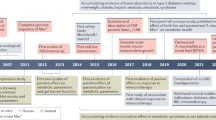
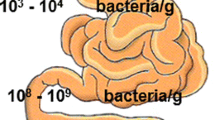
Abstract
Trillions of bacteria inhabit the mammalian gastrointestinal tract. In the majority of hosts, these symbionts contribute largely to beneficial functions promoting microbe-host homeostasis. However, an increasing number of human diseases is associated with altered microbiota composition and enrichment of certain bacterial species. A well-known example of this is Mucispirillum schaedleri, which has been associated with inflammatory conditions in the intestine. Mucispirillum spp. belong to the phylum Deferribacteres and are prevalent but low abundant members of the rodent, pig and human microbiota. Recently, M. schaedleri was causally linked to the development of Crohn’s disease—like colitis in immunodeficient mice. While this study certifies a considerable pathogenic potential, the same organism can also promote health in the immunocompetent host: M. schaedleri protects from Salmonella enterica serovar Typhimurium (S. Tm)-induced colitis by interfering with the expression of the pathogen´s invasion machinery. In this review, we summarize the current knowledge on the mammalian gut symbiont M. schaedleri and its role in intestinal homeostasis and discuss open questions and perspectives for future research.


Similar content being viewed by others
Data availability
The datasets analysed were retrieved from the IMGS portal.
References
Huber H, Stetter KO (2001) Class I. Deferribacteres class. nov. Bergey’s Manual Syst Bacteriol 1:465
Caccavo F, Coates JD, Rossello-Mora RA et al (1996) Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch Microbiol 165:370–376. https://doi.org/10.1007/s002030050340
Fiala G, Woese CR, Langworthy TA et al (1990) Flexistipes sinusarabici, a novel genus and species of eubacteria occurring in the Atlantis II Deep brines of the Red Sea. Arch Microbiol 154:120–126. https://doi.org/10.1007/BF00423320
Myhr S, Torsvik T (2000) Denitrovibrio acetiphilus, a novel genus and species of dissimilatory nitrate-reducing bacterium isolated from an oil reservoir model column. Int J Syst Evol Microbiol 50(Pt 4):1611–1619. https://doi.org/10.1099/00207713-50-4-1611
Takai K, Kobayashi H, Nealson KH et al (2003) Deferribacter desulfuricans sp. nov., a novel sulfur-, nitrate- and arsenate-reducing thermophile isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 53:839–846. https://doi.org/10.1099/ijs.0.02479-0
Iino T, Nakagawa T, Mori K et al (2008) Calditerrivibrio nitroreducens gen. nov., sp. nov., a thermophilic, nitrate-reducing bacterium isolated from a terrestrial hot spring in Japan. Int J Syst Evol Microbiol 58:1675–1679. https://doi.org/10.1099/ijs.0.65714-0
Tamazawa S, Mayumi D, Mochimaru H et al (2017) Petrothermobacter organivorans gen. nov., sp. nov., a thermophilic, strictly anaerobic bacterium of the phylum Deferribacteres isolated from a deep subsurface oil reservoir. Int J Syst Evol Microbiol 67:3982–3986. https://doi.org/10.1099/ijsem.0.002234
Rauschenbach I, Posternak V, Cantarella P et al (2013) Seleniivibrio woodruffii gen. nov., sp. nov., a selenate- and arsenate-respiring bacterium in the Deferribacteraceae. Int J Syst Evol Microbiol 63:3659–3665. https://doi.org/10.1099/ijs.0.043547-0
Rosshart SP, Vassallo BG, Angeletti D et al (2017) Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 171:1015-1028.e13. https://doi.org/10.1016/j.cell.2017.09.016
Harrell L, Wang Y, Antonopoulos D et al (2012) Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS ONE 7:e32545. https://doi.org/10.1371/journal.pone.0032545
Michel AJ, Ward LM, Goffredi SK et al (2018) The gut of the finch: uniqueness of the gut microbiome of the Galápagos vampire finch. Microbiome 6:167. https://doi.org/10.1186/s40168-018-0555-8
Umu ÖCO, Mydland LT, Øverland M et al (2020) Rapeseed-based diet modulates the imputed functions of gut microbiome in growing-finishing pigs. Sci Rep 10:9372. https://doi.org/10.1038/s41598-020-66364-4
Robertson BR, O’Rourke JL, Neilan BA et al (2005) Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int J Syst Evol Microbiol 55:1199–1204. https://doi.org/10.1099/ijs.0.63472-0
Linnenbrink M, Wang J, Hardouin EA et al (2013) The role of biogeography in shaping diversity of the intestinal microbiota in house mice. Mol Ecol 22:1904–1916. https://doi.org/10.1111/mec.12206
Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A et al (2015) The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16:164–177 (10.15252/embr.201439263)
Herp S, Brugiroux S, Garzetti D et al (2019) Mucispirillum schaedleri antagonizes salmonella virulence to protect mice against colitis. Cell Host Microbe 25:681-694.e8. https://doi.org/10.1016/j.chom.2019.03.004
Lagkouvardos I, Joseph D, Kapfhammer M et al (2016) IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep 6:33721. https://doi.org/10.1038/srep33721
Zmora N, Zilberman-Schapira G, Suez J et al (2018) Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 174:1388-1405.e21. https://doi.org/10.1016/j.cell.2018.08.041
Reitmeier S, Hitch TCA, Fikas N et al (2020) Handling of spurious sequences affects the outcome of high-throughput 16S rRNA gene amplicon profiling. https://doi.org/10.21203/rs.2.21240/v1
Krych L, Hansen CHF, Hansen AK et al (2013) Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLoS ONE 8:e62578. https://doi.org/10.1371/journal.pone.0062578
Zaborin A, Penalver Bernabe B, Keskey R et al (2020) Spatial compartmentalization of the microbiome between the lumen and crypts is lost in the murine cecum following the process of surgery, including overnight fasting and exposure to antibiotics. mSystems. https://doi.org/10.1128/mSystems.00377-20
Loy A, Pfann C, Steinberger M et al (2017) Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems. https://doi.org/10.1128/mSystems.00171-16
Berry D, Stecher B, Schintlmeister A et al (2013) Host-compound foraging by intestinal microbiota revealed by single-cell stable isotope probing. Proc Natl Acad Sci USA 110:4720–4725. https://doi.org/10.1073/pnas.1219247110
Aviello G, Knaus UG (2017) ROS in gastrointestinal inflammation: rescue or sabotage? Br J Pharmacol 174:1704–1718. https://doi.org/10.1111/bph.13428
Winter SE, Winter MG, Xavier MN et al (2013) Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339:708–711. https://doi.org/10.1126/science.1232467
Orcutt RP, Gianni FJ, Judge RJ (1987) Development of an “altered Schaedler flora” for NCI gnotobiotic rodents. Microecol Ther 17(59)
Wymore Brand M, Wannemuehler MJ, Phillips GJ et al (2015) The altered schaedler flora: continued applications of a defined murine microbial community. ILAR J 56:169–178. https://doi.org/10.1093/ilar/ilv012
Gomes-Neto JC, Kittana H, Mantz S et al (2017) A gut pathobiont synergizes with the microbiota to instigate inflammatory disease marked by immunoreactivity against other symbionts but not itself. Sci Rep 7:17707. https://doi.org/10.1038/s41598-017-18014-5
Bolsega S, Basic M, Smoczek A et al (2019) Composition of the intestinal microbiota determines the outcome of virus-triggered colitis in mice. Front Immunol 10:1708. https://doi.org/10.3389/fimmu.2019.01708
Chassaing B, Gewirtz AT (2018) Mice harboring pathobiont-free microbiota do not develop intestinal inflammation that normally results from an innate immune deficiency. PLoS ONE 13:e0195310. https://doi.org/10.1371/journal.pone.0195310
Kabbert J, Benckert J, Rollenske T et al (2020) High microbiota reactivity of adult human intestinal IgA requires somatic mutations. J Exp Med. https://doi.org/10.1084/jem.20200275
Berry D, Kuzyk O, Rauch I et al (2015) Intestinal microbiota signatures associated with inflammation history in mice experiencing recurring colitis. Front Microbiol 6:1408. https://doi.org/10.3389/fmicb.2015.01408
Berry D, Schwab C, Milinovich G et al (2012) Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J 6:2091–2106. https://doi.org/10.1038/ismej.2012.39
El Aidy S, Derrien M, Aardema R et al (2014) Transient inflammatory-like state and microbial dysbiosis are pivotal in establishment of mucosal homeostasis during colonisation of germ-free mice. Benef Microbes 5:67–77. https://doi.org/10.3920/BM2013.0018
Rooks MG, Veiga P, Wardwell-Scott LH et al (2014) Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J 8:1403–1417. https://doi.org/10.1038/ismej.2014.3
Vereecke L, Vieira-Silva S, Billiet T et al (2014) A20 controls intestinal homeostasis through cell-specific activities. Nat Commun 5:5103. https://doi.org/10.1038/ncomms6103
Selvanantham T, Lin Q, Guo CX et al (2016) NKT cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol 197:4464–4472. https://doi.org/10.4049/jimmunol.1601410
Bunker JJ, Flynn TM, Koval JC et al (2015) Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 43:541–553. https://doi.org/10.1016/j.immuni.2015.08.007
Belzer C, Gerber GK, Roeselers G et al (2014) Dynamics of the microbiota in response to host infection. PLoS ONE 9:e95534. https://doi.org/10.1371/journal.pone.0095534
Lin C-H, Chen C-C, Chiang H-L et al (2019) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflamm 16:129. https://doi.org/10.1186/s12974-019-1528-y
Xiao M, Fu X, Ni Y et al (2018) Protective effects of Paederia scandens extract on rheumatoid arthritis mouse model by modulating gut microbiota. J Ethnopharmacol 226:97–104. https://doi.org/10.1016/j.jep.2018.08.012
He Z, Kong X, Shao T et al (2019) Alterations of the gut microbiota associated with promoting efficacy of prednisone by bromofuranone in MRL/lpr mice. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00978
Kim J, Lee H, An J et al (2019) Alterations in gut microbiota by statin therapy and possible intermediate effects on hyperglycemia and hyperlipidemia. Front Microbiol 10:1947. https://doi.org/10.3389/fmicb.2019.01947
Jašarević E, Howard CD, Misic AM et al (2017) Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep 7:44182. https://doi.org/10.1038/srep44182
Werbner M, Barsheshet Y, Werbner N et al (2019) Social-stress-responsive microbiota induces stimulation of self-reactive effector T helper cells. mSystems. https://doi.org/10.1128/mSystems.00292-18
Ussar S, Griffin NW, Bezy O et al (2015) Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab 22:516–530. https://doi.org/10.1016/j.cmet.2015.07.007
Caruso R, Mathes T, Martens EC et al (2019) A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci Immunol. https://doi.org/10.1126/sciimmunol.aaw4341
Jergens AE, Wilson-Welder JH, Dorn A et al (2006) Helicobacter bilis triggers persistent immune reactivity to antigens derived from the commensal bacteria in gnotobiotic C3H/HeN mice. Gut 56:934–940. https://doi.org/10.1136/gut.2006.099242
Jochum L, Stecher B (2020) Label or concept—what is a pathobiont? Trends Microbiol 28:789–792. https://doi.org/10.1016/j.tim.2020.04.011
Rogers AWL, Tsolis RM, Bäumler AJ (2021) Salmonella versus the microbiome. Microbiol Mol Biol Rev. https://doi.org/10.1128/MMBR.00027-19
Hapfelmeier S, Stecher B, Barthel M et al (2005) The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J Immunol 174:1675–1685. https://doi.org/10.4049/jimmunol.174.3.1675
Galán JE (2001) Salmonella interactions with host cells: type III secretion at work. Annu Rev Cell Dev Biol 17:53–86. https://doi.org/10.1146/annurev.cellbio.17.1.53
Chen I-MA, Chu K, Palaniappan K et al (2021) The IMG/M data management and analysis system vol 6.0: new tools and advanced capabilities. Nucleic Acids Res 49:D751–D763. https://doi.org/10.1093/nar/gkaa939
Funding
Bärbel Stecher acknowledges funding by the German Center of Infection Research (DZIF), the DFG Priority Program SPP1656, the Collaborative Research Center CRC1371 and the European Research Council (Grant no. 865615).
Author information
Authors and Affiliations
Contributions
Data analysis was performed by AR and SW. Literature search was performed by SW and SH. This work was drafted by SH and BS, and critically revised by SW, AR and MS. MS designed the graphical abstract.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Edited by: Volkhard A. J. Kempf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Herp, S., Durai Raj, A.C., Salvado Silva, M. et al. The human symbiont Mucispirillum schaedleri: causality in health and disease. Med Microbiol Immunol 210, 173–179 (2021). https://doi.org/10.1007/s00430-021-00702-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-021-00702-9