Abstract
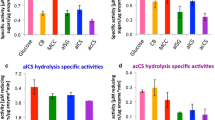
The cellulosome is a supramolecular multienzyme complex formed via species-specific interactions between the cohesin modules of scaffoldin proteins and the dockerin modules of a wide variety of polysaccharide-degrading enzymes. Here, we report a comparative analysis of cellulosomes prepared from the thermophilic anaerobic bacteria Clostridium (Ruminiclostridium) clariflavum DSM 19732 and Clostridium (Ruminiclostridium) thermocellum ATCC 27405 grown on delignified rice straw. The results indicate that the isolated C. clariflavum cellulosome exhibits lower activity for insoluble cellulosic substrates and higher activity for hemicellulosic substrates, especially for xylan, compared to the isolated C. thermocellum cellulosome. The C. clariflavum cellulosome was separated into large and small complexes by size exclusion chromatography, and the high xylanase activity of the intact complex is mainly attributed to the small complex. Furthermore, both C. clariflavum and C. thermocellum cellulosomes efficiently converted delignified rice straw into soluble sugars with different compositions, whereas a mixture of these cellulosomes exhibited essentially no synergy for the saccharification of delignified rice straw. This is the first study to report that isolated C. clariflavum cellulosomes exhibit greater xylanase activity than isolated C. thermocellum cellulosomes. We also report the effect of a combination of intact cellulosome complexes isolated from different species on the saccharification of plant biomass.





Similar content being viewed by others
References
Bayer, E. A., Belaich, J. P., Shoham, Y., & Lamed, R. (2004). The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annual Review of Microbiology, 58(1), 521–554.
Demain, A. L., Newcomb, M., & Wu, J. H. (2005). Cellulase, clostridia, and ethanol. Microbiology and Molecular Biology Reviews, 69(1), 124–154.
Artzi, L., Bayer, E. A., & Moraïs, S. (2017). Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nature Reviews. Microbiology, 15(2), 83–95.
Pagès, S., Bélaïch, A., Bélaïch, J. P., Morag, E., Lamed, R., Shoham, Y., & Bayer, E. A. (1997). Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain. Proteins, 29(4), 517–527.
Jindou, S., Soda, A., Karita, S., Kajino, T., Béguin, P., Wu, J. H., Inagaki, M., Kimura, T., Sakka, K., & Ohmiya, K. (2004). Cohesin-dockerin interactions within and between Clostridium josui and Clostridium thermocellum: binding selectivity between cognate dockerin and cohesin domains and species specificity. The Journal of Biological Chemistry, 279(11), 9867–9874.
Zverlov, V. V., Klupp, M., Krauss, J., & Schwarz, W. H. (2008). Mutations in the scaffoldin gene, cipA, of Clostridium thermocellum with impaired cellulosome formation and cellulosome hydrolysis: insertions of a new transposable element, IS1447, and implications for cellulase synergism on crystalline cellulose. Journal of Bacteriology, 190(12), 4321–4327.
Hirano, K., Nihei, S., Hasegawa, H., Haruki, M., & Hirano, N. (2015). Stoichiometric assembly of the cellulosome generates maximum synergy for the degradation of crystalline cellulose, as revealed by in vitro reconstitution of the Clostridium thermocellum cellulosome. Applied and Environmental Microbiology, 81(14), 4756–4766.
Hirano, K., Kurosaki, M., Nihei, S., Hasegawa, H., Shinoda, S., Haruki, M., & Hirano, N. (2016). Enzymatic diversity of the Clostridium thermocellum cellulosome is crucial for the degradation of crystalline cellulose and plant biomass. Scientific Reports, 6(1), 35709.
Gold, N. D., & Martin, V. J. (2007). Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. Journal of Bacteriology, 189(19), 6787–6795.
Raman, B., Chongle, P., Hurst, G. B., Rodriguez, M., McKeown, C. M., Lankford, P. K., Samatova, N. F., & Mielenz, J. R. (2009). Impact of pretreated switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: a quantitative proteomic analysis. PLoS One, 4(4), e5271.
Artzi, L., Morag, E., Barak, Y., Lamed, R., & Bayer, E. A. (2015). Clostridium clariflavum: key cellulosome players are revealed by proteomic analysis. MBio, 6(3), e00411–e00415.
Olson, D. G., Giannone, R. J., Hettich, R. L., & Lynd, L. R. (2013). Role of the CipA scaffoldin protein in cellulose solubilization, as determined by targeted gene deletion and complementation in Clostridium thermocellum. Journal of Bacteriology, 195(4), 733–739.
Morag, E., Lapidot, A., Govorko, D., Lamed, R., Wilchek, M., Bayer, E. A., & Shoham, Y. (1995). Expression, purification, and characterization of the cellulose-binding domain of the scaffoldin subunit from the cellulosome of Clostridium thermocellum. Applied and Environmental Microbiology, 61(5), 1980–1986.
Hong, W., Zhang, J., Feng, Y., Mohr, G., Lambowitz, A. M., Cui, G. Z., Liu, Y. J., & Cui, Q. (2014). The contribution of cellulosomal scaffoldins to cellulose hydrolysis by Clostridium thermocellum analyzed by using thermotargetrons. Biotechnology for Biofuels, 7(1), 80.
Shiratori, H., Ikeno, H., Ayame, S., Kataoka, N., Miya, A., Hosono, K., Beppu, T., & Ueda, K. (2006). Isolation and characterization of a new Clostridium sp. that performs effective cellulosic waste digestion in a thermophilic methanogenic bioreactor. Applied and Environmental Microbiology, 72(5), 3702–3709.
Shiratori, H., Sasaya, K., Ohiwa, H., Ikeno, H., Ayame, S., Kataoka, N., Miya, A., Beppu, T., & Ueda, K. (2009). Clostridium clariflavum sp. nov. and Clostridium caenicola sp. nov., moderately thermophilic, cellulose-/cellobiose-digesting bacteria isolated from methanogenic sludge. International Journal of Systematic and Evolutionary Microbiology, 59(7), 1764–1770.
Izquierdo, J. A., Goodwin, L., Davenport, K. W., Teshima, H., Bruce, D., Detter, C., Tapia, R., Han, S. S., Land, M., Hauser, L., Jeffries, C. D., Han, J., Pitluck, S., Nolan, M., Chen, A., Huntemann, M., Mavromatis, K., Mikhailova, N., Liolios, K., Woyke, T., & Lynd, L. R. (2012). Complete genome sequence of Clostridium clariflavum DSM 19732. Standards in Genomic Sciences, 19, 104–115.
Artzi, L., Dassa, B., Borovok, I., Shamshoum, M., Lamed, R., & Bayer, E. A. (2014). Cellulosomics of the cellulolytic thermophile Clostridium clariflavum. Biotechnology for Biofuels, 7(1), 100.
Simpson, P. J., Xie, H., Bolam, D. N., Gilbert, H. J., & Williamson, M. P. (2000). The structural basis for the ligand specificity of family 2 carbohydrate-binding modules. The Journal of Biological Chemistry, 275(52), 41137–41142.
Izquierdo, J. A., Pattathil, S., Guseva, A., Hahn, M. G., & Lynd, L. R. (2014). Comparative analysis of the ability of Clostridium clariflavum strains and Clostridium thermocellum to utilize hemicellulose and unpretreated plant material. Biotechnology for Biofuels, 7(1), 136.
Morita, K., Yamamoto, T., Fusada, N., Komatsu, M., Ikeda, H., Hirano, N., & Takahashi, H. (2009). In vitro characterization of the site-specific recombination system based on actinophage TG1 integrase. Molecular Genetics and Genomics, 282(6), 607–616.
Waeonukul, R., Kosugi, A., Tachaapaikoon, C., Pason, P., Ratanakhanokchai, K., Prawitwong, P., Deng, L., Saito, M., & Mori, Y. (2012). Efficient saccharification of ammonia soaked rice straw by combination of Clostridium thermocellum cellulosome and Thermoanaerobacter brockii β-glucosidase. Bioresource Technology, 107, 352–357.
Breves, R., Bronnenmeier, K., Wild, N., Lottspeich, F., Staudenbauer, W. L., & Hofemeister, J. (1997). Genes encoding two different beta-glucosidases of Thermoanaerobacter brockii are clustered in a common operon. Applied and Environmental Microbiology, 63(10), 3902–3910.
Hirano, N., Ohshima, H., & Takahashi, H. (2006). Biochemical analysis of the substrate specificity and sequence preference of endonuclease IV from bacteriophage T4, a dC-specific endonuclease implicated in restriction of dC-substituted T4 DNA synthesis. Nucleic Acids Research, 34(17), 4743–4751.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Kahar, P., Taku, K., & Tanaka, S. (2013). Multiple effects of swelling by sodium bicarbonate after delignification on enzymatic saccharification of rice straw. Journal of Bioscience and Bioengineering, 116(6), 725–733.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP) Technical Report NREL/TP-510-42618.
Miller, G. L., Golder, R. H., & Miller, E. E. (1951). Determination of Pentoses. Effect of varying proportions of components of bial’s color reagent. Analytical Chemistry, 23(6), 903–905.
Morris, D. L. (1948). Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science, 107(2775), 254–255.
Morag, E., Bayer, E. A., & Lamed, R. (1992). Affinity digestion for the near-total recovery of purified cellulosome from Clostridium thermocellum. Enzyme and Microbial Technology, 14(4), 289–292.
Wood, T. M. (1988). Preparation of crystalline, amorphous, and dyed cellulase substrates. Methods in Enzymology, 160, 19–25.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428.
Kataeva, I., Li, X. L., Chen, H., Choi, S. K., & Ljungdahl, L. G. (1999). Cloning and sequence analysis of a new cellulose gene encoding CelK, a major cellulosome component of Clostridium thermocellum: evidence for gene duplication and recombination. Journal of Bacteriology, 181(17), 5288–5295.
Kruus, K., Wang, W. K., Ching, J., & Wu, J. H. (1995). Exoglucanase activities of the recombinant Clostridium thermocellum CelS, a major cellulosome component. Journal of Bacteriology, 177(6), 1641–1644.
Guimaraes, B. G., Souchon, H., Lytle, B. L., Wu, J. H., & Alzari, P. M. (2002). The crystal structure and catalytic mechanism of cellobiohydrolase CelS, the major enzymatic component of the Clostridium thermocellum cellulosome. Journal of Molecular Biology, 320(3), 587–596.
Wei, H., Fu, Y., Magnusson, L., Baker, J. O., Maness, P. C., Xu, Q., Yang, S., Bowersox, A., Bogorad, I., Wang, W., Tucker, M. P., Himmel, M. E., & Ding, S. Y. (2014). Comparison of transcriptional profiles of Clostridium thermocellum grown on cellobiose and pretreated yellow poplar using RNA-Seq. Frontiers in Microbiology, 5, 142.
Shi, J., Ebrik, M. A., Yang, B., Garlock, R. J., Balan, V., Dale, B. E., Pallapolu, V. R., Lee, Y. Y., Kim, Y., Mosier, N. S., Ladisch, M. R., Holtzapple, M. T., Falls, M., Sierra-Ramirez, R., Donohoe, B. S., Vinzant, T. B., Elander, R. T., Hames, B., Thomas, S., Warner, R. E., & Wyman, C. E. (2011). Application of cellulase and hemicellulase to pure xylan, pure cellulose, and switchgrass solids from leading pretreatments. Bioresource Technology, 102(24), 11080–11088.
Nataf, Y., Bahari, L., Kahel-Raifer, H., Borovok, I., Lamed, R., Bayer, E. A., Sonenshein, A. L., & Shoham, Y. (2010). Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic polysaccharides via alternative sigma factors. Proceedings of the National Academy of Sciences of the United States of America, 107(43), 18646–18651.
Sand, A., Holwerda, E. K., Ruppertsberger, N. M., Maloney, M., Olson, D. G., Nataf, Y., Borovok, I., Sonenshein, A. L., Bayer, E. A., Lamed, R., Lynd, L. R., & Shoham, Y. (2015). Three cellulosomal xylanase genes in Clostridium thermocellum are regulated by both vegetative SigA (σ (A)) and alternative SigI6 (σ (I6)) factors. FEBS Letters, 589(20PartB), 3133–3140.
Ortiz de Ora, L., Muñoz-Gutiérrez, I., Bayer, E. A., Shoham, Y., Lamed, R., & Borovok, I. (2017). Revisiting the regulation of the primary scaffoldin gene in Clostridium thermocellum. Applied and Environmental Microbiology, 83, e03088–e03016.
Pohlschröder, M., Canale-Parola, E., & Leschine, S. B. (1995). Ultrastructural diversity of the cellulase complexes of Clostridium papyrosolvens C7. Journal of Bacteriology, 177(22), 6625–6629.
Fendri, I., Tardif, C., Fierobe, H. P., Lignon, S., Valette, O., Pagès, S., & Perret, S. (2009). The cellulosomes from Clostridium cellulolyticum: identification of new components and synergies between complexes. The FEBS Journal, 276(11), 3076–3086.
Mechaly, A., Fierobe, H. P., Belaich, A., Belaich, J. P., Lamed, R., Shoham, Y., & Bayer, E. A. (2001). Cohesin-dockerin interaction in cellulosome assembly: a single hydroxyl group of a dockerin domain distinguishes between nonrecognition and high affinity recognition. The Journal of Biological Chemistry, 276(13), 9883–9888.
Jindou, S., Kajino, T., Inagaki, M., Karita, S., Beguin, P., Kimura, T., Sakka, K., & Ohmiya, K. (2004). Interaction between a type-II dockerin domain and a type-II cohesin domain from Clostridium thermocellum cellulosome. Bioscience, Biotechnology, and Biochemistry, 68(4), 924–926.
Waller, B. H., Olson, D. G., Currie, D. H., Guss, A. M., & Lynd, L. R. (2013). Exchange of type II dockerin-containing subunits of the Clostridium thermocellum cellulosome as revealed by SNAP-tags. FEMS Microbiology Letters, 338(1), 46–53.
Sakka, K., Kishino, Y., Sugihara, Y., Jindou, S., Sakka, M., Inagaki, M., Kimura, T., & Sakka, K. (2009). Unusual binding properties of the dockerin module of Clostridium thermocellum endoglucanase CelJ (Cel9D-Cel44A). FEMS Microbiology Letters, 300(2), 249–255.
Sakka, K., Sugihara, Y., Jindou, S., Sakka, M., Inagaki, M., Sakka, K., & Kimura, T. (2011). Analysis of cohesin-dockerin interactions using mutant dockerin proteins. FEMS Microbiology Letters, 314(1), 75–80.
Funding
This work was funded by PRESTO grant JPMJPR12B7 from the Japan Science and Technology Agency and a grant (26550103) for Challenging Exploratory Research and a Grant-in-Aid for Scientific Research (B) (16H04732) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This research was also funded by grants for Strategic Research Foundations at Private Universities (2013–2017 and 2014–2018) from MEXT, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Shinoda, S., Kurosaki, M., Kokuzawa, T. et al. Comparative Biochemical Analysis of Cellulosomes Isolated from Clostridium clariflavum DSM 19732 and Clostridium thermocellum ATCC 27405 Grown on Plant Biomass. Appl Biochem Biotechnol 187, 994–1010 (2019). https://doi.org/10.1007/s12010-018-2864-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2864-6