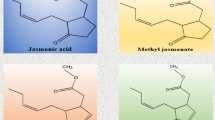
Abstract
The moss Physcomitrella patens (P. patens) is a useful model to study abiotic stress responses since it is highly tolerant to drought, salt and osmotic stress. However, very little is known about the defense mechanisms activated in this moss after pathogen assault. In this study, we show that P. patens activated multiple and similar responses against Pythium irregulare and Pythium debaryanum, including the reinforcement of the cell wall, induction of the defense genes CHS, LOX and PAL, and accumulation of the signaling molecules jasmonic acid (JA) and its precursor 12-oxo-phytodienoic acid (OPDA). However, theses responses were not sufficient and infection could not be prevented leading to hyphae colonization of moss tissues and plant decay. Pythium infection induced reactive oxygen species production and caused cell death of moss tissues. Taken together, these data indicate that Pythium infection activates in P. patens common responses to those previously characterized in flowering plants. Microscopic analysis also revealed intracellular relocation of chloroplasts in Pythium-infected tissues toward the infection site. In addition, OPDA, JA and its methyl ester methyl jasmonate induced the expression of PAL. Our results show for the first time JA and OPDA accumulation in a moss and suggest that this defense pathway is functional and has been maintained during the evolution of plants.






Similar content being viewed by others
Abbreviations
- P. patens :
-
Physcomitrella patens
- P. irregulare :
-
Pythium irregulare
- P. debaryanum :
-
Pythium debaryanum
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- OPDA:
-
12-Oxo-phytodienoic acid
- SA:
-
Salicylic acid
- ET:
-
Ethylene
- ROS:
-
Reactive oxygen species
- LOX:
-
Lipoxygenase
- PAL:
-
Phenylalanine ammonia-lyase
- CHS:
-
Chalcone synthase
References
Adie BA, Pérez-Pérez J, Pérez-Pérez MM, Godoy M, Sánchez-Serrano JJ, Schmelz EA, Solano R (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19:1665–1681
Albrecht A, Heiser I, Baker R, Nemec S, Elstner EF, Osswald W (1998) Effects of the Fusarium solani toxin dihydrofusarubin on tobacco leaves and spinach chloroplasts. J Plant Physiol 153:462–468
Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K (2005) Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol 138:1058–1070
Benhamou N, Bélanger R (1998) Induction of systemic resistance to Pythium damping-off in cucumber plants by benzothiadiazole: ultrastructure and cytochemistry of the host response. Plant J 14:13–21
Campion C, Massiot P, Rouxel F (1997) Aggressiveness and production of cell-wall degrading enzymes by Pythium violae, Pythium sulcatum and Pythium ultimum, responsible for cavity spot on carrots. Eur J Plant Pathol 103:725–735
Chico JM, Chini A, Fonseca A, Solano R (2008) JAZ repressors set the rhythm in jasmonate signaling. Curr Opin Plant Biol 11:1–9
Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS (2007) Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol 176:275–287
Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Feussner I, Wasternack C (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53:275–297
Frank W, Ratnadewi D, Reski R (2005) Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 220:384–394
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Govrin E, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Gross P, Julius C, Schmelzer E, Hahlbrock K (1993) Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation in infected, cultured parsley cells. EMBO J 12:1735–1744
Hoch HC, Galvani CD, Szarowski DH, Turner JN (2005) Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia 97:580–588
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15:2503–2513
Jiang C, Schommer CK, Kim SY, Suh DY (2006) Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry 67:2531–2540
Kamoun S, Huitema E, Vleeshouwers VG (1999) Resistance to oomycetes: a general role for the hypersensitive response? Trends Plant Sci 4:196–200
Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by downy mildew fungus. Plant Cell 2:437–455
Lang D, Eisinger J, Reski R, Rensing SA (2005) Representation and high-quality annotation of the Physcomitrella patens transcriptome demonstrates a high proportion of proteins involved in metabolism in mosses. Plant Biol (Stuttg) 7:238–250
Lang D, Zimmer AD, Rensing SA, Reski R (2008) Exploring plant biodiversity: the Physcomitrella genome and beyond. Trends Plant Sci 13:542–549
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Loake G, Grant M (2007) Salicylic acid in plant defence—the players and protagonists. Curr Opin Plant Biol 10:466–472
López MA, Bannenberg G, Castresana C (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11:420–427
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8:532–540
Lucena MA, Romero-Aranda R, Mercado JA, Cuartero J, Valpuesta V, Quesada MA (2003) Structural and physiological changes in the roots of tomato plants over-expressing a basic peroxidase. Physiol Plant 118:422–429
Martin F (1994) Pythium. In: Komoto K, Singh US, Singh RP (eds) Pathogenesis and host specificity in plant diseases: histopathological, biochemical, genetic and molecular bases. Pergamon Press, Oxford, pp 17–36
Mellersh DG, Foulds IV, Higgins VJ, Heath MC (2002) H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J 29:257–268
Montesano M, Scheller HV, Wettstein R, Palva ET (2004) Down-regulation of photosystem I by Erwinia carotovora-derived elicitors correlates with H2O2 accumulation in chloroplasts of potato. Mol Plant Pathol 5:115–123
Morel JB, Dangl JL (1997) The hypersensitive response and the induction of cell death in plants. Cell Death Differ 4:671–683
Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K, Kohara Y, Hasebe M (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100:8007–8012
O’Donnell PJ, Schmelz E, Block A, Miersch O, Wasternack C, Jones JB, Klee HJ (2003) Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiol 133:1181–1189
Pandey AK, Ger MJ, Huang HE, Yip MK, Zeng J, Feng TY (2005) Expression of the hypersensitive response-assisting protein in Arabidopsis results in harpin-dependent hypersensitive cell death in response to Erwinia carotovora. Plant Mol Biol 59:771–780
Pilloff RK, Devadas SK, Enyedi A, Raina R (2002) The Arabidopsis gain-of-function mutant Dll1 spontaneously develops lesions mimicking cell death associated with disease. Plant J 30:61–70
Ponce de León I, Oliver JP, Castro A, Gaggero C, Bentancor M, Vidal S (2007) Erwinia carotovora elicitors and Botrytis cinerea activate defense responses in Physcomitrella patens. BMC Plant Biol 8:52
Rensing SA, Lang D, Zimmer AD et al (2008) The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319:64–69
Saavedra L, Svensson J, Carballo V, Izmendi D, Welin B, Vidal S (2006) A dehydrin gene in Physcomitrella patens is required for salt and osmotic stress tolerance. Plant J 45:237–249
Sambrook J, Fitsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor
Sato Y, Wada M, Kadota A (2001) External Ca(2+) is essential for chloroplast movement induced by mechanical stimulation but not by light stimulation. Plant Physiol 127:497–504
Schaefer DG (2002) A new moss genetics: targeted mutagenesis in Physcomitrella patens. Annu Rev Plant Biol 53:477–501
Schaefer D, Zryd JP, Knight CD, Cove DJ (1991) Stable transformation of the moss Physcomitrella patens. Mol Gen Genet 226:418–424
Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39:790–808
Senger T, Wichard T, Kunze S, Gobel C, Lerchl J, Pohnert G, Feussner I (2005) A multifunctional lipoxygenase with fatty acid hydroperoxide cleaving activity from the moss Physcomitrella patens. J Biol Chem 280:7588–7596
Staswick PE, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15:747–754
Stumpe M, Bode J, Göbel C, Wichard T, Schaaf A, Frank W, Frank M, Reski R, Pohnert G, Feussner I (2006) Biosynthesis of C9-aldehydes in the moss Physcomitrella patens. Biochim Biophys Acta 1761:301–312
Sun MM, Li LH, Xie H, Ma RC, He YK (2007) Differentially expressed genes under cold acclimation in Physcomitrella patens. J Biochem Mol Biol 30:986–1001
Torres MA, Jones JD, Dangl JL (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141:373–378
Toth IK, Bell KS, Holeva MC, Birch PRJ (2003) Soft rot erwiniae: from genes to genomes. Mol Plant Pathol 4:17–30
van Kan JAL (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11:247–253
van Loon LC, Geraats BP, Linthorst HJ (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11:184–191
Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 9:7209–7214
Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54:455–468
Wichard T, Göbel C, Feussner I, Pohnert G (2004) Unprecedented lipoxygenase/hydroperoxide lyase pathways in the moss Physcomitrella patens. Angew Chem Int Ed 44:158–161
Acknowledgments
We gratefully acknowledge Roberto Solano (Centro Nacional de Biotecnología-CSIC, Spain) and Alicia Arias (IIBCE, Uruguay) for their generous gift of Pythium irregulare (Centro Nacional de Biotecnología-CSIC; Madrid, Spain) and Pythium debaryanum (collection INIA; Las Brujas, Uruguay), respectively. We thank Harvey C. Hoch (NYSAES, Cornell University, USA) for supplying us with the dye solophenyl flavine 7GFE500. We also thank Marcos Montesano (Facultad de Ciencias, UdelaR, Uruguay), Roberto Solano and Sabina Vidal (Facultad de Ciencias, UdelaR, Uruguay) for helpful discussion and critical reading of the manuscript. The authors thank DINACYT (Fondo Clemente Estable 9008) Uruguay, ANII (Fondo Clemente Estable FCE2007_376) Uruguay and UdelaR Uruguay/CSIC Spain (Joint project) for financial support. A. Castro was supported by a PEDECIBA fellowship, Uruguay. The Physcomitrella ESTs were obtained from the RIKEN Biological Research Center, Tsukuba, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors Juan Pablo Oliver and Alexandra Castro contributed equally to this work.
Rights and permissions
About this article
Cite this article
Oliver, J.P., Castro, A., Gaggero, C. et al. Pythium infection activates conserved plant defense responses in mosses. Planta 230, 569–579 (2009). https://doi.org/10.1007/s00425-009-0969-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0969-4