Abstract
Introduction
Permanent/nonresorbable hernia repair materials rely on profibrotic wound healing, and repair sites are commonly composed of disorganized tissue with inferior mechanical strength and risk of reherniation. Resorbable electrospun scaffolds represent a novel class of biomaterials, which may provide a unique platform for the design of advanced soft tissue repair materials. These materials are simple, inexpensive, nonwoven materials composed of polymer fibers that readily mimic the natural extracellular matrix. The primary goal of the present study was to evaluate the physiomechanical properties of novel electrospun scaffolds to determine their suitability for hernia repair. Based on previous experimentation, scaffolds possessing ≥20 N suture retention strength, ≥20 N tear resistance, and ≥50 N/cm tensile strength are appropriate for hernia repair.
Methods
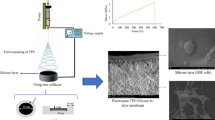
Six novel electrospun scaffolds were fabricated by varying combinations of polymer concentration (10–12 %) and flow rate (3.5–10 mL/h). Briefly, poly(ε-caprolactone) (PCL) was dissolved in a solvent mixture and electrospun onto a planar metal collector, yielding sheets with randomly oriented fibers. Physiomechanical properties were evaluated through scanning electron microscopy, laser micrometry, and mechanical testing.
Results
Scanning electron micrographs demonstrated fiber diameters ranging from 1.0 ± 0.1 μm (10 % PCL, 3.5 mL/h) to 1.5 ± 0.2 μm (12 % PCL, 4 mL/h). Laser micrometry demonstrated thicknesses ranging from 0.72 ± 0.07 mm (12 % PCL, 10 mL/h) to 0.91 ± 0.05 mm (10 % PCL, 3.5 mL/h). Mechanical testing identified two scaffolds possessing suture retention strengths ≥20 N (12 % PCL, 10 mL/h and 12 % PCL, 6 mL/h), and no scaffolds possessing tear resistance values ≥20 N (range, 4.7 ± 0.9 N to 10.6 ± 1.8 N). Tensile strengths ranged from 35.27 ± 2.08 N/cm (10 % PCL, 3.5 mL/h) to 81.76 ± 15.85 N/cm (12 % PCL, 4 mL/h), with three scaffolds possessing strengths ≥50 N/cm (12 % PCL, 10 mL/h; 12 % PCL, 6 mL/h; 12 % PCL, 4 mL/h).
Conclusions
Two electrospun scaffolds (12 % PCL, 10 mL/h and 12 % PCL, 6 mL/h) possessed suture retention and tensile strengths appropriate for hernia repair, justifying evaluation in a large animal model. Additional studies examining advanced methods of fabrication may further improve the unique properties of these scaffolds, propelling them into applications in a variety of clinical settings.










Similar content being viewed by others
References
Luijendijk RW, Hop WC, van den Tol MP et al (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343:392–398
Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240:578–583
Usher FC, Ochsner J, Tuttle LJ (1958) Use of Marlex mesh in the repair of incisional hernias. Am Surg 24:969–974
Langenbach MR, Schmidt J, Ubrig B, Zirngibl H (2008) Sixty-month follow-up after endoscopic inguinal hernia repair with three types of mesh: a prospective randomized trial. Surg Endosc 22:1790–1797
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Robinson TN, Clarke JH, Schoen J, Walsh MD (2005) Major mesh-related complications following hernia repair: events reported to the Food and Drug Administration. Surg Endosc 19:1556–1560
Welty G, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V (2001) Functional impairment and complaints following incisional hernia repair with different polypropylene meshes. Hernia 5:142–147
Rice RD, Ayubi FS, Shaub ZJ, Parker DM, Armstrong PJ, Tsai JW (2010) Comparison of Surgisis, AlloDerm, and Vicryl Woven Mesh grafts for abdominal wall defect repair in an animal model. Aesthetic Plast Surg 34:290–296
Laschke MW, Haufel JM, Scheuer C, Menger MD (2009) Angiogenic and inflammatory host response to surgical meshes of different mesh architecture and polymer composition. J Biomed Mater Res B Appl Biomater 91:497–507
Dayton MT, Buchele BA, Shirazi SS, Hunt LB (1986) Use of an absorbable mesh to repair contaminated abdominal-wall defects. Arch Surg 121:954–960
Zieren J, Castenholz E, Baumgart E, Muller JM (1999) Effects of fibrin glue and growth factors released from platelets on abdominal hernia repair with a resorbable PGA mesh: experimental study. J Surg Res 85:267–272
Tobias AM, Low DW (2003) The use of a subfascial Vicryl mesh buttress to aid in the closure of massive ventral hernias following damage-control laparotomy. Plast Reconstr Surg 112:766–776
Klinge U, Schumpelick V, Klosterhalfen B (2001) Functional assessment and tissue response of short- and long-term absorbable surgical meshes. Biomaterials 22:1415–1424
Franklin ME Jr, Trevino JM, Portillo G, Vela I, Glass JL, Gonzalez JJ (2008) The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc 22:1941–1946
Ueno T, Pickett LC, de la Fuente SG, Lawson DC, Pappas TN (2004) Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg 8:109–112
Jenkins ED, Yip M, Melman L, Frisella MM, Matthews BD (2010) Informed consent: cultural and religious issues associated with the use of allogeneic and xenogeneic mesh products. J Am Coll Surg 210:402–410
Matthews JA, Wnek GE, Simpson DG, Bowlin GL (2002) Electrospinning of collagen nanofibers. Biomacromolecules 3:232–238
Weigel T, Schinkel G, Lendlein A (2006) Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev Med Devices 3:835–851
Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL (2005) Electrospinning polydioxanone for biomedical applications. Acta Biomater 1:115–123
Xie J, Macewan MR, Schwartz AG, Xia Y (2010) Electrospun nanofibers for neural tissue engineering. Nanoscale 2:35–44
Xie J, Macewan MR, Willerth SM, Li X, Moran DW, Sakiyama-Elbert SE, Xia Y (2009) Conductive Core-Sheath Nanofibers and Their Potential Application in Neural Tissue Engineering. Adv Funct Mater 19:2312–2318
Xie J, Macewan MR, Li X, Sakiyama-Elbert SE, Xia Y (2009) Neurite outgrowth on nanofiber scaffolds with different orders, structures, and surface properties. ACS Nano 3:1151–1159
Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y (2009) The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials 30:354–362
Li X, Xie J, Lipner J, Yuan X, Thomopoulos S, Xia Y (2009) Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett 9:2763–2768
Li X, Xie J, Yuan X, Xia Y (2008) Coating electrospun poly(epsilon-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir 24:14145–14150
Xie J, Macewan MR, Ray WZ, Liu W, Siewe DY, Xia Y (2010) Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano 4:5027–5036
Deeken CR, Abdo M, Frisella M, Matthews BD (2011) Physicomechanical evaluation of polypropylene, polyester, and polytetrafluoroethylene meshes for inguinal hernia repair. J Am Coll Surg 212:68–79
Deeken CR, Abdo MS, Frisella MM, Matthews BD (2011) Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair. Surg Endosc 25:1541–1552
Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC (2001) The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42:261–272
Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B (2002) Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43:4403–4412
Garg K, Bowlin GL (2011) Electrospinning jets and nanofibrous structures. Biomicrofluidics 5:13403
Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK (2002) Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res 60:613–621
Reneker DH, Yarin AL, Fong H, Koombhongse S (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Physics 87:4531–4547
Reneker DH, Chun I (1996) Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7:216–223
(2011) Vicryl Woven Mesh instructions for use
(2011) TIGR Matrix Surgical Mesh instructions for use
(2011) Gore Bio-A Tissue Reinforcement instructions for use
Acknowledgments
This research was supported by research grants from the Washington University Bear Cub grant program, the Washington University Institute for Minimally Invasive Surgery, and the Washington University Dean’s Summer Fellowship program.
Disclosures
Dr. Deeken is a consultant for Atrium Medical Corporation and C.R. Bard/Davol, Inc. and has received honoraria from Covidien and Musculoskeletal Transplant Foundation, as well as grant support from Atrium Medical Corporation, Covidien, Kensey Nash Corporation, and Musculoskeletal Transplant Foundation. Dr. Matthews is a consultant for Atrium Medical Corporation and Ethicon, Inc. He also receives honoraria and research/equipment support from Atrium Medical Corporation, Ethicon EndoSurgery, Karl Storz Endoscopy, Stryker Endoscopy, and W.L. Gore & Associates, Inc. Margaret M. Frisella is a consultant for Atrium Medical Corporation and receives honoraria from W.L. Gore & Associates. Gregory C. Ebersole, Evan G. Buettmann, Matthew R. MacEwan, and Michael E. Tang have no conflict of interest or financial ties to disclose.
Funding
Supported by research grants from the Washington University Bear Cub grant program, the Washington University Institute for Minimally Invasive Surgery, and the Washington University Dean’s Summer Fellowship program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gregory C. Ebersole and Evan G. Buettmann contributed equally to this work.
Presented at the SAGES 2012 Annual Meeting, March 7–March 10, 2012, San Diego, CA.
Rights and permissions
About this article
Cite this article
Ebersole, G.C., Buettmann, E.G., MacEwan, M.R. et al. Development of novel electrospun absorbable polycaprolactone (PCL) scaffolds for hernia repair applications. Surg Endosc 26, 2717–2728 (2012). https://doi.org/10.1007/s00464-012-2258-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2258-8