Abstract
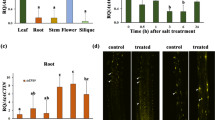
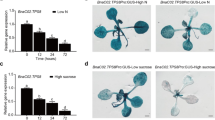
Allantoin, a metabolite generated in the purine degradation pathway, was primarily considered an intermediate for recycling of the abundant nitrogen assimilated in plant purines. More specifically, tropical legumes utilize allantoin and allantoic acid as major nodule-to-shoot nitrogen transport compounds. In other species, an increase in allantoin content was observed under different stress conditions, but the underlying molecular mechanisms remain poorly understood. In this work, Arabidopsis thaliana was used as a model system to investigate the effects of salt stress on allantoin metabolism and to know whether its accumulation results in plant protection. Plant seedlings treated with NaCl at different concentrations showed higher allantoin and lower allantoic acid contents. Treatments with NaCl favored the expression of genes involved in allantoin synthesis, but strongly repressed the unique gene encoding allantoinase (AtALN). Due to the potential regulatory role of this gene for allantoin accumulation, AtALN promoter activity was studied using a reporter system. GUS mediated coloration was found in specific plant tissues and was diminished with increasing salt concentrations. Phenotypic analysis of knockout, knockdown and stress-inducible mutants for AtALN revealed that allantoin accumulation is essential for salt stress tolerance. In addition, the possible role of allantoin transport was investigated. The Ureide Permease 5 (UPS5) is expressed in the cortex and endodermis of roots and its transcription is enhanced by salt treatment. Ups5 knockout plants under salt stress presented a susceptible phenotype and altered allantoin root-to-shoot content ratios. Possible roles of allantoin as a protectant compound in oxidative events or signaling are discussed.








Similar content being viewed by others
References
Bollard E (1957) Translocation of organic nitrogen in the xylem. Austral J Biol Sci 10:292–301
Brychkova G, Alikulov Z, Fluhr R, Sagi M (2008) A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J 54:496–509
Claeys H, Van Landeghem S, Dubois M, Maleux K, Inzé D (2014) What is stress? Dose–response effects in commonly used in vitro stress assays. Plant Physiol 165:519–527
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Collier R, Tegeder M (2012) Soybean ureide transporters play a critical role in nodule development, function and nitrogen export. Plant J 72:355–367
Desimone M et al (2002) A novel superfamily of transporters for allantoin and other oxo derivatives of nitrogen heterocyclic compounds in Arabidopsis. Plant Cell 14:847–856
Dinneny JR et al (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320:942–945
Duan L, Dietrich D, Ng CH, Chan PMY, Bhalerao R, Bennett MJ, Dinneny JR (2013) Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25:324–341
Glantzounis G, Tsimoyiannis E, Kappas A, Galaris D (2005) Uric acid and oxidative stress. Curr Pharm Des 11:4145–4151
Han G, Wang M, Yuan F, Sui N, Song J, Wang B (2014) The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol Biol 86:237–253
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Hesberg C, Hänsch R, Mendel RR, Bittner F (2004) Tandem orientation of duplicated xanthine dehydrogenase genes from Arabidopsis thaliana differential gene expression and enzyme activities. J Biol Chem 279:13547–13554
Kanani H, Dutta B, Klapa MI (2010) Individual vs. combinatorial effect of elevated CO2 conditions and salinity stress on Arabidopsis thaliana liquid cultures: comparing the early molecular response using time-series transcriptomic and metabolomic analyses. BMC Syst Biol 4:177
Kilian J et al (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50:347–363
Lamberto I, Percudani R, Gatti R, Folli C, Petrucco S (2010) Conserved alternative splicing of Arabidopsis transthyretin-like determines protein localization and S-allantoin synthesis in peroxisomes. Plant Cell 22:1564–1574
Martin T, Wohner RV, Hummel S, Willmitzer L, Frommer WB (1992) The GUS reporter system as a tool to study plant gene expression. In: Gallagher SR (ed) GUS protocols: using the GUS gene as a reporter of gene expression. Academic Press, New York, pp 23–43
McClure PR, Israel DW (1979) Transport of nitrogen in the xylem of soybean plants. Plant Physiol 64:411–416
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nikiforova VJ et al (2005) Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiol 138:304–318
Pandey S et al (2010) Boolean modeling of transcriptome data reveals novel modes of heterotrimeric G-protein action. Mol Syst Biol 6:372
Pélissier HC, Frerich A, Desimone M, Schumacher K, Tegeder M (2004) PvUPS1, an allantoin transporter in nodulated roots of French bean. Plant Physiol 134:664–675
Rose MT et al (2012) Root metabolic response of rice (Oryza sativa L.) genotypes with contrasting tolerance to zinc deficiency and bicarbonate excess. Planta 236:959–973
Sagi M, Omarov RT, Lips SH (1998) The Mo-hydroxylases xanthine dehydrogenase and aldehyde oxidase in ryegrass as affected by nitrogen and salinity. Plant Sci 135:125–135
Schmidt A et al (2004) UPS1 and UPS2 from Arabidopsis mediate high affinity transport of uracil and 5-fluorouracil. J Biol Chem 279:44817–44824
Schmidt A, Baumann N, Schwarzkopf A, Frommer WB, Desimone M (2006) Comparative studies on Ureide Permeases in Arabidopsis thaliana and analysis of two alternative splice variants of AtUPS5. Planta 224:1329–1340
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schubert KR (1986) Products of biological nitrogen fixation in higher plants: synthesis, transport, and metabolism. Annu Rev Plant Physiol 37:539–574
Serventi F, Ramazzina I, Lamberto I, Puggioni V, Gatti R, Percudani R (2010) Chemical basis of nitrogen recovery through the ureide pathway: formation and hydrolysis of S-ureidoglycine in plants and bacteria. ACS Chem Biol 5:203–214
Smith PM, Atkins CA (2002) Purine biosynthesis. Big in cell division, even bigger in nitrogen assimilation. Plant Physiol 128:793–802
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35:44–56
Ventura Y, Myrzabayeva M, Alikulov Z, Omarov R, Khozin-Goldberg I, Sagi M (2014) Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 6:plu053
Vernon LP (1960) Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal Chem 32:1144–1150
Vogels G, Van der Drift C (1970) Differential analyses of glyoxylate derivatives. Anal Biochem 33:143–157
Watanabe S, Matsumoto M, Hakomori Y, Takagi H, Shimada H, Sakamoto A (2014) The purine metabolite allantoin enhances abiotic stress tolerance through synergistic activation of abscisic acid metabolism. Plant Cell Environ 37:1022–1036
Werner AK, Witte C-P (2011) The biochemistry of nitrogen mobilization: purine ring catabolism. Trends Plant Sci 16:381–387
Werner AK, Romeis T, Witte C-P (2010) Ureide catabolism in Arabidopsis thaliana and Escherichia coli. Nat Chem Biol 6:19–21
Werner AK, Medina-Escobar N, Zulawski M, Sparkes IA, Cao F-Q, Witte C-P (2013) The ureide-degrading reactions of purine ring catabolism employ three amidohydrolases and one aminohydrolase in Arabidopsis, soybean, and rice. Plant Physiol 163:672–681
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yobi A et al (2013) Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol Plant 6:369–385
Zhu J-K (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zrenner R, Stitt M, Sonnewald U, Boldt R (2006) Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol 57:805–836
Acknowledgments
This work was supported by Grants (PICT-2009-0114) of the National Fund of Science and Technology (FONCyT, Argentina) and of the Secretary of Science and Technology of the National University of Córdoba (SECyT-UNC, Argentina). C.I.L. is grateful for a scholarship at the Multidisciplinary Institute of Plant Biology (IMBIV-CONICET). We thank Dr. Alejandra Trenchi for microscopy assistance.
Author contributions
C.I.L. did the cloning work, generation of transgenic plants and expression studies. Plant phenotyping was carried out by C.I.L., C.M. and C.A.G. The writing of the manuscript was performed by C.I.L., C.A.G. and M.D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Characterization of two aln T-DNA insertion lines and AtALN stress-inducible lines used in this study. (a) Two independent T-DNA insertion lines in the AtALN gene, Salk_146783 (designated aln-1) and Salk_142607 (designated aln-2) were analyzed. The insertion in aln-1 (aln-2) was localized in the twelfth (ninth) exon (intron). (b) AtALN T-DNA lines verification by PCR. Primers were designed using SALK’s T-DNA Primer tool. LP = Left genomic Primer, RP = Right genomic primer, LB = Left border primer of the T-DNA insertion. (c) Expression of AtALN mRNA in WT and aln T-DNA insertion lines. RNA was extracted from 14 day-old plants. A 641 bp AtACT2 (control) and a 682 bp AtALN fragments of wt and aln T-DNA insertion lines were amplified by PCR (28 and 32 cycles) or adding two extra PCR cycles (30 and 34 cycles). (d) Map of the construct pCRD29A::ALN used for the generation of transgenic plants with a stress-inducible-AtALN. (e) Expression of AtALN mRNA in WT, aln T-DNA insertion lines and RD29A::ALN/aln-2 under salt stress. 14 day-old seedlings were transferred to 0.5× MS vertical plates supplemented with 0 and 150 mM NaCl. Material harvested after 24 h salt treatment was used for RT-PCR. AtALN fragment was amplified by PCR (32 cycles) and AtACT2 fragment was used as control (28 cycles) (TIFF 16821 kb)
Fig. S2
Characterization of lines with T-DNA insertions in the AtUPS5 gene used in this study. (a) Two AtUPS5 T-DNA insertion lines Salk_044810 (designated ups5-1) and Salk_123120 (designated ups5-2) were analyzed. The insertion in ups5-1 and ups5-2 were localized in the UTR 5′ and in the promoter region of AtUPS5, respectively. (b) AtUPS5 T-DNA lines verification by PCR. Primers were designed using SALK’s T-DNA Primer tool. LP = Left genomic Primer, RP = Right genomic primer, LB = Left border primer of the T-DNA insertion. (c) Expression of AtUPS5 mRNA in WT and ups5 mutants. RNA was extracted from 14 day-old plants. A 641 bp AtACT2 fragment (control) and a 510 bp AtUPS5 fragment were amplified by RT-PCR (28 and 30 cycles), or adding two extra PCR cycles (30 and 32 cycles) (TIFF 14852 kb)
Fig. S3
Phenotype of ups5 and aln mutants grown with allantoin as a sole nitrogen source. WT, the two ups5 and the two aln T-DNA insertion lines previously described were grown in either solid 0.5× MS standard medium containing 30 mM total inorganic nitrogen (control) or 0.5× MS medium without nitrogen supplemented with 7.5 mM allantoin as sole nitrogen source for 7 days. (a) Representative seedlings grown on vertical plates (bar = 1 cm), (b) Root length and (c) Fresh weight per seedling are shown. Values of root length and fresh weight are relative to control plants. Bars represent the means and standard errors of at least 15 (b) or 3 (c) independent measurements. Asterisks indicates significant differences between genotypes and treatments (N = 221, *P < 0.05, Kruskal–Wallis for b; N = 31, *P < 0.05, DGC Test for c) (TIFF 88510 kb)
Rights and permissions
About this article
Cite this article
Lescano, C.I., Martini, C., González, C.A. et al. Allantoin accumulation mediated by allantoinase downregulation and transport by Ureide Permease 5 confers salt stress tolerance to Arabidopsis plants. Plant Mol Biol 91, 581–595 (2016). https://doi.org/10.1007/s11103-016-0490-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0490-7