Abstract
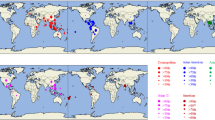
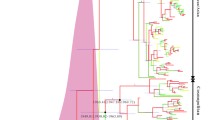
The spread of dengue disease has become a global public health concern. Dengue is caused by dengue virus, which is a mosquito-borne arbovirus of the genus Flavivirus, family Flaviviridae. There are four dengue virus serotypes (1-4), each of which is known to trigger mild to severe disease. Dengue virus serotype 4 (DENV-4) has four genotypes and is increasingly being reported to be re-emerging in various parts of the world. Therefore, the population structure and factors shaping the evolution of DENV-4 strains across the world were studied using genome-based population genetic, phylogenetic and selection pressure analysis methods. The population genomics study helped to reveal the spatiotemporal structure of the DENV-4 population and its primary division into two spatially distinct clusters: American and Asian. These spatial clusters show further time-dependent subdivisions within genotypes I and II. Thus, the DENV-4 population is observed to be stratified into eight genetically distinct lineages, two of which are formed by American strains and six of which are formed by Asian strains. Episodic positive selection was observed in the structural (E) and non-structural (NS2A and NS3) genes, which appears to be responsible for diversification of Asian lineages in general and that of modern lineages of genotype I and II in particular. In summary, the global DENV-4 population is stratified into eight genetically distinct lineages, in a spatiotemporal manner with limited recombination. The significant role of adaptive evolution in causing diversification of DENV-4 lineages is discussed. The evolution of DENV-4 appears to be governed by interplay between spatiotemporal distribution, episodic positive selection and intra/inter-genotype recombination.




Similar content being viewed by others
References
World Health Organization (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. WHO/HTM/NTD/DEN/2009.1 (World Health Organization 2009). http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O (2013) The global distribution and burden of dengue. Nature 496:504–507
Wilder-Smith A, Macary P (2014) Dengue: challenges for policy makers and vaccine developers. Curr Infect Dis Rep 16:1–8
Ghosh A, Dar L (2015) Dengue vaccines: challenges, development, current status and prospects. Indian J Med Microbiol 33:3
Thomas SJ (2015) Preventing dengue-is the possibility now a reality? N Engl J Med 372:172–173
Kanade T, Shah I (2011) Dengue encephalopathy. J Vector Borne Dis 48:180–181
Verma R, Sahu R, Holla V (2014) Neurological manifestations of dengue infection: a review. J Neurol Sci 346:26–34
Henchal EA, Putnak JR (1990) The dengue viruses. Clin Microbiol Rev 3:376–396
Normile D (2013) Surprising new dengue virus throws a spanner in disease control efforts. Science 342:415
Chen R, Vasilakis N (2011) Dengue—Quo tu et quo vadis? Viruses 3:1562–1608
Holmes EC, Burch SS (2000) The causes and consequences of genetic variation in dengue virus. Trends Microbiol 8:74–77
Holmes EC, Twiddy SS (2003) The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 3:19–28
Worobey M, Rambaut A, Holmes EC (1999) Widespread intra-serotype recombination in natural populations of dengue virus. Proc Natl Acad Sci 96:7352–7357
AbuBakar S, Wong P-F, Chan Y-F (2002) Emergence of dengue virus type 4 genotype IIA in Malaysia. Indian J Med Microbiol 83:2437–2442
Behura SK, Severson DW (2013) Nucleotide substitutions in dengue virus serotypes from Asian and American countries: insights into intracodon recombination and purifying selection. BMC microbiol 13:37. doi:10.1186/1471-2180-13-37
De Figueiredo RM, Naveca FG, Oliveira CM, Bastos Mde S, Mourão MP, Viana Sde S, Melo Mdo N, Itapirema EF, Saatkamp CJ, Farias IP (2011) Co-infection of dengue virus by serotypes 3 and 4 in patients from Amazonas, Brazil. Rev Inst Med Trop Sao Paulo 53:321–323
Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, Kabra SK, Broor S (2008) Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J 5:1–5
Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE (2014) Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 22:138–146
Cecilia D, Kakade MB, Bhagat AB, Vallentyne J, Singh A, Patil JA, Todkar SM, Varghese SB, Shah PS (2011) Detection of dengue-4 virus in Pune, Western India after an absence of 30 years-its association with two severe cases. Virol J 8:46
Nunes MR, Faria NR, Vasconcelos HB, Medeiros DB, Silva de Lima CP, Carvalho VL, Pinto da Silva EV, Cardoso JF, Sousa EC Jr, Nunes KN, Rodrigues SG, Abecasis AB, Suchard MA, Lemey P, Vasconcelos PF (2012) Phylogeography of dengue virus serotype 4, Brazil, 2010–2011. Emerg Infect Dis 18:1858–1864
Ramos C, Sánchez G, Pando RH, Baquera J, Hernández D, Mota J, Ramos J, Flores A, Llausás E (1998) Dengue virus in the brain of a fatal case of hemorrhagic dengue fever: case report. J Neurovirol 4:465–468
Hapuarachchi HC, Oh HM, Thein TL, Pok K-Y, Lai Y-L, Tan L-K, Lee K-S, Leo Y-S, Ng L-C (2013) Clinico-genetic characterisation of an encephalitic Dengue virus 4 associated with multi-organ involvement. J Clin Virol 57:91–94
Lum LH, Smitasin N (2014) Dengue breathing down our neck: a case report of dengue encephalitis. Int J Case Rep Imag 5:600–603
Dash P, Sharma S, Srivastava A, Santhosh S, Parida M, Neeraja M, Subbalaxmi M, Lakshmi V, Rao P (2011) Emergence of dengue virus type 4 (genotype I) in India. Epidemiol Infect 139:857–861
Villabona-Arenas CJ, de Andrade Zanotto PM (2011) Evolutionary history of dengue virus type 4: insights into genotype phylodynamics. Infect Genet Evol 11:878–885
Foster JE, Bennett SN, Vaughan H, Vorndam V, McMillan WO, Carrington CV (2003) Molecular evolution and phylogeny of dengue type 4 virus in the Caribbean. Virology 306:126–134
Patil J, Cherian S, Walimbe A, Bhagat A, Vallentyne J, Kakade M, Shah P, Cecilia D (2012) Influence of evolutionary events on the Indian subcontinent on the phylogeography of dengue type 3 and 4 viruses. Infect Genet Evol 12:1759–1769
Resch W, Zaslavsky L, Kiryutin B, Rozanov M, Bao Y, Tatusova TA (2009) Virus variation resources at the National Center for Biotechnology Information: dengue virus. BMC Microbiol 9:65. doi:10.1186/1471-2180-9-65
Kolekar P, Kale M, Kulkarni-Kale U (2012) Alignment-free distance measure based on return time distribution for sequence analysis: applications to clustering, molecular phylogeny and subtyping. Mol Phylogenet Evol 65:510–522
Waman VP, Kolekar PS, Kale MM, Kulkarni-Kale U (2014) Population structure and evolution of Rhinoviruses. PloS One 9:e88981. doi:10.1371/journal.pone.0088981
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Haubold B, Hudson RR (2000) LIAN 3.0: detecting linkage disequilibrium in multilocus data. Bioinformatics 16:847–849
Devlin B, Risch N (1995) A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics 29:311–322
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Gilks W, Richardson S, Spiegelhalter D (1996) Markov chain Monte Carlo in practice. Chapman Hall, New York, p 486
Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2013) GenBank. Nucleic Acids Res 41(Database issue):D36–D42
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer
FigTree v1.2.3. http://tree.bio.ed.ac.uk/software/figtree/
Martin DP, Williamson C, Posada D (2005) RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 21:260–262
Padidam M, Sawyer S, Fauquet CM (1999) Possible emergence of new geminiviruses by frequent recombination. Virology 265:218–225
Martin D, Posada D, Crandall K, Williamson C (2005) A modified bootscan algorithm for automated identification of recombinant sequences and recombination breakpoints. AIDS Res Hum Retroviruses 21:98–102
Smith JM (1992) Analyzing the mosaic structure of genes. J Mol Evol 34:126–129
Posada D, Crandall KA (2001) Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc Natl Acad Sci 98:13757–13762
Gibbs MJ, Armstrong JS, Gibbs AJ (2000) Sister-scanning: a Monte Carlo procedure for assessing signals in recombinant sequences. Bioinformatics 16:573–582
Boni MF, Posada D, Feldman MW (2007) An exact nonparametric method for inferring mosaic structure in sequence triplets. Genetics 176:1035–1047
Privman E, Penn O, Pupko T (2012) Improving the performance of positive selection inference by filtering unreliable alignment regions. Mol Biol Evol 29:1–5
Löytynoja A, Goldman N (2010) webPRANK: a phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinform 11:579
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Penn O, Privman E, Ashkenazy H, Landan G, Graur D, Pupko T (2010) GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Res 38:W23–W28
Pond SLK, Frost SD (2005) Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 21:2531–2533
Pond SLK, Muse SV (2005) HyPhy: hypothesis testing using phylogenies. Statistical methods in molecular evolution. Springer, Berlin, pp 125–181
Pond SLK, Frost SD (2005) Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol 22:1208–1222
Pond SLK, Frost SD, Grossman Z, Gravenor MB, Richman DD, Brown AJL (2006) Adaptation to different human populations by HIV-1 revealed by codon-based analyses. PLoS Comput Biol 2:e62
Murrell B, Wertheim JO, Moola S, Weighill T, Scheffler K, Pond SLK (2012) Detecting individual sites subject to episodic diversifying selection. PLoS Genet 8:e1002764. doi:10.1371/journal.pgen.1002764
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat T, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW (2002) Genetic structure of human populations. Science 298:2381–2385
Becquet C, Patterson N, Stone AC, Przeworski M, Reich D (2007) Genetic structure of chimpanzee populations. PLoS Genet 3:e66
Peacock E, Sonsthagen SA, Obbard ME, Boltunov A, Regehr EV, Ovsyanikov N, Aars J, Atkinson SN, Sage GK, Hope AG (2015) Implications of the circumpolar genetic structure of polar bears for their conservation in a rapidly warming arctic. PloS One 10:e112021. doi:10.1371/journal.pgen.0030066
Szmaragd C, Balloux F (2007) The population genomics of hepatitis B virus. Mol Ecol 16:4747–4758
Weaver SC, Vasilakis N (2009) Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol 9:523–540
Vazquez S, Ruiz D, Barrero R, Ramirez R, Calzada N, del Rosario Peña B, Reyes S, Guzman MG (2010) Kinetics of dengue virus NS1 protein in dengue 4-confirmed adult patients. Diagn Microbiol Infect Dis 68:46–49
Garcia G, Vaughn DW, Del Angel RM (1997) Recognition of synthetic oligopeptides from nonstructural proteins NS1 and NS3 of dengue-4 virus by sera from dengue virus-infected children. Am J Trop Med Hyg 56:466–470
Edeling MA, Diamond MS, Fremont DH (2014) Structural basis of flavivirus NS1 assembly and antibody recognition. Proc Natl Acad Sci 111:4285–4290
Akey DL, Brown WC, Dutta S, Konwerski J, Jose J, Jurkiw TJ, DelProposto J, Ogata CM, Skiniotis G, Kuhn RJ (2014) Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 343:881–885
Xie X, Gayen S, Kang C, Yuan Z, Shi P-Y (2013) Membrane topology and function of dengue virus NS2A protein. J Virol 87:4609–4622
Malavige G, McGowan S, Atukorale V, Salimi M, Peelawatta M, Fernando N, Jayaratne S, Ogg G (2012) Identification of serotype-specific T cell responses to highly conserved regions of the dengue viruses. Clin Exp Immunol 168:215–223
Mongkolsapaya J, Dejnirattisai W, X-n Xu, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S (2003) Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9:921–927
Huang Y, Niu B, Gao Y, Fu L, Li W (2010) CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26:680–682
Shu P-Y, Su C-L, Liao T-L, Yang C-F, Chang S-F, Lin C-C, Chang M-C, Hu H-C, Huang J-H (2009) Molecular characterization of dengue viruses imported into Taiwan during 2003–2007: geographic distribution and genotype shift. Am J Trop Med Hyg 80(6):1039–1046
Lee K-S, Lo S, Tan SS-Y, Chua R, Tan L-K, Xu H, Ng L-C (2012) Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infect Genet Evol 12(1):77–85
Jing Q-L, Yang Z-C, Luo L, Xiao X-C, Di B, He P, Fu C-X, Wang M, Lu J-H (2012) Emergence of dengue virus 4 genotype II in Guangzhou, China, 2010: Survey and molecular epidemiology of one community outbreak. BMC Infect Dis 12:87. doi:10.1186/1471-2334-12-87
Meltzer E, Lustig Y, Glichinsky O, Steiner F, Schwartz E (2014) Probable importation of Dengue virus type 4 to Angola from Brazil. Emerg Infect Dis 20:1775–1776
Pinho A, Sardi S, Paula F, Peixoto I, Brandão C, Fernandez F, Campos G (2015) Asian genotypes of dengue virus 4 in Brazil. Epidemiol Infect 143(14):3114–3117
Lara-Ramírez EE, Salazar MI, López-López MdJ, Salas-Benito JS, Sánchez-Varela A, Guo X (2014) Large-scale genomic analysis of codon usage in dengue virus and evaluation of its phylogenetic dependence. Biomed Res Int 2014:851425. doi:10.1155/2014/851425
Allicock OM, Lemey P, Tatem AJ, Pybus OG, Bennett SN, Mueller BA, Suchard MA, Foster JE, Rambaut A, Carrington CV (2012) Phylogeography and population dynamics of dengue viruses in the Americas. Mol Biol Evol 29(6):1533–1543. doi:10.1093/molbev/msr320
Carrington CV, Foster JE, Pybus OG, Bennett SN, Holmes EC (2005) Invasion and maintenance of dengue virus type 2 and type 4 in the Americas. J Virol 79(23):14680–14687
Bennett SN, Holmes EC, Chirivella M, Rodriguez DM, Beltran M, Vorndam V, Gubler DJ, McMillan WO (2003) Selection-driven evolution of emergent dengue virus. Mol Biol Evol 20:1650–1658
McCutchan FE (2006) Global epidemiology of HIV. J Med Virol 78:S7–S12
Acknowledgments
This work was supported by a Center of Excellence (CoE) grant from the Department of Biotechnology (DBT), Government of India, New Delhi. UKK acknowledges DBT CoE for financial assistance. VPW acknowledges DBT fellowship. SMK acknowledges the Bioinformatics Resources and Applications Facility (BRAF), C-DAC, Pune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by a Center of Excellence (CoE) grant from the Department of Biotechnology (DBT), Government of India, New Delhi, India.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2016_2886_MOESM1_ESM.doc
Online resource 1: Table S1. Dataset of 134 strains of DENV-4. Table enlists serial number, GenBank accession number, name, country, collection date, genotype and subpopulation (assigned using STRUCTURE program). Serial numbers are used to represent the corresponding strain in bar plot given in Fig. 2 (DOC 217 kb)
705_2016_2886_MOESM2_ESM.tif
Online resource 2: Figure S1. Complete genome-based phylogenetic tree of DENV-4 obtained using Maximum-likelihood method. 134 DENV-4 genomes were used to generate tree using ML with 1000 bootstrap replicates. OTU is labelled as: ‘DENV4| (genotype)| (GenBank accession number)’ for every DENV-4 strain. Branches in tree are color-coded as per the eight subpopulations obtained using STRUCTURE program. They are GS (red) GIII (green), GI-A (blue), GI-B (magenta), GII-A1 (yellow), older GII-A2 (orange), modern GII-A3 (purple) and an admixed GII-A4 (cyan) (TIFF 423 kb)
705_2016_2886_MOESM3_ESM.tif
Online resource 3: Figure S2. Complete genome-based tree of DENV-4 obtained using Maximum-parsimony method. 134 DENV-4 genomes were used to generate tree using MP with 1000 bootstrap replicates. OTU is labelled as: ‘DENV4|(genotype)|(GenBank accession number)’ for every strain. Branches in tree are color-coded as per eight subpopulations obtained using STRUCTURE program: GS (red) GIII (green), GI-A (blue), GI-B (magenta), GII-A1 (yellow), GII-A2 (orange), GII-A3 (purple) and an GII-A4 (cyan) (TIFF 251 kb)
705_2016_2886_MOESM4_ESM.tif
Online resource 4: Figure S3. Envelope gene-based tree of DENV-4 obtained using Neighbor-joining method. Tree is generated using NJ with 1000 bootstrap replicates. OTU is labelled as: ‘DENV4|(genotype)|(GenBank accession number)’ for every strain. Branches are color-coded as per eight subpopulations obtained using STRUCTURE program: GS (red), GIII (green), GI-A (blue), GI-B (magenta), GII-A1 (yellow), older GII-A2 (orange), modern GII-A3 (purple) and GII-A4 (cyan) (TIFF 282 kb)
705_2016_2886_MOESM5_ESM.tif
Online resource 5: Figure S4. Envelope gene-based time-scaled tree of DENV-4 obtained using BEAST v18.2. Envelope-gene sequences were extracted from DENV-4 genomic entries having year information. Time-scale tree using uncorrelated lognormal relaxed clock model with constant population size, was generated. OTU is labelled as: ‘D4|(genotype)|(GenBank accession number)|(year)|(country)’ for every strain. Few country fields were labelled as ‘NA’ to indicate their non-availability. Nodes and branches are labelled with posterior probability values. Branches are color-coded as per eight subpopulations obtained using STRUCTURE program: GS (red), GIII (green), GI-A (blue), GI-B (magenta), GII-A1 (yellow), GII-A2 (orange), GII-A3 (purple) and GII-A4 (cyan) (TIFF 411 kb)
705_2016_2886_MOESM6_ESM.pdf
Online resource 6: Figure S5. Episodic positive selection on codon-90 in NS2A, obtained using MEME. Selection on NS2A codon-90 was observed to be stronger on branch (red) leading to modern GI-A lineage (PDF 51 kb)
705_2016_2886_MOESM7_ESM.pdf
Online resource 7: Figure S6. Episodic positive selection on codon-142 in NS3, obtained using MEME. Selection on NS3 codon-142 was observed to be stronger on branch (red) leading to cluster of Asian DENV-4 subpopulations (PDF 58 kb)
Rights and permissions
About this article
Cite this article
Waman, V.P., Kasibhatla, S.M., Kale, M.M. et al. Population genomics of dengue virus serotype 4: insights into genetic structure and evolution. Arch Virol 161, 2133–2148 (2016). https://doi.org/10.1007/s00705-016-2886-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-016-2886-8