Abstract
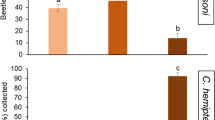
Yeast-insect interactions have been well characterized in drosophilid flies, but not in tephritid fruit flies, which include many highly polyphagous pest species that attack ripening fruits. Using the Queensland fruit fly (Bactrocera tryoni) as our model tephritid species, we identified yeast species present in the gut of wild-collected larvae and found two genera, Hanseniaspora and Pichia, were the dominant isolates. In behavioural trials using adult female B. tryoni, a fruit-agar substrate inoculated with Pichia kluyveri resulted in odour emissions that increased the attraction of flies, whereas inoculation with Hanseniaspora uvarum, produced odours that strongly deterred flies, and both yeasts led to decreased oviposition. Larval development trials showed that the fruit-agar substrate inoculated with the ‘deterrent odour’ yeast species, H. uvarum, resulted in significantly faster larval development and a greater number of adult flies, compared to a substrate inoculated with the ‘attractive odour’ yeast species, P. kluyveri, and a yeast free control substrate. GC-MS analysis of volatiles emitted by H. uvarum and P. kluyveri inoculated substrates revealed significant quantitative differences in ethyl-, isoamyl-, isobutyl-, and phenethyl- acetates, which may be responsible for the yeast-specific olfactory responses of adult flies. We discuss how our seemingly counterintuitive finding that female B. tryoni flies avoid a beneficial yeast fits well with our understanding of female choice of oviposition sites, and how the contrasting behavioural effects of H. uvarum and P. kluyveri raises interesting questions regarding the role of yeast-specific volatiles as cues to insect vectors. A better understanding of yeast-tephritid interactions could assist in the future management of tephritid fruit fly pests through the formulation of new “attract and kill” lures, and the development of probiotics for mass rearing of insects in sterile insect control programs.




Similar content being viewed by others
References
Anagnostou C, Dorsch M, Rohlfs M (2010) Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol Exp Appl 136:1–11. doi:10.1111/j.1570-7458.2010.00997.x
Balagawi S, Jackson K, Haq IU, Hood-Nowotny R, Resch C, Clarke AR (2014) Nutritional status and the foraging behaviour of Bactrocera tryoni with particular reference to protein bait spray. Physiol Entomol 39:33–43
Barker JSF, Vacek DC, East PD (1988) Attraction of larvae of Drosophila buzzatii and D. aldrichi to yeast species isolated from their natural environment. Aust J Zool 36:53–63
Bateman MA (1972) Ecology of fruit flies. Annu Rev Entomol 17:493–518. doi:10.1146/annurev.en.17.010172.002425
Becher PG et al (2012) Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol 26:822–828. doi:10.1111/j.1365-2435.2012.02006.x
Buser CC, Newcomb RD, Gaskett AC, Goddard MR (2014) Niche construction initiates the evolution of mutualistic interactions. Ecol Lett 17:1257–1264. doi:10.1111/ele.12331
Cadez N, Poot GA, Raspor P, Smith MT (2003) Hanseniaspora meyeri sp. nov. Hanseniaspora clermontiae sp. nov., Hanseniaspora lachancei sp. nov. and Hanseniaspora opuntiae sp. nov., novel apiculate yeast species. Int J Syst Evol Microbiol 53:1671–1680. doi:10.1099/ijs.0.02618-0
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. doi:10.1093/bioinformatics/btp348
Chandler JA, Eisen JA, Kopp A (2012) Yeast communities of diverse Drosophila species: comparison of two symbiont groups in the same hosts. Appl Environ Microbiol 78:7327–7336
Christiaens JF et al (2014) The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep 9:425–432. doi:10.1016/j.celrep.2014.09.009
Clarke AR, Armstrong KF, Carmichael AE, Milne JR, Raghu S, Roderick GK, Yeates DK (2005) Invasive phytophagous pests arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis Complex of fruit flies. Annu Rev Entomol 50(1):293–319. doi:10.1146/annurev.ento.50.071803.130428
Clarke AR, Powell KS, Weldon CW, Taylor PW (2011) The ecology of Bactrocera tryoni (Diptera: Tephritidae): what do we know to assist pest management? Ann Appl Biol 158:26–54. doi:10.1111/j.1744-7348.2010.00448.x
Coluccio AE, Rodriguez RK, Kernan MJ, Neiman AM (2008) The yeast spore wall enables spores to survive passage through the digestive tract of Drosophila. PLoS One 3:e2873. doi:10.1371/journal.pone.0002873
Cunningham JP, Carlsson MA, Villa TF, Dekker T, Clarke AR (2016) Do fruit ripening volatiles enable resource specialism in polyphagous fruit flies? J Chem Ecol 42:931–940
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect Semiochemicals. J Chem Ecol 39:840–859. doi:10.1007/s10886-013-0306-z
Deutscher AT, Reynolds OL, Chapman TA (2016) Yeast: an overlooked component of Bactrocera tryoni (Diptera: Tephritidae) larval gut microbiota. J Econ Entomol 110:298–300. doi:10.1093/jee/tow262
Dominiak BC, Westcott AE, Barchia IM (2003) Release of sterile Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), at Sydney, Australia. Aust J Exp Agric 43:519–528. doi:10.1071/ea01146
Drew RAI (1987) Reduction in fruit-fly (Tephritidae, Dacinae) populations in their endemic rain-forest habitat by frugivorous vertebrates. Aust J Zool 35:283–288
Duetz W, Bouwmeester H, Van Beilen J, Witholt B (2003) Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl Microbiol Biotechnol 61:269–277
Duyck PF, David P, Junod G, Brunel C, Dupont R, Quilici S (2006) Importance of competition mechanisms in successive invasions by polyphagous tephritids in la Reunion. Ecology 87:1770–1780. doi:10.1890/0012-9658(2006)87[1770:iocmis]2.0.co;2
Dyck VA, Hendrichs J, Robinson AS (2005) Sterile insect technique. Principles and practice in area-wide integrated pest management. Springer, The Netherlands
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi:10.1093/nar/gkh340
Fitt GP (1984) Oviposition behavior of two tephritid fruit-flies, Dacus tryoni and Dacus jarvisi, as influenced by the presence of larvae in the host fruit. Oecologia 62:37–46. doi:10.1007/bf00377370
Gibson CM, Hunter MS (2010) Extraordinarily widespread and fantastically complex: comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol Lett 13:223–234. doi:10.1111/j.1461-0248.2009.01416.x
Haidani AE et al (2008) Isolation and characterisation of yeast strains for the olive fly Bactrocera oleae biological control. Moroccan J Biol 2:19–29
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT.Nucl acids Symp Ser 41:95-98
Hamby KA, Becher PG (2016) Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J Pest Sci 89:621–630. doi:10.1007/s10340-016-0768-1
Hamby KA, Hernández A, Boundy-Mills K, Zalom FG (2012) Associations of yeasts with spotted-wing drosophila (Drosophila suzukii; Diptera: Drosophilidae) in cherries and raspberries. Appl and Environ Microbiol 78:4869–4873. doi:10.1128/aem.00841-12
Hendrichs J, Franz G, Rendon P (1995) Increased effectiveness and applicability of the sterile insect technique through male-only releases for control of Mediterranean fruit flies during fruiting seasons. J Appl Entomol 119:371–377
Holighaus G, Rohlfs M (2016) Fungal allelochemicals in insect pest management. Appl Microbiol Biotechnol 100:5681–5689. doi:10.1007/s00253-016-7573-x
Kurtzman CP, Robnett CJ (1997) Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 35:1216–1223. doi:0095–1137/97
Lachance MA, Gilbert DG, Starmer WT (1995) Yeast communities associated with Drosophila species and related flies in an eastern oak-pine forest: a comparison with western communities. J Ind Microbiol 14:484–494. doi:10.1007/bf01573963
Löoke M, Kristjuahan K, Kristjuhan A (2011) Extraction of genomic Dna from yeasts for Pcr- based applications. BioTechniques 50:325–328. doi:10.2144/000113672.extraction
Malacrida AR, Gomulski LM, Bonizzoni M, Bertin S, Gasperi G, Gugliclmino CR (2007) Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica 131:1–9. doi:10.1007/s10709-006-9117-2
Masry A, Furlong MJ, Clarke AR, Cunningham JP (2016) An improved culturing method for opiine fruit fly parasitoids and its application to parasitoid monitoring in the field. Insect Science. doi:10.1111/1744-7917.12403
Meats A, Leighton SM (2004) Protein consumption by mated, unmated, sterile and fertile adults of the Queensland fruit fly, Bactrocera tryoni and its relation to egg production. Physiol Entomol 29:176–182. doi:10.1111/j.1365-3032.2004.00383.x
Molnárová J, Vadkertiová R, Stratilová E (2014) Extracellular enzymatic activities and physiological profiles of yeasts colonizing fruit trees. J Basic Microbiol 54:S74–S84. doi:10.1002/jobm.201300072
Mori BA, Whitener AB, Leinweber Y, Revadi S, Beers EH, Witzgall P, Becher PG (2016) Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J Appl Ecol 54:170–177. doi:10.1111/1365-2664.12688
Murphy KA, Tabuloc CA, Cervantes KR, Chiu JC (2016) Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci Rep 6:22587. doi:10.1038/srep22587
Muthuthantri S, Clarke AR (2012) Five commercial citrus rate poorly as hosts of the polyphagous fruit fly Bactrocera tryoni (Froggatt)(Diptera: Tephritidae) in laboratory studies. Austral Entomology 51:289–298. doi:10.1111/j.1440-6055.2012.00866.x
Navarro-Llopis V, Primo J, Vacas S (2015) Bait station devices can improve mass trapping performance for the control of the Mediterranean fruit fly. Pest Manag Sci 71:923–927. doi:10.1002/ps.3864
Parker AG (2005) Mass-rearing for sterile insect release. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, The Netherlands, pp 209–232
Pérez-Staples D, Weldon CW, Smallridge C, Taylor PW (2009) Pre-release feeding on yeast hydrolysate enhances sexual competitiveness of sterile male Queensland fruit flies in field cages. Entomol Exp Appl 131:159–166. doi:10.1111/j.1570-7458.2009.00841.x
Reuter M, Bell G, Greig D (2007) Increased outbreeding in yeast in response to dispersal by an insect vector. Curr Biol 17:81–83. doi:10.1016/j.cub.2006.11.059
Rohlfs M, Kürschner L (2010) Saprophagous insect larvae, Drosophila melanogaster, profit from increased species richness in beneficial microbes. J Appl Entomol 134:667–671
Rstudio Team (2016) RStudio: integrated development environment for R. RStudio, Inc., Boston http://rstudio.com/
Sarles L, Verhaeghe A, Francis F, Verheggen FJ (2015) Semiochemicals of Rhagoletis fruit flies: potential for integrated pest management. Crop Prot 78:114–118. doi:10.1016/j.cropro.2015.09.001
Scheidler NH, Liu C, Hamby KA, Zalom FG, Syed Z (2015) Volatile codes: correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci Rep 5:14059. doi:10.1038/srep14059
Stamps JA, Yang LH, Morales VM, Boundy-Mills KL (2012) Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS One 7:e42238
Starmer W, Aberdeen V (1990) The nutritional importance of pure and mixed cultures of yeasts in the development of Drosophila mulleri larvae in Opuntia tissues and its relationship to host plant shifts. In: Barker JSF, MacIntyre RJ, Starmer WT (eds) Ecological and evolutionary genetics of drosophila. Plenum Press, New York, pp 145–116
Starmer WT, Fogleman JC (1986) Coadaptation of Drosophila and yeasts in their natural habitat. J Chem Ecol 12:1037–1055. doi:10.1007/bf01638995
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Varikou K, Garantonakis N, Birouraki A, Ioannou A, Kapogia E (2016) Improvement of bait sprays for the control of Bactrocera oleae (Diptera: Tephritidae). Crop Prot 81:1–8. doi:10.1016/j.cropro.2015.11.007
Vega FE, Dowd PF (2005) the role of yeasts as insect endosymbionts. In: F.E. Vega, M. Blackwell (eds.) Insect-fungal associations: ecology and evolution, Oxford University Press, New York, pp. 211–243
Vijaysegaran S, Walter GH, Drew RAI (1997) Mouthpart structure, feeding mechanisms, and natural food sources of adult Bactrocera (Diptera: Tephritidae). Ann Entomol Soc Am 90:184–201. doi:10.1093/aesa/90.2.184
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl 18:315–322
White IM, Elson-Harris MM (1992) Fruit flies of economic significance: their identification and bionomics. CAB international, Wallingford
Witzgall P et al (2012) “this is not an apple” -yeast mutualism in codling moth. J Chem Ecol 38:949–957. doi:10.1007/s10886-012-0158-y
Yamada R, Deshpande SA, Bruce KD, Mak EM, William WJ (2015) Microbes promote amino acid harvest to rescue undernutrition in Drosophila. Cell Rep 10:865–872
Acknowledgements
The research was supported by the Plant Biosecurity CRC (CRC3152). We would like to thank Mark Blacket and Linda Semeraro for assistance in the identification of B. tryoni larvae.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclosure
The research was supported by the Plant Biosecurity CRC (CRC3152).
Electronic supplementary material
ESM 1
(DOCX 204 kb)
Rights and permissions
About this article
Cite this article
Piper, A.M., Farnier, K., Linder, T. et al. Two Gut-Associated Yeasts in a Tephritid Fruit Fly have Contrasting Effects on Adult Attraction and Larval Survival. J Chem Ecol 43, 891–901 (2017). https://doi.org/10.1007/s10886-017-0877-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0877-1