cha S, Lee MS, Cho GJ, Choi WS, Suk K. Role of antiproliferative B cell translocation gene-1 as an apoptotic sensitizer in activation-induced cell death of brain microglia. J Immunol. 2003;171:5802–11.
Nahta R, Yuan LX, Fiterman DJ, Zhang L, Symmans WF, Ueno NT, et al. B cell translocation gene 1 contributes to antisense Bcl-2-mediated apoptosis in breast cancer cells. Mol Cancer Ther. 2006;5:1593–601.
Ermiah E, Buhmeida A, Khaled BR, Abdalla F, Salem N, Pyrhönen S, et al.. Prognostic value of bcl-2 expression among women with breast cancer in Libya. Tumour Biol. 2013;34:1569–1578.
Gu Y, Pan Y, Meng B, Guan B, Fu K, Sun B, et al. High levels of bcl-2 protein expression do not correlate with genetic abnormalities but predict worse prognosis in patients with lymphoblastic lymphoma. Tumour Biol. 2013;34:1441–1450.
Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–9.
Alok C, Bharat B. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Phamacol. 2002;64:883–8.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, G.G., Lu, Y.F., Cheng, Y.J. et al. The expression of BTG1 is downregulated in NSCLC and possibly associated with tumor metastasis. Tumor Biol. 35, 2949–2957 (2014). https://doi.org/10.1007/s13277-013-1379-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1379-6