Abstract
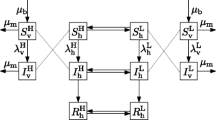
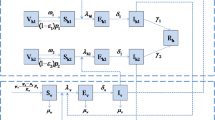
Dengue is considered one of the most important vector-borne infection, affecting almost half of the world population with 50 to 100 million cases every year. In this paper, we present one of the simplest models that can encapsulate all the important variables related to vector control of dengue fever. The model considers the human population, the adult mosquito population and the population of immature stages, which includes eggs, larvae and pupae. The model also considers the vertical transmission of dengue in the mosquitoes and the seasonal variation in the mosquito population. From this basic model describing the dynamics of dengue infection, we deduce thresholds for avoiding the introduction of the disease and for the elimination of the disease. In particular, we deduce a Basic Reproduction Number for dengue that includes parameters related to the immature stages of the mosquito. By neglecting seasonal variation, we calculate the equilibrium values of the model’s variables. We also present a sensitivity analysis of the impact of four vector-control strategies on the Basic Reproduction Number, on the Force of Infection and on the human prevalence of dengue. Each of the strategies was studied separately from the others. The analysis presented allows us to conclude that of the available vector control strategies, adulticide application is the most effective, followed by the reduction of the exposure to mosquito bites, locating and destroying breeding places and, finally, larvicides. Current vector-control methods are concentrated on mechanical destruction of mosquitoes’ breeding places. Our results suggest that reducing the contact between vector and hosts (biting rates) is as efficient as the logistically difficult but very efficient adult mosquito’s control.
Similar content being viewed by others
References
Adams, B., & Boots, M. (2010). How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics, 2, 1–10.
Aguiar, M., Kooi, B. W., Rocha, F., Ghaffari, P., & Stollenwerk, N. (2013). How much complexity is needed to describe the fluctuations observed in dengue hemorrhagic fever incidence data? Ecol. Complex., 16, 31–40.
Amaku, M., Azevedo, R. S., Castro, R. M., Massad, E., & Coutinho, F. A. B. (2009). Relationship among epidemiological parameters in a non-immunized Brazilian community. Mem. Inst. Oswaldo Cruz, 104, 897–900.
Amaku, M., Burattini, M. N., Coutinho, F. A. B., & Massad, E. (2013a). A comment on the estimation of the Basic Reproduction Number for vector-borne infections. Phil. Trans. R. Soc. A, eLetter. http://rsta.royalsocietypublishing.org/content/368/1933/5679.abstract/reply. Accessed 17 Aug 2013.
Amaku, M., Burattini, M. N., Coutinho, F. A. B., Lopez, L. F., & Massad, E. (2013b). Maximum equilibrium prevalence of mosquito-borne microparasite infections in humans. Comput. Math. Methods Med., 2013, 659038. doi:10.1155/2013/659038.
Anguelov, R., Dumont, Y., & Lubuma, J. M.-S. (2012). Mathematical modeling of sterile insect technology for control of anopheles mosquito. Comput. Math. Appl., 64(3), 374–389.
Bacaër, N., & Guernaoui, S. (2006). The epidemic threshold of vector-borne diseases with seasonality: the case of cutaneous leishmaniasis in Chichaoua, Morocco. J. Math. Biol., 53, 421–436.
Beatty, M. E., Letson, G. W., & Margolis, H. S. (2008). Estimating the global burden of dengue. Abstract book: dengue 2008. In Proceedings of the 2nd international conference on dengue and dengue haemorrhagic fever, Phuket, Thailand.
Beatty, M. E., Beutels, P., Meltzer, M. I., Shepard, D. S., Hombach, J., et al. (2011). Health economics of dengue: a systematic literature review and expert panel’s assessment. Am. J. Trop. Med. Hyg., 84, 473–488.
Brownstein, J. S., Heth, E., & O’Neill, L. (2003). The potential of virulent Wolbachia to modulate disease transmission by insects. J. Invertebr. Pathol., 84, 24–29.
Burattini, M. N., Chen, M., Chow, A., Coutinho, F. A. B., Goh, K. T., et al. (2008). Modelling the control strategies against dengue in Singapore. Epidemiol. Infect., 136, 309–319.
Chitnis, N., Hyman, J. M., & Cushing, J. M. (2008). Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol., 70, 1272–1296.
Cousins, R. D., & James, F. (2006). Comment on “The distribution of composite measurements: how to be certain of the uncertainties in what we measure,” by M. P. Silverman, W. Strange, and T. C. Lipscombe [Am. J. Phys. 72(8), 1068–1081 (2004)]. Am. J. Phys., 72(8), 1068–1081.
Coutinho, F. A. B., Lopez, L. F., & Massad, E. (2004). Comment on “The distribution of composite measurements: how to be certain of the uncertainties in what we measure,” by M. P. Silverman, W. Strange, and T. C. Lipscombe [Am. J. Phys. 72(8), 1068–1081 (2004)] Am. J. Phys., 72(8), 1068–1081.
Coutinho, F. A. B., Burattini, M. N., Lopez, L. F., & Massad, E. (2005). An approximate threshold condition for non-autonomous system: an application to a vector-borne infection. Math. Comput. Simul., 70, 149–158.
Coutinho, F. A. B., Burattini, M. N., Lopez, L. F., & Massad, E. (2006). Threshold conditions for a non-autonomous epidemic system describing the population dynamics of dengue. Bull. Math. Biol., 68, 2263–2282.
Dumont, Y., & Chiroleu, F. (2010). Vector control for the Chikungunya disease. Math. Biosci. Eng., 7(2), 313–345.
Ellis, A. M., Garcia, A. J., Focks, D. A., Morrison, A. C., & Scott, T. W. (2011). Parameterization and sensitivity analysis of a complex simulation model for mosquito population dynamics, dengue transmission and their control. Am. J. Trop. Med. Hyg., 82, 257–264.
Erickson, R. A., Presley, S. M., Allen, L. J. S., Long, K. R., & Cox, S. B. (2010). A dengue model with a dynamic Aedes albopictus vector population. Ecol. Model., 221, 2899–2908.
Fegan, G., Noor, A. M., Akhwale, W. S., Cousens, S., & Snow, R. W. (2007). Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet, 370, 1035–1039.
Forattini, O. P. (1996). Medical culicidology. São Paulo: EDUSP.
Garba, S. M., Gumel, A. B., & Abu Bakar, M. R. (2008). Backward bifurcations in dengue transmission dynamics. Math. Biosci., 215, 11–25.
Gubler, D. J. (2002). The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res., 33, 330–342.
Gubler, D. J. (2011). Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop. Med. Health, 39 (4 Suppl), 3–11.
Guy, B., Almond, J., & Lang, J. (2011). Dengue vaccine prospects. Lancet, 377, 381–382.
Halstead, S. B. (1990). Dengue. In K. S. Warren & A. A. F. Mahmoud (Eds.), Tropical and geographical medicine (pp. 675–684). New York: McGraw-Hill.
Index Mundi (2011). http://www.indexmundi.com/map/?v=30&l=pt. Accessed 18 Aug 2011.
Integrated Vector Management (2012). http://www.ivmproject.net/about/index.cfm?fuseaction=static&label=dengue. Accessed 1 Apr 2012.
Khasnis, A. A., & Nettlelman, M. D. (2005). Global warming and infectious disease. Arch. Med. Res., 36, 689–696.
Kooi, B. W., Aguiar, M., & Stollenwerk, N. (2013). Bifurcation analysis of a family of multistrain epidemiology models. J. Comput. Appl. Math., 252, 148–158.
Lambrechts, L., Scott, T. W., & Gubler, D. J. (2010). Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis., 4, e646.
Lopez, L. F., Coutinho, F. A. B., Burattini, M. N., & Massad, E. (2002). Threshold conditions for infection persistence in complex host-vectors interactions. C. R. Biol., 325, 1073–1084.
Luz, P. M., Vanni, T., Medlock, J., Paltiel, A. D., & Galvani, A. P. (2011). Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet, 377, 1673–1680.
Macdonald, G. (1952). The analysis of equilibrium in malaria. Trop. Dis. Bull., 49, 813–828.
Massad, E., & Coutinho, F. A. B. (2011). The cost of dengue control. Lancet, 377, 1630–1631.
Massad, E., Behrens, R. H., Burattini, M. N., & Coutinho, F. A. B. (2009). Modeling the risk of malaria for travelers to areas with stable malaria transmission. Malar. J., 8, 296.
Massad, E., Coutinho, F. A. B., Lopez, L. F., & da Silva, D. R. (2011). Modeling the impact of global warming on vector-borne infections. Phys. Life Rev., 8, 169–199.
Ocampo, C. B., & Wesson, D. M. (2004). Population dynamics of Aedes aegypti from a dengue hyperendemic urban setting in Colombia. Am. J. Trop. Med. Hyg., 7(1), 506–513.
Pinho, S. T. R., Ferreira, C. P., Esteva, L., Barreto, F. R., Morato e Silva, V. C., & Teixeira, M. G. L. (2010). Modelling the dynamics of dengue real epidemics. Philos. Trans. R. Soc. A, Math. Phys. Eng. Sci., 368, 5679–5693.
Reiter, P., & Gubler, D. J. (2001). Surveillance and control of urban dengue vectors. In D. J. Gubler & G. Kuno (Eds.), Dengue and dengue hemorrhagic fever (pp. 425–462). Wallingford: CABI Publishing.
Rodrigues, H. S., Monteiro, M. T., & Torres, D. F. M. (2012). Dengue in Cape Verde: vector control and vaccination. arXiv:1204.0544v1.
Ross, R. (1911). The prevention of malaria (2nd ed.). London: Murray. With addendum on the theory of happenings.
Silverman, M. P., Strange, W., & Lipscombe, T. C. (2004). The distribution of composite measurements: how to be certain of the uncertainties in what we measure. Am. J. Phys., 72, 1068–1081.
Suaya, J. A., Shepard, D. S., & Siqueira, J. B. (2009). Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am. J. Trop. Med. Hyg., 80, 846–855.
UNWTO World Tourism Organization (2011). Tourism highlights. www.world-tourism.org/facts/menu.html. Accessed 11 Mar 2011.
Wahl, L. M., & Nowak, M. A. (2000). Adherence and drug resistance: predictions for therapy outcome. Proc. - Royal Soc., Biol. Sci., 267, 835–843.
Wang, W., & Zhao, X.-Q. (2008). Threshold dynamics for compartmental epidemic models in periodic environments. J. Dyn. Differ. Equ., 20, 699–717.
WHO (2009). Dengue and dengue haemorrhagic fever. Fact sheet No. 117. http://who.int/mediacentre/factsheets/fs117/en/print.html. Accessed 11 Mar 2011.
WHO (2012). Dengue and severe dengue. Fact sheet No. 117. http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed 10 Apr 2012.
Wilder-Smith, A., Ooi, E. E., Vasudevan, S. G., & Gubler, D. J. (2010). Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr. Infect. Dis. Rep., 12, 157–164.
Wilder-Smith, A., Renhorn, K. E., Tissera, H., Abu Bakar, S., Alphey, L., et al. (2012). DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob. Health Action, 5, 17273.
Yasuno, M., & Tonn, R. J. (1970). A study of biting habits of Aedes aegypti in Bangkok, Thailand. Bull. World Health Organ., 43, 319–325.
Acknowledgements
The research from which these results were obtained has received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 282589, from LIM01 HCFMUSP and CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
M.A., F.A.B.C., S.M.R., L.F.L., M.N.B. and E.M. designed the work, performed the analysis, and wrote the paper.
Appendix: Some Comments on the Meaning of the Model’s Equations
Appendix: Some Comments on the Meaning of the Model’s Equations
In this appendix, we show how to include spatial heterogeneities in the model and, by doing so, we clarify the meaning of the model’s equations.
First, we assume that mosquitoes have a limited range of flight, which implies that the probability of transmission of infection from one infected mosquito to one susceptible host varies according to the distance between them.
Consider the first equation of system (1),
All the variables are densities. This implies that we are considering a very large region where the populations of mosquitoes and hosts are constant, that is, do not vary from point to point. Then, one might think that in Eq. (40) a mosquito in a certain place can bite a host which can be very far from it. This is not reasonable and it is not true for Eq. (40). To see this, consider the parameter a, the mosquitoes’ biting rate. We can write this as a=a′A, where a′ is the biting rate per unit area and A is the area where the mosquitoes’ flight ranges. Therefore, only humans inside this area are bitten by this mosquito. But, since the humans and mosquitoes populations are assumed as homogeneously distributed, this does not appear in the equations because in parameter a this effect is hidden.
Let us now introduce spatial heterogeneity. For this we should specify the position \(\vec {r}\), representing the spatial location of individuals. Thus, let \(S_{H}(\vec {r} )\,ds\) be the number of human susceptibles in the small area ds around the position \(\vec {r}\).
Let us now consider how \(S_{H}(\vec {r} )\,ds\) varies with time. Let \(I_{M}(\vec {r}' )\,ds'\) be the number of infected mosquitoes in the small area ds′ around the position \(\vec {r}'\). The total number of bites the infected mosquitoes population inflicts in a time interval dt is \(a'I_{M}(\vec {r}' )\,ds'\,dt\). A fraction of those bites \(F(\vert \vec {r} - \vec {r} ' \vert )\) is inflicted on the hosts at position \(\vec {r}\), that is, \(S_{H}(\vec {r} )\,ds\). Of course, \(F(\vert \vec {r} - \vec {r} ' \vert )\) is a decreasing function of the distance \(\vert \vec {r} - \vec {r} ' \vert \) between infected mosquitoes and susceptible humans. Thus, Eq. (40) becomes
All the other equations in system (1) should be similarly modified and, of course, the result is very difficult to integrate. When \(a'(\vec {r} ')F(\vert \vec {r} - \vec {r} ' \vert )\) is equal to \(a'A\theta (\vert \vec {r} - \vec {r} ' \vert )\), and the densities are homogeneously distributed in space, we have
The above formalism is necessary when we are dealing with large regions of space, where heterogeneities are significant. However, for small regions, where heterogeneities can be neglected, the system of Eqs. (1) of the main text is a good approximation. The relative sensitivity of the transmission variables to the studied parameters, however, is not expected to be significantly influenced by spatial heterogeneities. Of course, the value of the transmission variables may vary from place to place but the relative sensitivity, the main objective of the present analysis, of these variables to the parameters should be the same.
Rights and permissions
About this article
Cite this article
Amaku, M., Coutinho, F.A.B., Raimundo, S.M. et al. A Comparative Analysis of the Relative Efficacy of Vector-Control Strategies Against Dengue Fever. Bull Math Biol 76, 697–717 (2014). https://doi.org/10.1007/s11538-014-9939-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-9939-5