Abstract
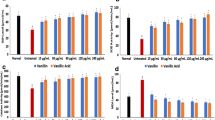
The neuroprotective activities of phenolics have been demonstrated in several studies, with their antioxidant properties playing an influential role. In this study, the therapeutic effect of ferulic acid was investigated on oxidative stress, purinergic and cholinergic enzymatic activities, and dysregulated metabolic pathways in oxidative brain injury. Ferulic acid significantly elevated the reduced glutathione (GSH) level, superoxide dismutase and catalase activities, and concomitantly depleted malondialdehyde and nitric oxide level. It also inhibited the activities of acetylcholinesterase and butyrylcholinesterase, and increased the activities of ATPase. LC-MS analysis of the metabolites revealed restoration of most depleted metabolites, with concomitant generation of dihydroferulic acid 4-O-glucuronide, diadenosine heptaphosphate, cis-4-decenoic acid, ganglioside GT3 (d18:0/23:0), phosphatidylinositol-3,4,5-trisphosphate, and phosphoribosyl-ATP on treatment with ferulic acid. Pathway analysis of the identified metabolites revealed reactivation of oxidative-inactivated pathways, with concomitant activation of histidine and inositol phosphate metabolic pathways. There was no cytotoxicity on incubation of ferulic acid with HT22 cells. Molecular docking studies revealed a high affinity for acetylcholinesterase, with a binding energy of − 7.4 kcal/mol. In silico simulation analysis predicted permeability of ferulic acid across blood brain barrier (BBB) and an oral LD50 calculated value of 1772 mg/kg, with a toxicity class of 4. These results indicate the antioxidative and protective effects of ferulic acid in oxidative brain injury.









Similar content being viewed by others
References
Adefegha SA, Omojokun OS, Oboh G, Fasakin O, Ogunsuyi O (2016) Modulatory effects of ferulic acid on cadmium-induced brain damage. J Evid Base Compl Altern Med 21:NP56–NP61
Adefegha SA, Oboh G, Oyeleye SI, Dada FA, Ejakpovi I, Boligon AA (2017) Cognitive enhancing and antioxidative potentials of velvet beans (Mucuna pruriens) and horseradish (Moringa oleifera) seeds extracts: a comparative study. J Food Biochem 41:e12292
Ademiluyi AO, Ogunsuyi OB, Oboh G (2016) Alkaloid extracts from Jimson weed (Datura stramonium L.) modulate purinergic enzymes in rat brain. Neurotoxicology 56:107–117
Adewoye O, Bolarinwa A, Olorunsogo O (2000) Ca++, Mg++-ATPase activity in insulin-dependent and non-insulin dependent diabetic Nigerians. Afr J Med Med Sci 29:195–199
Akomolafe S, Akinyemi A, Ogunsuyi O, Oyeleye S, Oboh G, Adeoyo O, Allismith Y (2017) Effect of caffeine, caffeic acid and their various combinations on enzymes of cholinergic, monoaminergic and purinergic systems critical to neurodegeneration in rat brain—in vitro. NeuroToxicology 62:6–13
Altamura S, Muckenthaler MU (2009) Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J Alzheimers Dis 16:879–895
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139:179–197
Bruning J et al (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125
Chan CX, Khan AA, Choi JH, Ng CD, Cadeiras M, Deng M, Ping P (2013) Technology platform development for targeted plasma metabolites in human heart failure. Clin Proteomics 10:7
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chong J et al (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:W486–W494
Chowdhury P, Soulsby M (2002) Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Ann Clin Lab Sci 32:188–192
Ćupić Miladinović D, Borozan S, Ivanović S (2018) Involvement of cholinesterases in oxidative stress induced by chlorpyrifos in the brain of Japanese quail. Poult Sci 97:1564–1571
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Report 7:42717
Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R (2014) ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res 42:W53–W58
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Erukainure OL, Mopuri R, Oyebode OA, Koorbanally NA, Islam MS (2017a) Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed Pharmacother 96:37–47
Erukainure OL, Oyebode OA, Sokhela MK, Koorbanally NA, Islam MS (2017b) Caffeine–rich infusion from Cola nitida (kola nut) inhibits major carbohydrate catabolic enzymes; abates redox imbalance; and modulates oxidative dysregulated metabolic pathways and metabolites in Fe 2+-induced hepatic toxicity. Biomed Pharmacother 96:1065–1074
Erukainure LO, Sanni O, Islam MS (2018) Clerodendrum volubile: phenolics and applications to health. In: Watson R, Preedy V, Zibadi S (eds) Polyphenols: mechanisms of action in human health and disease, 2nd edn. Elsevier. https://doi.org/10.1016/B978-0-12-813006-3.00006-4
Erukainure OL, Ijomone OM, Oyebode OA, Chukwuma CI, Aschner M, Islam MS (2019a) Hyperglycemia-induced oxidative brain injury: therapeutic effects of Cola nitida infusion against redox imbalance, cerebellar neuronal insults, and upregulated Nrf2 expression in type 2 diabetes rats. Food Chem Toxicol 127:206–217
Erukainure OL, Oyebode OA, Ibeji CU, Koorbanally NA, Islam MS (2019b) Vernonia Amygdalina Del. stimulated glucose uptake in brain tissues enhances antioxidative activities; and modulates functional chemistry and dysregulated metabolic pathways. Metab Brain Dis. https://doi.org/10.1007/s11011-018-0363-7
Frisch et al (2009) Gaussian 09, revision D. 01. Gaussian, Inc., Wallingford
Gay NH et al (2018) Neuroprotective effects of phenolic and carboxylic acids on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Neurochem Res 43:619–636
Grantham-McGregor S, Ani C (2001) A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 131:649S–668S
Greig NH, Lahiri DK, Sambamurti K (2002) Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int Psychogeriatr 14:77–91
Griffin JW, Bradshaw PC (2017, 2017) Amino acid catabolism in Alzheimer’s disease brain: friend or foe? Oxidative Med Cell Longev. https://doi.org/10.1155/2017/5472792
Hasegawa H, Oguro K, Naito Y, Ichiyama A (1999) Iron dependence of tryptophan hydroxylase activity in RBL2H3 cells and its manipulation by chelators. Eur J Biochem 261:734–739
Hidalgo C, Núñez MT (2007) Calcium, iron and neuronal function. IUBMB Life 59:280–285
Holeček M (2018) Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab 15:33
Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT (2004) Redox-active metals, oxidative stress, and Alzheimer’s disease pathology. Ann N Y Acad Sci 1012:153–163
Kakkar P, Das B, Viswanathan P (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kanski J, Aksenova M, Stoyanova A, Butterfield DA (2002) Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. J Nutr Biochem 13:273–281
Karamać M, Koleva L, Kancheva VD, Amarowicz R (2017) The structure–antioxidant activity relationship of ferulates. Molecules 22:527
Kryger G, Silman I, Sussman JL (1999) Structure of acetylcholinesterase complexed with E2020 (Aricept®): implications for the design of new anti-Alzheimer drugs. Structure 7:297–307
Latunde-Dada GO (2017) Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta (BBA)-Gen Subj 1861:1893–1900
Laursen M, Yatime L, Nissen P, Fedosova NU (2013) Crystal structure of the high-affinity Na+, K+-ATPase–ouabain complex with Mg2+ bound in the cation binding site. Proc Natl Acad Sci 110:10958–10963
Li D (1998) Effects of iron deficiency on iron distribution and gamma-aminobutyric acid (GABA) metabolism in young rat brain tissues. Hokk J Med Sci 73:215–225
Maaroufi K, Save E, Poucet B, Sakly M, Abdelmelek H, Had-Aissouni L (2011) Oxidative stress and prevention of the adaptive response to chronic iron overload in the brain of young adult rats exposed to a 150 kilohertz electromagnetic field. Neuroscience 186:39–47
Manna P, Jain SK (2015) Phosphatidylinositol-3, 4, 5-triphosphate and cellular signaling: implications for obesity and diabetes. Cell Physiol Biochem 35:1253–1275
McCaddon A, Regland B, Hudson P, Davies G (2002) Functional vitamin B12 deficiency and Alzheimer disease. Neurology 58:1395–1399
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45:117–127
Morabito MV, Berman DE, Schneider RT, Zhang Y, Leibel RL, Small SA (2014) Hyperleucinemia causes hippocampal retromer deficiency linking diabetes to Alzheimer’s disease. Neurobiol Dis 65:188–192
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Oboh G, Agunloye OM, Akinyemi AJ, Ademiluyi AO, Adefegha SA (2013) Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem Res 38:413–419
Ojha S, Javed H, Azimullah S, Khair SBA, Haque ME (2015) Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Design Dev Ther 9:5499
Patel M (2016) Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci 37:768–778
Pezacka E, Green R, Jacobsen DW (1990) Glutathionylcobalamin as an intermediate in the formation of cobalamin coenzymes. Biochem Biophys Res Commun 169:443–450
Prachayasittikul S, Suphapong S, Worachartcheewan A, Lawung R, Ruchirawat S, Prachayasittikul V (2009) Bioactive mewtabolites from Spilanthes acmella Murr. Molecules 14:850–867
Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) High therapeutic potential of Spilanthes acmella: a review. EXCLI J 12:291
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:57–61
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40:92–100
Strange RW, Antonyuk SV, Hough MA, Doucette PA, Valentine JS, Hasnain SS (2006) Variable metallation of human superoxide dismutase: atomic resolution crystal structures of Cu–Zn, Zn–Zn and as-isolated wild-type enzymes. J Mol Biol 356:1152–1162
Suwanjang W, Prachayasittikul S, Prachayasittikul V (2016) Effect of 8-hydroxyquinoline and derivatives on human neuroblastoma SH-SY5Y cells under high glucose. PeerJ 4:e2389
Szwajgier D, Borowiec K, Pustelniak K (2017) The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients 9:477
Torreilles F, Salman-Tabcheh S, Guérin M-C, Torreilles J (1999) Neurodegenerative disorders: the role of peroxynitrite. Brain Res Rev 30:153–163
Tsikas D (2005) Review Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res 39:797–815
Van Dyke K (1997) The possible role of peroxynitrite in Alzheimer’s disease: a simple hypothesis that could be tested more thoroughly. Med Hypotheses 48:375–380
Wishart DS et al (2012) HMDB 3.0—the human metabolome database in 2013. Nucleic Acids Res 41:D801–D807
Wu J, Holstein JD, Upadhyay G, Lin D-T, Conway S, Muller E, Lechleiter JD (2007) Purinergic receptor-stimulated IP3-mediated Ca2+ release enhances neuroprotection by increasing astrocyte mitochondrial metabolism during aging. J Neurosci 27:6510–6520
Yang B, Hao F, Li J, Chen D, Liu R (2013) Binding of chrysoidine to catalase: spectroscopy, isothermal titration calorimetry and molecular docking studies. J Photochem Photobiol B Biol 128:35–42
Acknowledgments
Dr. TA Olasehinde acknowledges Prof. David Schubert from Salk Institute, San Diego, USA, for providing HT-22 cells.
Funding
This work was supported by funding from the Research office, University of KwaZulu-Natal, Durban and the National Research Foundation- the World Academy of Science (NRF-TWAS), Pretoria, South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was carried out in accordance with the approved guidelines of the Animal Research Ethics Committee of the University of KwaZulu-Natal, Durban, South Africa (Protocol approval number: AREC/020/017D).
Conflict of Interest
The authors declare that they have no conflicts of interest relating to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 357 kb)
Rights and permissions
About this article
Cite this article
Salau, V.F., Erukainure, O.L., Ibeji, C.U. et al. Ferulic Acid Modulates Dysfunctional Metabolic Pathways and Purinergic Activities, While Stalling Redox Imbalance and Cholinergic Activities in Oxidative Brain Injury. Neurotox Res 37, 944–955 (2020). https://doi.org/10.1007/s12640-019-00099-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00099-7