Abstract
Lung cancer is the most common cause of cancer-related deaths worldwide with non-small cell lung cancer (NSCLC) making up most of these cases. Males have poorer overall survival compared to women following a lung cancer diagnosis. Many studies have focused on the effects of estrogen to explain higher survival rates among women, but few have looked at the effects of androgens. We describe the expression of the androgen receptor (AR) and Ki67 in lung cancer specimens in the Manitoba Tumor Bank (MTB) and correlate these factors with patient outcome. Using the MTB, we performed immunohistochemistry on lung cancer tissue to determine expression of the AR and Ki67. These were then correlated with patient outcome. Of the 136 cases, 55% were female and 55% were adenocarcinoma. AR expression was not independently associated with outcome. Ki67 was associated with a significantly higher hazard ratio for death and recurrence (HR 2.19, 95% CI 1.30–3.70; HR 1.92, 95% CI 1.07–3.46, respectively). AR expression modified the effect of Ki67 on outcome, such that when both were expressed, there was no association with recurrence or survival (HR 2.39, 95% CI 1.31–4.36 for AR− Ki67+ vs HR 1.54, 95% CI 0.44–5.37 for AR+ Ki67+). Ki67 was associated with poorer outcomes alone. AR status alone was not associated with outcome. Although the mechanism remains unclear, AR status seems to negate the association of a high Ki67 and poor outcome.
Similar content being viewed by others
Introduction
Lung cancer is the most common cause of cancer-related deaths worldwide with non-small cell lung cancer (NSCLC) making up the majority of these cases [1]. Although 5-year recurrence-free estimates range from > 70% in patients with stage I disease to < 50% for patients with stage II NSCLC, most lung cancer patients present with late stages of NSCLC where curative options are no longer available [2, 3]. In late stages, the median overall survival is 14.9 months with chemotherapy in those under the age of 70 [4].
While cigarette smoking is tied to 90% of lung cancers and is the number one cause, other risk factors are still involved [5]. Over the years, there has been an increase in female smokers and a dramatic rise in the overall number of lung cancer cases in women [6]. Females have been shown to present with more advanced disease and at a younger age [5,6,7]. Despite more advanced disease, women have better survival. It has recently been proposed that female sex is both an independent and favorable prognostic factor with NSCLC [5,6,7]. While female sex hormones, primarily estrogen, as well as many other factors may be responsible for the variations seen in NSCLC between males and females, the role of androgens has not been fully evaluated.
The androgen receptor (AR) has been detected within normal human lung cells as well as in a number of different lung cancers, including small cell, adenocarcinoma, and squamous cell carcinoma [8]. Adult lung tissues contain androgen-responsive cells primarily within the bronchial epithelium and the type II pneumocyte cells, which are important in maintaining the alveolar epithelium through surfactant production and the production of new epithelial cells [8]. Within murine lungs, androgens have been shown to be involved in multiple processes involved in tumor development and growth including upregulating genes for oxygen transport, heme biosynthesis, negative regulation of apoptosis, and downregulating DNA repair genes [8]. Previous work using the androgen-responsive lung cancer cell line A549 found that DHT induced a proliferative response through cross talk of AR and epidermal growth factor receptor (EGFR) [9]. Although early work found no relationship between the stage of lung cancer and the AR content within biopsy samples, lower AR content was found in the most undifferentiated tumor samples [10].
Ki67 is a known prognostic marker in other cancers, such as breast cancer and mantle cell lymphoma [11, 12]. However, Ki67’s role within NSCLC has had mixed results as a prognostic marker throughout the literature [13,14,15]. Ki67 is a DNA-binding nuclear protein which is expressed within proliferating cells throughout the cell cycle with the exception of the G0 phase [14].
Knowing that androgen signaling can influence several pathways linked to cancer development, we hypothesized that AR expression and Ki67 expression are associated with outcome in patients with lung cancer. We assessed the expression of the AR and Ki67 within a cohort of lung cancer specimens in the Manitoba Tumor Bank and correlated these with patient outcome.
Methods
Microarray Construction
Lung cancer samples collected from the Manitoba Tumor Bank (MTB) were used to create a lung cancer tissue microarray (TMA). Patients diagnosed with NSCLC were identified using the Manitoba Cancer Registry. Tissue samples from 136 cases were used to create formalin-fixed paraffin embedded (FFPE) blocks. These FFPE blocks were then used to generate the TMA at the MTB [16]. The MTB practices the policies and protocols of the Canadian Tumor Repository Network (ref: www.CTRNet.ca) and operates under approval from the Health Research Ethics Board, University of Manitoba. Ethics approval for this project was given by the Health Research Ethics Board, University of Manitoba. Clinical information from patients was obtained from CancerCare Manitoba electronic charts. Clinical information included the age at diagnosis, sex, tumor stage, treatment, and date of diagnosis and death.
The histopathology of MTB biospecimens was previously assessed and entered into a computerized database to enable selection based on tissue composition and clinical-pathological parameters. The FFPE blocks used in this study were assessed by a study pathologist and two cores (2 × 0.6 mm diameter) from tissues were used for the creation of the TMA.
Immunohistochemistry
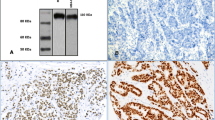
Construction and immunohistochemistry (IHC) for TMAs was performed as described previously [17,18,19]. Serial sections (~ 5 μm) were stained with antibodies as described in previous studies [18, 19]. Briefly, sections were submitted to antigen retrieval (AR mild CC1; Ki67 standard CC1, Ventana Medical Systems, AZ, USA) using an auto-immunostainer (Discovery Staining Module, Ventana Medical Systems, AZ, USA), followed by 1-h incubation with primary antibody and 32-min incubation with secondary antibody. Primary antibody concentrations initially applied to the Ventana instrument were 1:25 for antibodies AR (sc-816, Santa Cruz Biotechnology Inc. Santa Cruz, CA, USA) and 1:100 Ki67 (M7240001, Agilent Technologies Canada Inc. Mississauga, ON, Canada) translating into final concentrations of 1:75 and 1:300 respectively after 1:3 further dilution on the machine.
Quantification and Cut-off Selection
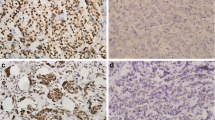
Slides were scored using standard light microscopy (Fig. 1). IHC-scores were derived from assessment of both average staining intensity across the two tumor cores (scale 0–3) and percentage of positive cells (0–100%). These two scores, when multiplied, generate an IHC or H-score of 0–300. Only nuclear staining for both AR and Ki67 was scored. TMAs were evaluated independently by three investigators (GQ, CP, AB). Where discordance was found, cases were re-evaluated to reach consensus. Since no relevant clinical cut-off points are presently reported in lung cancer for AR or Ki67, positivity reported in this study was empirically based on IHC-scores greater than the 50th percentile. For AR, the 50th percentile was 0; therefore any staining was regarded as positive for AR. For Ki67 H-score, values above 100 were considered positive. Relapse-free survival (RFS) was defined as time from diagnosis to the first recurrence or death and overall survival (OS) was defined as time from diagnosis to death, censored on date of the last follow-up.
Statistical Analysis
Frequency tables were generated and univariate and multivariable analyses were used to describe the associations between receptor status and clinical outcomes. A multivariable Cox model that included sex, age, and stage was used to assess the independent effects of AR and Ki67 on death and recurrence (SAS version 9.1). The analyses were stratified by sex, as well as the combination of AR and Ki67 expression to explore the interaction between these factors.
Results
A total of 136 tissue samples were collected from the MTB. Of these, 55.2% (n = 75) were female (Table 1). The mean age of diagnosis was 69.5 years. Over half (55.2%) of the samples were adenocarcinomas. Most patients were early stage using American Joint Committee on Cancer, 7th Edition (AJCC7), with the majority being stage Ia or Ib (Table 1).
The distribution of AR status or Ki67 status did not differ significantly by sex (Table 2). However, the distribution of Ki67 differed with cancer type, with low Ki67 status being more common in adenocarcinoma and less likely in squamous cell carcinoma and the “other” category (p = 0.001 Table 2). High Ki67 status was equally common in squamous cell carcinoma and adenocarcinoma and uncommon in among the “other” category. There was no statistically significant difference in AR expression between lung tumor subtypes in females compared to males (Supplemental Tables 1 and 2). Univariable and multivariable model results are presented in Table 3. Using multivariable models, Ki67 was independently associated with higher risk of death, though the effect on survival was limited to males when stratified by sex. AR was not independently associated with risk of recurrence or death.
When Ki67 and AR were combined (Table 4), negative AR status and high Ki67 was strongly associated with risk of lung cancer recurrence and death (Fig. 2) in multivariable models. In patients with high Ki67, AR expression modified the effect of Ki67 on death. Lack of AR expression in the group with high Ki67 was strongly associated with a higher risk death, and a higher risk of recurrence in females. However, in patients with combined expression of AR and high Ki67, no effect on death or recurrence was observed. Complete subgroup analysis of AR positive and high Ki67 could not be performed due to limited numbers.
Discussion
We sought to evaluate the association between AR expression and Ki67 in non-small cell lung cancer and patient outcome using a local collection of well-annotated lung cancer tissues collected by the CTRNet certified MTB. The expression of the AR was not independently associated with risk of death or recurrence in our cohort. Ki67 was independently associated with a worse survival and a higher risk of recurrence. We found an interaction between AR expression and Ki67 in this cohort. Patients with high Ki67 but low AR expression had a higher risk of death and recurrence; however, when AR and Ki67 were both expressed, the effect on survival or recurrence was no longer observed.
Ki67 staining is a common way to assess for proliferation within a tissue sample. However, the use of Ki67 staining has not been implemented in a clinical setting for many tumor types. The role of Ki67 has not always been clear, with mixed results of its prognostic role seen throughout the literature. However, recent publications, including a meta-analysis of the use of Ki67 staining in lung cancer has shown high Ki67 status to be a poor prognostic factor [13,14,15]. Our results align with this literature and show high Ki67 status is a poor prognostic factor for recurrence and survival outcomes. The effect appeared to be similar between males and females, though the association with female sex and survival did not quite reach statistical significance. These results provide further support for the use of Ki67 expression as a prognostic factor within NSCLC. Our data showed low levels of Ki67 staining were more commonly associated with adenocarcinoma. These data support previous findings that Ki67 staining varies depending on histology within NSCLC and found that adenocarcinoma in particular had lower levels of Ki67 staining than other histologies [13].
The expression of sex hormones in lung cancer tissue may provide an important biological link for the association between sex and outcome. A recent study found that AR positive NSCLC was associated with better overall survival [20]. We did not find the same association in this study; however, we included mainly early-stage NSCLC, while the Barardi study focused on late stage metastatic NSCLC. In our cohort, AR expression was not independently associated with outcome; however, it modified the prognostic value of Ki67 such that when AR is expressed, the prognostic value of Ki67 is no longer present. This appears to show some similarity to the estrogen receptor (ER) in breast cancer, where AR expression in NSCLC may reflect a more differentiated state of the tumor independent of other biological markers of prognosis, and therefore be a marker of a less aggressive form of cancer [21]. In our cohort, Ki67 expression was only found to be important for prognosis when AR was not expressed. This observation may also reflect that hormonal signaling plays a role in regulating tumor growth and survival, though the hypothesis that interfering with that signaling, as with hormone deprivation as seen in both breast and prostate cancer, could be an effective targeted therapy approach in a subgroup of NSCLC cases is speculative [22, 23]. We and others have made the observation that antiandrogens in men and antiestrogen use in women is associated with a better clinical outcome in NSCLC, though the current study cannot evaluate the possibility of this approach having an effect on patient outcomes [22]. We observed that the expression of AR is associated with outcome, perhaps by abrogating the effect of Ki67, providing more evidence that this pathway may be of importance in NSCLC.
The mechanism by which this observed association could be mediated remains unclear. Direct effects at the tumor level are possible given the detection of AR in these cells. However, sex dimorphism in the immune system is well known and may also contribute, as could the tumor microenvironment where AR and ERs have been detected in addition to cellular circuitry in the normal lung being different in males and females due to known sex-biased gene expression [24, 25]. Androgen treatment of murine lung resulted in the upregulation of genes involved in oxygen transport, heme biosynthesis, and the negative regulation of apoptosis [8]. DNA repair genes and double-strand break repair genes have been found to be downregulated with the addition of androgens [8]. While these data support a role of steroid hormones in both differentiation and survival in the normal lung, the molecular mechanisms by which androgen exposure may mediate similar or differential effects in lung cancer tissue requires further exploration.
It should be noted that while we found statistically significant associations, these results are hypothesis-generating and should not be taken as definitive. This study was performed on tissue from a well-established tumor bank with robust clinical annotation. Despite this, the definition of lung cancer recurrence remains challenging and may be incomplete, as not all patients with lung cancer recurrence would present back to the cancer center. This may explain why cancer recurrence did not always correlate with overall survival outcomes, although death without recurrence is also reasonably possible given the typical age and comorbidity of patients with lung cancer and could partially explain our results. Death was measured as all-cause mortality, and direct attribution to lung cancer as cause of death cannot be directly inferred. The tumor bank draws patients from a population-based regional cancer center, but by the nature of patients whose tissue is banked would suffer from selection bias. When compared to the total population of lung cancer patients in Manitoba, the study population’s median survival was somewhat longer. This is expected, since the tissue was collected from patients undergoing surgical resection, who are more likely to have lower stage/resectable disease and be in better health to receive aggressive treatment. AR expression was not found to be different between sex and morphology, though this study was not powered to detect a difference and numerically there was a trend towards greater AR expression in males and adenocarcinomas. Sex was not a significant predictor of outcome, contrary to previous studies; however, this study was not powered to detect this difference and sex was included in all multivariable models. Similarly, it remains possible that our study was underpowered to show and independent effect of AR expression on survival, and we did observe a potential association with improved RFS in females (Table 3). In addition, the AR antibody used in this and other studies recognizes an epitope to the N-terminus of the protein. Therefore, the nature of the AR in NSCLC is unclear, since the antibody would not only detect the full-length wild-type AR, but also C-terminally truncated AR variants and other mutant forms of AR, both of which have been found in prostate cancer that are shown to drive castrate resistant PC [26]. Therefore, the nature of AR-like proteins in NSCLC requires further exploration. Importantly, as this was a descriptive study of our cohort, we did not adjust for multiple comparisons, and validation in external cohorts is important to further explore the associations we observed.
We have demonstrated an association between AR and Ki67 expression in lung cancer tissue and patient outcome. Future work incorporating independent datasets is needed to confirm our findings and explore the potential mechanisms to explain these results to better define prognostic role of this pathway in NSCLC.
References
World Health Organization (2012) Estimated incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 2 Aug 2016
Pitz MW, Musto G, Demers AA, Kliewer EV (2009) Survival and treatment pattern of non-small cell lung cancer over 20 years. J Thorac Oncol 4(3):492–498
Ridge CA, Mcerlean AM, Ginsberg AM (2013) Epidemiology of lung cancer. Semin Interv Radiol 30(2):93–98
Dawe DE, Pond GR, Ellis PM (2016) Assessment of referral and chemotherapy treatment patterns for elderly patients with non small-cell lung cancer. Clin Lung Cancer 17(6):563–572
Bach PB, Kris MG (2016) Lung cancer in US women: a contemporary epidemic. JAMA 291(14):1763–1768
Thomas L, Doyle LA, Edelman MJ (2005) Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 128:370–381
Pitz MW, Musto G, Navaratnam S (2013) Sex as an independent prognostic factor in a population-based, non-small cell lung cancer cohort. Can Respir J 20(1):30–35
Mikkonen L, Pihlajamaa P, Sahu B, Zhang F, Jänne OA (2010) Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 314:14–24
Recchia AG, Musti AM, Lanzino M, Panno ML, Turano E, Zumpano R, Belfiore A, Andò S, Maggiolini M (2009) A cross-talk between the androgen receptor and the epidermal growth factor receptor leads to p38MAPK-dependent activation of mTOR and cyclinD1 expression in prostate and lung cancer cells. Int J Biochem Cell Biol 41:603–614
Beattie CW, Hansen NW, Thomas PA (1985) Steroid receptors in human lung cancer. Cancer Res 45:4206–4214
De Azambuja E, Cardoso F, de Castro G Jr, Colozza M, Durbecq V, Sotiriou C, Larsimont D, Paesmans M (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Br J Cancer 96:1504–1513
Klapper W, Hoster E, Determann O, Cabeçadas J, Campo E, Cogliatti S, Kodet R, Krivolapov YA, Loddenkemper C, Stein H, Müller-hermelink K, Rosenwald A (2009) Ki-67 as a prognostic marker in mantle cell lymphoma—consensus guidelines of the pathology panel of the European MCL Network. J Hematopathol 2:103–111
Warth A, Cortis J, Soltermann A, Meister M, Budczies J, Stenzinger A, Goeppert B, Thomas M (2014) Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer 111:1222–1229
Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert A, Lafitte J, Sculier J (2004) Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 91:2018–2025
Tabata K, Tanaka T, Hayashi T, Hori T, Nunomura S (2014) Ki-67 is a strong prognostic marker of non-small cell lung cancer when tissue heterogeneity is considered. BMC Clin Pathol 14(23):1–8
Pariesen M (2017) Manitoba Tumour Bank. https://www.umanitoba.ca/institutes/manitoba_institute_cell_biology/MICB/Platforms/MbTumourBank.html
Skliris GP, Leygue E, Watson PH, Murphy LC (2006) Expression of oestrogen receptor-b in oestrogen receptor-a negative human breast tumours. Br J Cancer 95:616–626
Skliris GP, Rowan BG, Al-dhaheri M, Williams C, Troup S (2012) Immunohistochemical validation of multiple phospho-specific epitopes for estrogen receptor α (ERα) in tissue microarrays of ERα positive human breast carcinomas. Breast Cancer Res Treat 118:443–453
Navaratnam S, Skliris G, Qing G, Shepherd FA, Nowatzki J, Demers A (2012) Differential role of estrogen receptor beta in early versus metastatic non-small cell lung cancer. Horm Cancer 3:93–100
Berardi R, Morgese F, Santinelli A, Onofri A, Biscotti A, Brunelli A, Caramanti M, Savini A, De Lisa M, Ballatore Z, Pompili C, Salati M, Torniai M, Cascinu S (2016) Hormonal receptors in lung adenocarcinoma: expression and difference in outcome by sex. Oncotarget 7:82648–82657
Maynard PV, Davies CJ, Blamey RW, Elston CW, Johnson J, Griffiths K (1978) Relationship between oestrogen-receptor content and histologic grade in human primary breast tumors. Br J Cancer 38:745–748
Harlos C, Musto G, Lambert P, Ahmed R, Pitz MW (2015) Androgen pathway manipulation and survival in patients with lung cancer. Horm Cancer 6:120–127
Campbell JL, Elston FC, Blamey CW, Morris RW, Nicholson AH, Griffiths RI, Haybittle K (1981) Quantitative oestradiol receptor values in primary breast cancer and response of metastases to endocrine therapy. Lancet 2:1317–1319
Schooling CM (2016) Could androgens be relevant to partly explain why men have lower life expectancy than women? J Epidemiol Community Health 70(4):324–328
Mayne BT, Bianco-miotto T, Buckberry S, Breen J, Clifton V, Shoubridge C, Roberts CT (2016) Large scale gene expression meta-analysis reveals expression in humans. Front Genet 7(183):1–14
Cao S, Zhan Y, Dong Y (2016) Emerging data on androgen receptor splice variants in prostate cancer. Endocr Relat Cancer 23(12):199–210
Acknowledgments
We would like to acknowledge the valuable contribution of the Manitoba Tumor Bank.
Funding
Funding was provided by the CancerCare Manitoba Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval for this project was given by the Health Research Ethics Board, University of Manitoba.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Electronic Supplementary Material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Grant, L., Banerji, S., Murphy, L. et al. Androgen Receptor and Ki67 Expression and Survival Outcomes in Non-small Cell Lung Cancer. HORM CANC 9, 288–294 (2018). https://doi.org/10.1007/s12672-018-0336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-018-0336-7