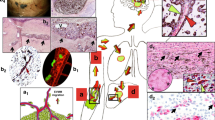
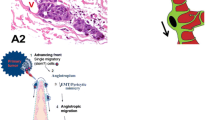
Abstract
Intravascular dissemination of tumor cells is the accepted mechanism of cancer metastasis. However, the phenomenon of angiotropism, pericyte mimicry (PM), and extravascular migratory metastasis (EVMM) has questioned the concept that tumor cells metastasize exclusively via circulation within vascular channels. This new paradigm of cancer spread and metastasis suggests that metastatic cells employ embryonic mechanisms for attachment to the abluminal surfaces of blood vessels (angiotropism) and spread via continuous migration, competing with and replacing pericytes, i.e., pericyte mimicry (PM). This is an entirely extravascular phenomenon (i.e., extravascular migratory metastasis or EVMM) without entry (intravasation) into vascular channels. PM and EVMM have mainly been studied in melanoma but also occur in other cancer types. PM and EVMM appear to be a reversion to an embryogenesis-derived program. There are many analogies between embryogenesis and cancer progression, including the important role of laminins, epithelial–mesenchymal transition, and the re-activation of embryonic signals by cancer cells. Furthermore, there is no circulation of blood during the first trimester of embryogenesis, despite the fact that there is extensive migration of cells to distant sites and formation of organs and tissues during this period. Embryonic migration therefore is a continuous extravascular migration as are PM and EVMM, supporting the concept that these embryonic migratory events appear to recur abnormally during the metastatic process. Finally, the perivascular location of tumor cells intrinsically links PM to vascular co-option. Taken together, these two new paradigms may greatly influence the development of new effective therapeutics for metastasis. In particular, targeting embryonic factors linked to migration that are detected during cancer metastasis may be particularly relevant to PM/EVMM.




Similar content being viewed by others
References
Chitty JL, Filipe EC, Lucas MC, Herrmann D, Cox TR, Timpson P (2018) Recent advances in understanding the complexities of metastasis. Version. https://doi.org/10.12688/f1000research.15064.2
Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR (2019) Vessel co-option in cancer. Nat Rev Clin Oncol 16(8):469–493
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med. 285(21):1182–1186
Lugassy C, Zadran S, Bentolila LA, Wadehra M, Prakash R, Carmichael ST, Kleinman HK, Péault B, Larue L, Barnhill RL (2014) Angiotropism, pericytic mimicry and extravascular migratory metastasis in melanoma: an alternative to intravascular cancer dissemination. Cancer Microenviron 7(3):139–152
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70(14):5649–5669
Barnhill RL, Lugassy C (2004) Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology 36(5):485–490
Barnhill RL, Ye M, Batistella A, Stern MH, Roman-Roman S, Dendale R, Lantz O, Piperno-Neumann S, Desjardins L, Cassoux N, Lugassy C (2017) The biological and prognostic significance of angiotropism in uveal melanoma. Lab Invest 97(6):746
Barnhill RL, Lemaitre S, Lévy-Gabrielle C, Rodrigues M, Desjardins L, Dendale R, Vincent-Salomon A, Roman-Roman S, Lugassy C, Cassoux N (2016) Satellite in transit metastases in rapidly fatal conjunctival melanoma: implications for angiotropism and extravascular migratory metastasis (description of a murine model for conjunctival melanoma). Pathology 48(2):166–176
Barnhill R, Vermeulen P, Daelemans S, van Dam PJ, Roman-Roman S, Servois V, Hurbain I, Gardrat S, Raposa G, Nicolas A, Dendale R, Pierron G, Desjardins L, Cassoux N, Piperno-Neumann S, Mariani P, Lugassy C (2018) Replacement and desmoplastic histopathological growth patterns: a pilot study of prediction of outcome in patients with uveal melanoma liver metastases. J Pathol Clin Res 4(4):227–240
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, Tüting T (2014) Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507(7490):109–113
Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA (2008) Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol 32(9):1396–1403
Moy AP, Duncan LM, Muzikansky A, Kraft S (2019) Angiotropism in primary cutaneous melanoma is associated with disease progression and distant metastases: a retrospective study of 179 cases. J Cutan Pathol 46(7):498–507. https://doi.org/10.1111/cup.13461
Barnhill RL, Ye M, Batistella A, Stern MH, Roman-Roman S, Dendale R, Lantz O, Piperno-Neumann S, Desjardins L, Cassoux N, Lugassy C (2017) The biological and prognostic significance of angiotropism in uveal melanoma. Lab Invest. https://doi.org/10.1038/labinvest.2017.16
Van Dam PJ, van der Stok EP, Teuwen LA, Van den Eynden GG, Illemann M, Frentzas S, Majeed AW, Eefsen RL, Coebergh van den Braak RRJ, Lazaris A, Fernandez MC, Galjart B, Laerum OD, Rayes R, Grünhagen DJ, Van de Paer M, Sucaet Y, Mudhar HS, Schvimer M, Nyström H, Kockx M, Bird NC, Vidal-Vanaclocha F, Metrakos P, Simoneau E, Verhoef C, Dirix LY, Van Laere S, Gao ZH, Brodt P, Reynolds AR, Vermeulen PB (2017) International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer 117(10):1427–1441
Barnhill R, Dy K, Lugassy C (2002) Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol 119(3):705–706
Payette MJ, Katz M 3rd, Grant-Kels JM (2009) Melanoma prognostic factors found in the dermatopathology report. Clin Dermatol 27(1):53–74
Wesseling P, Ruiter DJ, Burger PC (1997) Angiogenesis in brain tumors: pathobiological and clinical aspects. J Neurooncol 32:253–265
Gritsenko P, Leenders W, Friedl P (2017) Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma. Histochem Cell Biol 148(4):395–406
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 153:139–152
Lugassy C, Haroun RI, Brem H, Tyler BM, Jones RV, Fernandez PM, Patierno SR, Kleinman HK, Barnhill RL (2002) Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol 24(6):473–478
Beauchesne P (2011) Extra-neural metastases of malignant gliomas: myth or reality? Cancers 3(1):461–477
Tiwary S, Morales JE, Kwiatkowski SC, Lang FF, Rao G, McCarty JH (2018) Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci Rep 8(1):8267
Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, Ridge SM, Jablonski EM, Therrien J, Tannheimer S, McCall CM, Chenn A, Sipkins DA (2018) Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 560(7716):55–60
Galjart B, Nierop PMH, van der Stok EP, van den Braak RRJC, Höppener DJ, Daelemans S, Dirix LY, Verhoef C, Vermeulen PB, Grünhagen DJ (2019) Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis 22(2):355–368
Stessels F, Van den Eynden G, Van der Auwera I, Salgado R, Van den Heuvel E, Harris AL, Jackson DG, Colpaert CG, van Marck EA, Dirix LY, Vermeulen PB (2004) Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 90(7):1429–1436
Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, Giancotti FG, Schachner M, Malladi S, Massagué J (2018) Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol 20(8):966–978
Levy MJ, Gleeson FC, Zhang L (2009) Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol 7(2):246–248
Rustagi T, Gleeson FC, Chari ST, Lehrke HD, Takahashi N, Malikowski TM, Abu Dayyeh BK, Chandrasekhara V, Iyer PG, Kendrick ML, Pearson RK, Petersen BT, Rajan E, Smoot RL, Storm AC, Topazian MD, Truty MJ, Vege SS, Wang KK, Levy MJ (2019) Safety, diagnostic accuracy, and effects of endoscopic ultrasound fine-needle aspiration on detection of extravascular migratory metastases. Clin Gastroenterol Hepatol 17(12):2533–2540
Lugassy C, Vernon SE, Warner JW, Le CQ, Manyak M, Patierno SR, Barnhill RL (2005) Angiotropism of human prostate cancer cells: implications for extravascular migratory metastasis. BJU Int 95(7):1099–1103
Lugassy C, Kleinman HK, Fernandez PM, Patierno SR, Webber MM, Ghanem G, Spatz A, Barnhill RL (2002) Human melanoma cell migration along capillary-like structures in vitro: a new dynamic model for studying extravascular migratory metastasis. J Invest Dermatol 119(3):703–704
Fornabaio G, Barnhill RL, Lugassy C, Bentolila LA, Cassoux N, Roman-Roman S, Alsafadi S, Del Bene F (2018) Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci Rep 8(1):10448
Lugassy C, Kleinman HK, Engbring JA, Welch DR, Harms JF, Rufner R, Ghanem G, Patierno SR, Barnhill RL (2004) Pericyte-like location of GFP-tagged melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol 164(4):1191–1198
Lugassy C, Kleinman HK, Vernon SE, Welch DR, Barnhill RL (2007) C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br J Dermatol 157(780–782):6
Bentolila LA, Prakash R, Mihic-Probst D, Wadehra M, Kleinman HK, Carmichael TS, Péault B, Barnhill RL, Lugassy C (2016) Imaging of angiotropism/vascular co-option in a murine model of brain melanoma: implications for melanoma progression along extravascular pathways. Sci Rep 6:23834
Lugassy C, Lazar V, Dessen P, van den Oord JJ, Winnepenninckx V, Spatz A, Bagot M, Bensussan A, Janin A, Eggermont AM, Barnhill RL (2011) Gene expression profiling of human angiotropic primary melanoma: selection of 15 differentially expressed genes potentially involved in extravascular migratory metastasis. Eur J Cancer 47(8):1267–1275
Lugassy C, Wadehra M, Li X, Corselli M, Akhavan D, Binder SW, Péault B, Cochran AJ, Mischel PS, Kleinman HK, Barnhill RL (2013) Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron 6(1):19–29
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156(5):1002–1016
Seifert S, Sontheimer H (2014) Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J Physiol 592(22):5109–5127
Yadav VN, Zamler D, Baker GJ, Kadiyala P, Erdreich-Epstein A, DeCarvalho AC, Mikkelsen T, Castro MG, Lowenstein PR (2016) CXCR1 increases in vivo glioma perivascular invasion, and reduces radiation induced apoptosis: a genetic knockdown study. Oncotarget 7(50):83701–83719
Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, Krishnan S, Lindberg OR, Yuen TJ, Tien AC, Sabo JK, Wang N, Chen I, Kloepper J, Larrouquere L, Ghosh M, Tirosh I, Huillard E, Alvarez-Buylla A, Oldham MC, Persson AI, Weiss WA, Batchelor TT, Stemmer-Rachamimov A, Suvà ML, Phillips JJ, Aghi MK, Mehta S, Jain RK, Rowitch DH (2018) A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell 33(5):874–889 e7
Lai CJ, Lin CY, Liao WY, Hour TC, Wang HD, Chuu CP (2019) CD44 promotes migration and invasion of docetaxel-resistant prostate cancer cells likely via induction of hippo-yap signaling. Cells 8(4):295
Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH, Shi Y, Van den Eynden G, Daley F, Peckitt C, Tan X, Salman A, Lazaris A, Gazinska P, Berg TJ, Eltahir Z, Ritsma L, Van Rheenen J, Khashper A, Brown G, Nystrom H, Sund M, Van Laere S, Loyer E, Dirix L, Cunningham D, Metrakos P, Reynolds AR (2016) Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 22(11):1294–1302
Engbring JA, Kleinman HK (2003) The basement membrane matrix in malignancy. J Pathol. Jul;200(4):465-70
Qin Y, Rodin S, Simonson OE, Hollande F (2017) Laminins and cancer stem cells: partners in crime? Semin Cancer Biol 45:3–12
Lugassy C, Eyden BP, Christensen L, Escande JP (1997) Angio-tumoral complex in human malignant melanoma characterized by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol 29:19–28
Lugassy C, Dickersin GR, Christensen L, Karaoli T, LeCharpentier M, Escande JP, Barnhill RL (1999) Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol 26(2):78–83
Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V (1997) Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277(5323):225–228
Lugassy C, Torres-Muñoz JE, Kleinman HK, Ghanem G, Vernon SE, Barnhill RL (2009) Over-expression of malignancy-associated laminins and laminin receptors by angiotropic human melanoma cells in a chick chorioallantoic membrane model. J Cutan Pathol 36(12):1237–1243
Oikawa Y, Hansson J, Sasaki T, Rousselle P, Domogatskaya A, Rodin S, Tryggvason K, Patarroyo M (2011) Melanoma cells produce multiple laminin isoforms and strongly migrate on α5 laminin(s) via several integrin receptors. Exp Cell Res 317(8):1119–1133
Carpenter PM, Sivadas P, Hua SS, Xiao C, Gutierrez AB, Ngo T, Gershon PD (2017) Migration of breast cancer cell lines in response to pulmonary laminin 332. Cancer Med 6(1):220–234
Tsubota Y, Ogawa T, Oyanagi J, Nagashima Y, Miyazaki K (2010) Expression of laminin gamma2 chain monomer enhances invasive growth of human carcinoma cells in vivo. Int J Cancer 127(9):2031–2041
Teng Y, Wang Z, Ma L, Zhang L, Guo Y, Gu M, Wang Z, Wang Y, Yue W (2016) Prognostic significance of circulating laminin gamma2 for early-stage non-small-cell lung cancer. Onco Targets Ther 9:4151–4162
Sun T, Patil R, Galstyan A, Klymyshyn D, Ding H, Chesnokova A, Cavenee WK, Furnari FB, Ljubimov VA, Shatalova ES, Wagner S, Li D, Mamelak AN, Bannykh SI, Patil CG, Rudnick JD, Hu J, Grodzinski ZB, Rekechenetskiy A, Falahatian V, Lyubimov AV, Chen YL, Leoh LS, Daniels-Wells TR, Penichet ML, Holler E, Ljubimov AV, Black KL, Ljubimova JY (2019) Blockade of a Laminin-411-notch axis with CRISPR/Cas9 or a nanobioconjugate inhibits glioblastoma growth through tumor-microenvironment cross-talk. Cancer Res 79(6):1239–1251
Kikkawa Y, Ogawa T, Sudo R, Yamada Y, Katagiri F, Hozumi K, Nomizu M, Miner JH (2013) The lutheran/basal cell adhesion molecule promotes tumor cell migration by modulating integrin-mediated cell attachment to laminin-511 protein. J Biol Chem 288(43):30990–31001
Hutchins EJ, Bronner ME (2019) Draxin alters laminin organization during basement membrane remodeling to control cranial neural crest EMT. Dev Biol 446(2):151–158
Prager BC, Xie Q, Bao S, Rich JN (2019) Cancer stem cells: the architects of the tumor ecosystem. Cell Stem Cell 24(1):41–53
Roth L, Kalev-Altman R, Monsonego-Ornan E, Sela-Donenfeld D (2017) A new role of the membrane-type matrix metalloproteinase 16 (MMP16/MT3-MMP) in neural crest cell migration. Int J Dev Biol 61(35):245–256
Virchow RLK (1978) Cellular pathology, 1859 special ed. John Churchill, London, pp 204–207
Cofre J, Abdelhay E (2017) Cancer is to embryology as mutation is to genetics: hypothesis of the cancer as embryological phenomenon. ScientificWorldJournal 2017:3578090
Aiello NM, Stanger BZ (2016) Echoes of the embryo: using the developmental biology toolkit to study cancer. Dis Model Mech 9(2):105–114
Campbell K (2018) Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr Opin Cell Biol 55:30–35
Fane ME, Chhabra Y, Smith AG, Sturm RA (2019) BRN2, a POUerful driver of melanoma phenotype switching and metastasis. Pigment Cell Melanoma Res 32(1):9–24
Saitoh M (2018) Involvement of partial EMT in cancer progression. J Biochem 164(4):257–264
Lee G, Hall RR 3rd, Ahmed AU (2016) Cancer stem cells: cellular plasticity, niche, and its clinical relevance. J Stem Cell Res Ther 6(10):363
Lugassy C, Péault B, Wadehra M, Kleinman HK, Barnhill RL (2013) Could pericytic mimicry represent another type of melanoma cell plasticity with embryonic properties? Pigment Cell Melanoma Res 26(5):746–754
Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Péault B (2012) The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21(8):1299–1308
Di Gregorio A, Bowling S, Rodriguez TA (2016) Cell Competition and Its role in the regulation of cell fitness from development to cancer. Dev Cell 38(6):621–634
Lugassy C, Kleinman HK, Barnhill RL (2020) Pericyte mimicry: an embryonic-like mechanism for tumor metastasis. In: Ribatti D, Pezzella F (eds) Tumor vascularization. Academic Press, San Diego
Dupin E, Calloni GW, Coelho-Aguiar JM, Le Douarin NM (2018) The issue of the multipotency of the neural crest cells. Dev Biol 444(Suppl 1):S47–S59
Gandalovičová A, Vomastek T, Rosel D, Brábek J (2016) Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget 7(18):25022–25049
George L, Dunkel H, Hunnicutt BJ, Filla M, Little C, Lansford R, Lefcort F (2016) In vivo time-lapse imaging reveals extensive neural crest and endothelial cell interactions during neural crest migration and formation of the dorsal root andsympathetic ganglia. Dev Biol 413(1):70–85
Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ (2006) Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci USA 103(10):3752–3757
Bailey CM, Morrison JA, Kulesa PM (2012) Melanoma revives an embryonic migration program to promote plasticity and invasion. Pigment Cell Melanoma Res 25(5):573–583
Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SP (2016) Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351(6271):379–384
Szabó A, Theveneau E, Turan M, Mayor R (2019) Neural crest streaming as an emergent property of tissue interactions during morphogenesis. PLoS Comput Biol 15(4):e1007002
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133(3421):571–573
Ribatti D, Mangialardi G, Vacca A (2006) Stephen paget and the ‘seed and soil’ theory of metastatic dissemination. Clin Exp Med 6(4):145–149
Lugassy C (2019) Concepts of cancer and Metastasis: Historical perspective. Melanoma: From Biology to Target. January AACR
Mitchell MJ, Denais C, Chan MF, Wang Z, Lammerding J, King MR (2015) Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am J Physiol Cell Physiol 309(11):C736–C746
Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E (2002) Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab 87(6):2954–2959
Burton GJ, Jauniaux E (2018) Development of the human placenta and fetal heart: synergic or independent? Front Physiol 9:373
McLennan R, Bailey CM, Schumacher LJ, Teddy JM, Morrison JA, Kasemeier-Kulesa JC, Wolfe LA, Gogol MM, Baker RE, Maini PK, Kulesa PM (2017) DAN (NBL1) promotes collective neural crest migration by restraining uncontrolled invasion. J Cell Biol 216(10):3339–3354
Hadjimichael C, Chanoumidou K, Papadopoulou N, Arampatzi P, Papamatheakis J, Kretsovali A (2015) Common stemness regulators of embryonic and cancer stem cells. World J Stem Cells 7(9):1150–1184
Deryugina EI, Kiosses WB (2017) Intratumoral cancer cell intravasation can occur independent of invasion into the adjacent stroma. Cell Rep 19(3):601–616
Mintz B, Illmensee K (1975) Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci USA 72(9):3585–3589
Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ (2019) Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res 9(1):1–21
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147(5):992–1009
Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, Hayward SW, Li D, Webb DJ (2017) Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 216(11):3799–3816
Ray A, Slama ZM, Morford RK, Madden SA, Provenzano PP (2017) Enhanced directional migration of cancer stem cells in 3D aligned collagen matrices. Biophys J 112(5):1023–1036
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10(7):445–457
Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galván JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D (2019) Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573(7775):526–531
Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D (2017) Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer 16(1):176
Yeh AC, Ramaswamy S (2015) Mechanisms of cancer cell dormancy-another hallmark of cancer? Cancer Res 75(23):5014–5022
Ingangi V, Minopoli M, Ragone C, Motti ML, Carriero MV (2019) Role of microenvironment on the fate of disseminating cancer stem cells. Front Oncol 21(9):82
Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F (2010) Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 16(1):116–122
Clark AG, Vignjevic DM (2015) Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol 36:13–22
Kulesa PM, Teddy JM, Stark DA, Smith SE, McLennan R (2008) Neural crest invasion is a spatially-ordered progression into the head with higher cell proliferation at the migratory front as revealed by the photoactivatable protein. KikGR. Dev Biol 316(2):275–287
Li Y, Vieceli FM, Gonzalez WG, Li A, Tang W, Lois C, Bronner ME (2019) In vivo quantitative imaging provides insights into trunk neural crest migration. Cell Rep 26(6):1489–1500
Hindley CJ, Condurat AL, Menon V, Thomas R, Azmitia LM, Davis JA, Pruszak J (2016) The hippo pathway member YAP enhances human neural crest cell fate and migration. Sci Rep 6:23208
Warren JSA, Xiao Y, Lamar JM (2018) YAP/TAZ activation as a target for treating metastatic cancer. Cancers (Basel) 10(4):E115
Seo J, Kim MH, Hong H, Cho H, Park S, Kim SK, Kim J (2019) MK5 regulates YAP stability and is a molecular target in YAP-driven cancers. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-19-1339
Fisher ML, Grun D, Adhikary G, Xu W (2017) Eckert RL (2017) Inhibition of YAP function overcomes BRAF inhibitor resistance in melanoma cancer stem cells. Oncotarget 8(66):110257–110272
Chang HY, Chang HM, Wu TJ, Chaing CY, Tzai TS, Cheng HL, Raghavaraju G, Chow NH, Liu HS (2017) The role of lutheran/basal cell adhesion molecule in human bladder carcinogenesis. J Biomed Sci 24(1):61
Enomoto-Okawa Y, Maeda Y, Harashima N, Sugawara Y, Katagiri F, Hozumi K, Hui KM, Nomizu M, Ito Y, Kikkawa Y (2017) An anti-human lutheran glycoprotein phage antibody inhibits cell migration on laminin-511: epitope mapping of the antibody. PLoS ONE 12(1):e0167860
Maeda H, Khatami M (2018) Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med 7(1):11
Acknowledgements
We sincerely thank Maud Haon who designed the diagram for Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lugassy, C., Kleinman, H.K., Vermeulen, P.B. et al. Angiotropism, pericytic mimicry and extravascular migratory metastasis: an embryogenesis-derived program of tumor spread. Angiogenesis 23, 27–41 (2020). https://doi.org/10.1007/s10456-019-09695-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-019-09695-9