Abstract
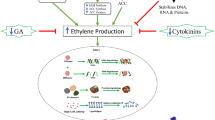
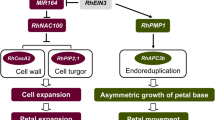
Floral senescence involves an ordered set of events coordinated at the plant, flower, organ and cellular level. This review assesses our current understanding of the input signals, signal transduction and cellular processes that regulate petal senescence and cell death. In many species a visible sign of petal senescence is wilting. This is accompanied by remobilization of nutrients from the flower to the developing ovary or to other parts of the plant. In other species, petals abscise while still turgid. Coordinating signals for floral senescence also vary across species. In some species ethylene acts as a central regulator, in others floral senescence is ethylene insensitive and other growth regulators are implicated. Due to the variability in this coordination and sequence of events across species, identifying suitable models to study petal senescence has been challenging, and the best candidates are reviewed. Transcriptomic studies provide an overview of the MAP kinases and transcription factors that are activated during petal senescence in several species including Arabidopsis. Our understanding of downstream regulators such as autophagy genes and proteases is also improving. This gives us insights into possible signalling cascades that regulate initiation of senescence and coordination of cell death processes. It also identifies the gaps in our knowledge such as the role of microRNAs. Finally future prospects for using all this information from model to non-model species to extend vase life in ornamental species is reviewed.


Similar content being viewed by others
References
Arrom L, Munné-Bosch S (2010) Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci 179:289–295
Arrom L, Munné-Bosch S (2012a) Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Science 188–189:41–47
Arrom L, Munné-Bosch S (2012b) Hormonal changes during flower development in floral tissues of Lilium. Planta 236:343–354
Azad AK, Ishikawa T, Sawa Y, Shibata H (2008) Intracellular energy depletion triggers programmed cell death during petal senescence in tulip. J Exp Bot 59:2085–2095
Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177:301–318
Battelli R, Lombardi L, Rogers HJ, Picciarelli P, Lorenzi R, Ceccarelli N (2011) Changes in ultrastructure, protease and caspase-like activities during flower senescence in Lilium longiflorum. Plant Sci 180:716–725
Bonneau L, Ge Y, Drury GE, Gallois P (2008) What happened to plant caspases? J Exp Bot 59:491–499
Borohov A, Tirosh T, Halevy AH (1976) Abscisic acid content of senescing petals on cut rose flowers as affected by sucrose and water stress. Plant Physiol 58:175–178
Breeze E, Wagstaff C, Harrison E, Bramke I, Rogers HJ, Stead AD, Thomas B, Buchanan-Wollaston V (2004) Gene expression patterns to define stages of post-harvest senescence in Alstroemeria petals. Plant Biotechnol J 2:155–168
Breeze E, Harrison E, McHattie S, Hughes L, Hickman R, Hill C, Kiddle S, Kim Y, Penfold CA, Jenkins D, Zhang C, Morris K, Jenner C, Jackson S, Thomas B, Tabrett A, Legaie R, Moore JD, Wild DL, Ott S, Rand D, Beynon J, Denby K, Mead A, Buchanan-Wollaston V (2011) High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23:873–894
Calderón Villalobos LIA, Lee S, De Oliveira C, Ivetac A, Brandt W, Armitage L, Sheard LB, Tan X, Parry G, Mao H, Zheng N, Napier R, Kepinski S, Estelle M (2012) A combinatorial TIR1/AFB–Aux/IAAco-receptor system for differential sensing of auxin. Nature Chem Biol 8:477–485
Chanasut U, Rogers HJ, Leverentz MK, Griffiths G, Thomas B, Wagstaff C, Stead AD (2003) Increasing flower longevity in Alstroemeria. Postharvest Biol Technol 29:325–333
Chandler S, Tanaka Y (2007) Genetic modification in floriculture. CRC Crit Rev Plant Sci 26:169–197
Chang H, Jones ML, Banowetz GM, Clark DG (2003) Overproduction of cytokinins in petunia flowers transformed with PSAG12: IPT delays corolla senescence. Plant Physiol 132:2174–2183
Chen J-C, Jiang C-Z, Gookin TE, Hunter DA, Clark DG, Reid MS (2004) Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol Biol 55:521–530
Chen MK, Hsu WH, Lee PF, Thiruvengadam M, Chen HI, Yang CH (2011a) The MADS box gene, FOREVER YOUNG FLOWER, acts as a repressor controlling floral organ senescence and abscission in Arabidopsis. Plant J 68:168–185
Chen MK, Lee PF, Yang CH (2011b) Delay of flower senescence and abscission in Arabidopsis transformed with an FOREVER YOUNG FLOWER homolog from Oncidium orchid. Plant Signal Behav 6:1841–1843
Cho SK, Larue CT, Chevalier D, Wang H, Jinn T-L, Zhang S, Walker JC (2008) Regulation of floral organ abscission in Arabidopsis thaliana. Proc Natl Acad Sci USA 105:15629–15634
Coll NS, Vercammen D, Smidler A, Clover C, Van Breusegem F, Dangl JL, Epple P (2010) Arabidopsis Type I metacaspases control cell death. Science 330:1393–1397
Conner AJ, Albert NW, Deroles SC (2009) Transformation and regeneration of petunia. In: Gerats T, Strommer J (eds) Petunia evolutionary, developmental and physiological genetics. Springer, New York, pp 301–324
Cuperus JT, Fahlgren N, Carrington JC (2011) Evolution and functional diversification of MIRNA genes. Plant Cell 23:431–442
Eason JR, Ryan DJ, Pinkney TT, O’Donoghu EM (2002) Programmed cell death during flower senescence: isolation and characterization of cysteine proteinases from Sandersonia aurantiaca. Funct Plant Biol 29:1055–1064
Edwards D, Batley J (2010) Plant genome sequencing: applications for crop improvement. Plant Biotechnol J 8:2–9
El-Sharkawy I, Sherif S, Mila I, Bouzayen M, Jayasankar S (2009) Molecular characterization of seven genes encoding ethylene responsive transcriptional factors during plum fruit development and ripening. J Exp Bot 60:907–922
Fiil BK, Petersen K, Petersen M, Mundy J (2009) Gene regulation by MAP kinase cascades. Curr Opin Plant Biol 12:615–621
Graham LE, Schippers JHM, Dijkwel PP, Wagstaff C (2012) Ethylene and senescence processes. Annu Plant Rev 44:305–341
Gubrium EK, Clevenger DJ, Clark DG, Barrett JE, Nell TA (2000) Reproduction and horticultural performance of transgenic ethylene-insensitive petunias. J Am Soc Hortic Sci 125:277–281
Guo A, He K, Liu D, Bai S, Gu X, Wei L, Luo J (2005) DATF: a database of Arabidopsis transcription factors. Bioinformatics 21:2568–2569
Hammond JP, Broadley MR, Craigon DJ, Higgins J, Emmerson ZF, Townsend HJ, White PJ, May ST (2005) Using genomic DNA-based probe-selection to improve the sensitivity of high-density oligonucleotide arrays when applied to heterologous species. Plant Method 1:10
Hay A, Tsiantis M (2009) A KNOX family TALE. Curr Opin Plant Biol 12:593–598
Hoeberichts FA, van Doorn WG, Vorst O, Hall RD, van Wordragen MF (2007) Sucrose prevents up-regulation of senescence-associated genes in carnation petals. J Exp Bot 58:2873–2885
Hoekstra FA, Weges R (1986) Lack of control by early pistillate ethylene of the accelerated wilting of Petunia hybrida flowers. Plant Physiol 80:403–408
Hunter DA, Steele BC, Reid MS (2002) Identification of genes associated with perianth senescence in daffodil (Narcissus pseudonarcissus L. ‘Dutch Master’). Plant Sci 163:13–21
Hunter DA, Ferrante A, Vernieri P, Reid MS (2004a) Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus ‘Dutch Master’). Physiol Plant 121:313–321
Hunter DA, Yi MF, Xu XJ, Reid MS (2004b) Role of ethylene in perianth senescence of daffodil (Narcissus pseudonarcissus L. ‘Dutch Master’). Postharvest Biol Technol 32:269–280
Iordachescu M, Verlinden S (2005) Transcriptional regulation of three EIN3-like genes of carnation (Dianthus caryophyllus L. cv. Improved White Sim) during flower development and upon wounding, pollination, and ethylene exposure. J Exp Bot 56:2011–2018
Jones ML (2004) Changes in gene expression during senescence. In: Nooden LD (ed) Plant cell death processes. Elsevier, Amsterdam, pp 51–71
Jones ML, Stead AD, Clark DG (2009) Petunia flower senescence. In: Gerats T, Strommer J (eds) Petunia: evolutionary, developmental and physiological genetics. Springer, New York, pp 301–324
Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG (2009) Trifurcate feed-forward regulation of age-dependent cell death involving mir164 in Arabidopsis. Science 323:1053–1057
Liu J, Li J, Wang H, Fu Z, Liu J, Yu Y (2011) Identification and expression analysis of ERF transcription factor genes in petunia during flower senescence and in response to hormone treatments. J Exp Bot 62:825–840
Ma N, Tan H, Liu X, Xue J, Li Y, Gao J (2006) Transcriptional regulation of ethylene receptor and CTR genes involved in ethylene-induced flower opening in cut rose (Rosa hybrida) cv Samantha. J Exp Bot 57:2763–2773
MAPK Group (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Mayak S, Halevy AH (1970) Cytokinin activity in rose petals and its relation to senescence. Plant Physiol 46:497–499
Miao Y, Zentgraf U (2007) The antagonist function of Arabidopsis WRKY53 and ESR/ESP in leaf senescence is modulated by the jasmonic and salicylic acid equilibrium. Plant Cell 19:819–830
Miao Y, Laun TM, Smykowski A, Zentgraf U (2007) Arabidopsis MEKK1 can take a short cut: it can directly interact with senescence-related WRKY53 transcription factor on the protein level and can bind to its promoter. Plant Mol Biol 65:63–76
Mor Y, Spiegelstein H, Halevy AH (1983) Inhibition of ethylene biosynthesis in carnation petals by cytokinin. Plant Physiol 71:541–546
Müller R, Stummann BM, Serek M (2000) Characterization of an ethylene receptor family with differential expression in rose (Rosa hybrida L.) flowers. Plant Cell Rep 19:1232–1239. doi:10.1007/s002990000251
Müller R, Owen CA, Xue Z-T, Welander M, Stummann BM (2002) Characterization of two CTR-like protein kinases in Rosa hybrida and their expression during flower senescence and in response to ethylene. J Exp Bot 53:1223–1225
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Pak C, van Doorn WG (2005) Delay of Iris flower senescence by protease inhibitors. New Phytol 165:473–480
Panavas T, Walker ER, Rubinstein B (1998) Possible role of abscisic acid in senescence of daylily petals. J Exp Bot 49:1987–1997
Picchioni GA, Mackay WA, Valenzuela-Vázquez M (2007) Correlative supply and demand functions in Lupinus havardii: a forgotten side of cut flower physiology? J Am Soc Hortic Sci 132:102–111
Price AM, Aros Orellana DF, Stevens R, Acock R, Buchanan-Wollaston V, Stead AD, Rogers HJ (2008) A comparison of leaf and petal senescence in wallflowers (Erysimum linifolium) reveals common and distinct patterns of gene expression and physiology. Plant Physiol 147:1898–1912
Rogers HJ (2006) Programmed cell death in floral organs: how and why do flowers die? Ann Bot 97:309–315
Rogers HJ (2012) Is there a important role for reactive oxygen species and redox regulation during floral senescence? Plant, Cell Environ 35:217–233
Rogers HJ, Stead AD (2011) Petal abscission: falling to their death or cast out to die? In: Yaish MW (ed) The flowering process and its control in plants: gene expression and hormone interaction. Research Signpost, Kerala, pp 229–258
Rojo E, Martín R, Carter C, Zouhar J, Pan S, Plotnikova J, Jin H, Paneque M, Sánchez-Serrano JJ, Baker B, Ausubel FM, Raikhel NV (2004) VPE gamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr Biol 14:1897–1906
Ronen M, Mayak S (1981) Interrelationship between abscisic acid and ethylene in the control of senescence processes in carnation flowers. J Exp Bot 32:759–765. doi:10.1093/jxb/32.4.759
Sanmartín M, Jaroszewski L, Raikhel NV, Rojo E (2005) Caspases, regulating death since the origin of life? Plant Physiol 137:841–847
Schmid M, Simpson D, Gietl C (1999) Programmed cell death in castor bean endoserm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc Natl Acad Sci USA USA 96:14159–14164
Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann J (2005) A gene expression map of Arabidopsis development. Nat Genet 37:501–506
Schommer C, Palatnik JF, Aggarwal P, Chételat A, Cubas P, Farmer EE, Nath U, Weigel D (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6:e230
Seglie L, Martina K, Devecchi M, Roggero C, Trotta F, Scariot V (2011) The effects of 1-MCP in cyclodextrin-based nanosponges to improve the vase life of Dianthus caryophyllus cut flowers. Postharvest Biol Technol 59:200–205
Senthil-Kumar M, Mysore KS (2011) New dimensions for VIGS in plant functional genomics. Trends Plant Sci 16:656–665. doi:10.1016/j.tplants.2011.08.006
Shibuya K, Nagata M, Tanikawa N, Yoshioka T, Hashiba T, Satoh S (2002) Comparison of mRNA levels of three ethylene receptors in senescing flowers of carnation (Dianthus caryophyllus L.). J Exp Bot 53:399–406
Shibuya K, Barry KG, Ciardi JA, Loucas HM, Underwood BA, Nourizadeh S, Ecker JR, Klee HJ, Clark DG (2004) The central role of PhEIN2 in ethylene responses throughout plant development in petunia. Plant Physiol 136:2900–2912
Shibuya K, Yamada T, Ichimura K (2009a) Autophagy regulates progression of programmed cell death during petal senescence in Japanese morning glory. Autophagy 5:546–547
Shibuya K, Yamada T, Suzuki T, Shimizu K, Ichimura K (2009b) InPSR26, a putative membrane protein, regulates programmed cell death during petal senescence in Japanese morning glory. Plant Physiol 149:816–824
Shvarts M, Weiss D, Borochov A (1997) Temperature effects on growth, pigmentation and post-harvest longevity of petunia flowers. Sci Hortic 69:217–227
Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabídopsis. Plant Cell 2:755–767
Stead AD, van Doorn WG (1994) Strategies of flower senescence-a review. In: Scott RJ, Stead AD (eds) Molecular and cellular aspects of plant reproduction. Cambridge University Press, Cambridge, pp 215–238
Taverner EA, Letham DS, Wang J, Cornish E (2000) Inhibition of carnation petal inrolling by growth retardants and cytokinins. Aus J Plant Physiol 27:357–362
ten Have A, Woltering EJ (1997) Ethylene biosynthetic genes are differentially expressed during carnation (Dianthus caryophyllus L.) flower senescence. Plant Mol Biol 34:89–97
Thomas H, Ougham HJ, Wagstaff C, Stead AD (2003) Defining senescence and death. J Exp Bot 54:1127–1132
Tieman DM, Ciardi JA, Taylor MG, Klee HJ (2001) Members of the tomato LeEIL gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26:47–58
To JPC, Kieber JJ (2008) Cytokinin signaling: two-components and more. Trends Plant Sci 13:85–92
Trick M, Cheung F, Drou N, Fraser F, Lobenhofer E K, Hurban P, Magusin A, Town CD and Bancroft I (2009) A newly-developed community microarray resource for transcriptome profiling in Brassica species enables the confirmation of Brassica-specific expressed sequences. BMC Plant Biol: 50
Trivellini A, Ferrante A, Vernieri P, Serra G (2011) Effects of abscisic acid on ethylene biosynthesis andperception in Hibiscus rosa-sinensis L. flower development. J Exp Bot 62:5437–5452
Tsiatsiani L, Van Breusegem F, Gallois P, Zavialov A, Lam E, Bozhkov PV (2011) Metacaspases. Cell Death Diff 18:1279–1288
Valpuesta V, Lange NE, Guerrero C, Reid MS (1995) Upregulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis) flowers. Plant Mol Biol 28:575–582
van Doorn WG (2011) Classes of programmed cell death in plants, compared to those in animals. J Exp Bot 62:4749–4761
van Doorn WG, Woltering EJ (2004) Senescence and programmed cell death: substance or semantics? J Exp Bot 55:2147–2153
van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10:117–122
van Doorn WG, Woltering EJ (2008) Physiology and molecular biology of petal senescence. J Exp Bot 59:453–480
van Doorn WG, Balk PA, van Houwelingen AM, Hoeberichts FA, Hall RD, Vorst O, van der Schoot C, van Wordragen MF (2003) Gene expression during anthesis and senescence in Iris flowers. Plant Mol Biol 53:845–863
van Staden J (1995) Hormonal control of carnation flower senescence. Acta Hortic 405:232–239
van Staden J, Dimalla GG (1980) The effect of silver thiosulphate preservative on the physiology of cut carnations. II. Influence on endogenous cytokinin. Z Pflanzenphysiol 99:19–26
van Staden J, Featonby-Smith BC, Mayak S, Spiegelstein H, Halevy AH (1987) Cytokinins in cut carnation flowers. II. Relation between endogenous ethylene and cytokinin levels in the petals. Plant Growth Regul 5:75–86
Vardi Y, Mayak S (1989) Involvement of abscisic acid during water stress and recovery in petunia flowers. Acta Hortic 261:107–112
Verlinden S (2003) Changes in mineral concentrations in petunia corollas during development and senescence. Hortic Sci 38:71–74
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136:669–687
Wagstaff C, Leverentz MK, Griffiths G, Thomas B, Chanasut U, Stead AD, Rogers HJ (2002) Protein degradation during senescence of Alstroemeria petals. J Exp Bot 53:233–240
Wagstaff C, Malcolm P, Rafiq A, Leverentz M, Griffiths G, Thomas B, Stead A, Rogers HJ (2003) Programmed cell death (PCD) processes begin extremely early in Alstroemeria petal senescence. New Phytol 160:49–59
Wagstaff C, Yang TJW, Stead AD, Buchanan-Wollaston V, Roberts JA (2009) A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J 57:690–705
Wagstaff C, Bramke I, Breeze E, Thornber S, Harrison L, Thomas B, Buchanan-Wollaston V, Stead AD, Rogers HJ (2010) A unique group of genes respond to cold drought stress in cut Alstroemeria flowers whereas ambient drought stress accelerates developmental expression patterns. J Exp Bot 61:2905–2921
Weaver LM, Gan S, Quirino B, Amasino RM (1998) A comparison of the expression patterns of several senescence associated genes in response to stress and hormone treatment. Plant Mol Biol 37:455–469
Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15:444–447
Woltering EJ, van Doorn WG (1988) Role of ethylene and senescence of petals: morphological and taxonomical relationships. J Exp Bot 39:1605–1616
Woodson WR, Lawton KA (1988) Ethylene-induced gene expression in carnation petals. Relationship to autocatalytic ethylene production and senescence. Plant Physiol 87:498–503
Wulster G, Sacalis J, Janes HW (1982) Senescence in isolated carnation petals effects of indoleacetic acid and inhibitors of protein synthesis. Plant Physiol 70:1039–1043
Xu X, Gookin T, Jiang C-Z, Reid M (2007a) Genes associated with opening and senescence of Mirabilis jalapa flowers. J Exp Bot 58:2193–2201
Xu X, Jiang C-Z, Donnelly L, Reid MS (2007b) Functional analysis of a RING domain ankyrin repeat protein that is highly expressed during flower senescence. J Exp Bot 58:3623–3630
Yamada T, van Ichimura K, Doorn WG (2007) Relationship between petal abscission and programmed cell death in Prunus yedoensis and Delphinium. Planta 226:1195–1205
Yamada T, Ichimura K, Kanekatsu M, van Doorn WG (2009) Homologues of genes associated with programmed cell death in animal cells are differentially expressed during senescence of Ipomoea nil petals. Plant Cell Physiol 50:610–625
Yang TF, Gonzalez-Caranza ZH, Maunders MJ, Roberts JA (2008) Ethylene and the regulation of senescence processes in transgenic Nicotiana sylvestris plants. Ann Bot 101:301–310
Yin X, Allan AC, Chen K, Ferguson IB (2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol 153:1280–1292
Yoo SD, Cho Y, Sheen J (2009) Emerging connections in the ethylene signaling network. Trends Plant Sci 14:270–279
Zaccai M, Jia G, Chen X, Genis O, Feibin D, Gesua R (2007) Regeneration and transformation system in Mirabilis jalapa. Sci Hortic 111:304–309
Zenoni S, D’Agostino N, Tornielli GB, Quattrocchio F, Chiusano ML, Koes R, Zethof J, Guzzo F, Delledonne M, Frusciante L, Gerats T, Pezzotti M (2011) Revealing impaired pathways in the an11 mutant by high-throughput characterization of Petunia axillaris and Petunia inflata transcriptomes. Plant J 68:11–27
Zhou C, Cai Z, Guo Y, Gan S (2009) An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol 150:167–177
Zonneveld BJM, Leitch IJ, Bennett MD (2005) First nuclear DNA amounts in more than 300 angiosperms. Ann Bot 96:229–244
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogers, H.J. From models to ornamentals: how is flower senescence regulated?. Plant Mol Biol 82, 563–574 (2013). https://doi.org/10.1007/s11103-012-9968-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9968-0