Abstract
Aging is a biological process that affects most cells, organisms and species. Telomeres have been postulated as a universal biological clock that shortens in parallel with aging in cells. Telomeres are located at the end of the chromosomes and consist of an evolutionary conserved repetitive nucleotide sequence ranging in length from a few hundred base pairs in yeast till several kilo base pairs in vertebrates. Telomeres associate with shelterin proteins and form a complex protecting the chromosomal deoxyribonucleic acid (DNA) from recognition by the DNA damage-repair system. Due to the “end-replication problem” telomeres shorten with each mitotic cycle resulting in cumulative telomere attrition during aging. When telomeres reach a critical length the cell will not further undergo cell divisions and become senescent or otherwise dysfunctional. Telomere shortening has not only been linked to aging but also to several age associated diseases, including tumorigenesis, coronary artery disease, and heart failure. In the current review, we will discuss the role of telomere biology in relation to aging and aging associated diseases.
Similar content being viewed by others
“Death takes place because a worn-out tissue cannot forever renew itself, and because a capacity for increase by means of cell division is not everlasting but finite”
A. Weismann. Clarendon, Oxford 1881
A brief historical perspective
Telomeres are special deoxyribonucleic acid (DNA) structures that “cap” the ends of our chromosomes in conjunction with specialized proteins, the telomere-shelterin complex. This complex protects the chromosomes from erosion and end-to-end fusion. The term telomere originates from the Greek telos, which means “end” and meros which means “part”. The existence of these end-parts of the chromosomes was first suggested in 1938 by Muller [60]. In 1961, Hayflick undermined a major paradigm of his time by providing convincing evidence that primary cells were not immortal, but could undergo only a limited number of cell divisions. This phenomenon, currently being referred to as the Hayflick limit [38], predicts the existence of an internal counting mechanism within the cell. Olovnikov, a Russian researcher, was the first who linked the end of the chromosomes to the cell cycle arrest described by Leonard Hayflick [66]. The term “end-replication problem” describes the effect that linear chromosomes cannot replicate their terminal ends of the chromosome and consequently shorten at each mitotic cycle. The first identification of the sequence of the terminal end of the chromosome (the telomere) in the Tetrahymena was discovered by Elizabeth Blackburn and Joseph Gal in 1978 [6]. Ten years later, Robert Moyazis and colleagues revealed that the sequence of the human telomere consists of TTAGGG repeats [59]. Up to date, the sequence of many species and organisms have been established and we can conclude that the telomeres are an evolutionary well-conserved sequence [55] (Table 1). The next major breakthrough in telomere biology was the discovery of the reverse transcriptase telomerase by Carol Greider working as a postdoctoral student at the laboratory of Elizabeth Blackburn in 1985 [31]. In contrast to DNA-polymerase, telomerase is capable of elongating the telomeres. Bypassing the end-replication problem for germ cells is essential to maintain telomere length for offspring. In 1997 Maria Blasco in the lab of Carol Greider created a telomerase deficient mouse, which had inactive telomerase and consequently reduced telomere length in each following generation [8]. The most striking characteristic of the telomeres in somatic cells is the shortening with age and in cell culture the telomere length is directly linked to the replicative capacity. In this review, we will discuss the function of the telomeres and will focus on the importance of telomere biology in normal aging and in pathology.
Telomere; structure and T-loop
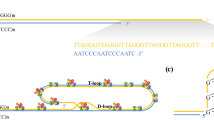
In vertebrates, the end of the chromosome, a G-rich strand, ends in a single strand extension of 75–200 bp, the G-tail (Fig. 1). In the nonmitotic phase of the cell cycle this G-tail is shielded by a crucial so-called telomere shelterin complex in which the telomere binds internally by forming two internal loops, the D-loop and the T-loop [33]. The telomere shelterin complex is designed to protect the chromosomal ends from erosion and end-to-end fusion [21] and is formed by different proteins associated with the telomeres, such as telomeric repeat binding factor 1 (TRF1) and 2 (TRF2) that can bind to double stranded telomere DNA. Another telomere associated protein, protein protection of telomeres 1 (POT1), can bind directly to single stranded DNA, and it is suggested that POT1 binding to the 3’overhang is important for forming the D-loop. Other proteins involved in the shelterin complex that are recruited by TRF1 and TRF2 are repressor activator protein 1 (Rap1), TPP1, and TRF1-interacting nuclear factor 2 (TIN2) [21] (Fig. 2). Telomere shortening will result in destabilization of the chromosomes and an inability to recruit the proteins of the shelterin complex. As a result, the T-loop cannot be formed as easily and the chromosome ends will be left uncapped. This is a situation that resembles double stranded DNA breaks, and presents a highly unstable cellular state that may lead to activation of the p53 or p16ink4a pathway and eventually can result in senescence or apoptosis [20].
Telomeres are located at the very final ends of the linear chromosomes and consists of TTAGGG repeats in vertebrates. Reproduced with permission [42]
Telomere length and aging
In contrast to the similarity of the sequence, the telomere length is highly variable among species, within species, within an organism, and even between chromosomes. In a study that evaluated telomere length in different organs from humans of different age, telomere length varied between 8 and 15 kbp and was highly variable between organs from one subject [82]. This may be explained by variable telomere attrition rate—in humans it is estimated that telomere length shortens 30–150 bp per replication cycle in fibroblasts and lymphocytes [35, 97]. There is a high amount of variance in telomere length in humans. Already at birth, remarkable differences in telomere length are observed. In addition, females have longer telomeres than men and in African Americans telomeres generally are longer than in White Americans [41]. As there is no gender difference at birth, it is most likely due to differences in environmental factors such as differences in estrogens levels [48]. Strikingly, macaques have approximately the same life span as humans but have longer telomeres in addition to a longer subtelomeric region [29]. Telomere attrition rate is not stable for each chromosome, in human cells the chromosomes 17p, 13p, and 19p have been identified as being shorter compared to the other chromosomes [30, 52].
In humans, telomere length is measured extensively in leukocytes in relation to aging and various pathologies. Leukocyte telomere length obviously has the advantage of being relatively easily obtained and processing is a relatively simple process. Telomere length in leukocytes is highly variable among individuals and decreases throughout life. Especially large differences develop the first few years after birth [70] after which telomere length are relatively stable throughout childhood, preadolescent, and adolescent years. Eventually, telomere length attrition increases at very old age (Fig. 3). An important aging hypothesis is that telomere attrition increases at the onset of disease. Therefore, telomere length of the leukocytes could be a good marker for disease. Telomere length in different aging diseases is discussed later in the review. The major disadvantage of using leukocyte telomere length is that it is a measure of the activity state of the immune system and one might argue that leukocyte telomere length is rather a representation of increased inflammation than of aging.
Telomere length in lymphocytes and granulocytes during human lifespan. Reproduced with permission from [4]
Most animal research on telomeres has been performed in rodents, especially on inbred mice and rat species that have highly variable telomere lengths. Laboratory rats have relatively long telomeres that vary between 20 and 100 kb and telomere length in mice is even more variable and can extend up to 150 kb (of the C57BL/6 mice) [17, 39]. In contrast, the outbred (wild type) mice strain Mus spretus has telomere length that is comparable to human cells. Comparing multiple mice strains showed that most mouse species do not have long telomeres, and long telomeres in mice strains originate from excessive breeding [39]. In rats, telomere length shortens with aging in several organs like kidney, liver, pancreas, and lungs. Research into telomere length from blood derived cells from multiple bird species with different life expectancies shows that telomere attrition rate is a better predictor of life expectancy than the age of the animal [37]. One remarkable animal is the Leach’s storm-petrel, a long lived bird species with a maximum observed lifespan of 36 years. Instead of telomere attrition, it is suggested that the telomere length increases during aging in this species [37]. The Leach’s storm-petrel has increased levels of telomerase activity in their bone marrow cells compared to other birds [36]. It is tempting to speculate that these animals have managed to increase their lifespan by dealing with telomere erosion. However, the assumption that absolute telomere length has an effect on life span is still elusive. For example, mice strains with longer telomeres do not seem to have an increased lifespan compared to mice strains with shorter telomeres. How these differences in telomere length affect lifespan are still unknown, the most accepted hypothesis is that the shortest telomeres are contributing most to the expected lifespan [40].
Quantifying telomere length
The most commonly used techniques to measure telomere length are Southern blot, polymerase-chain reaction (PCR) based techniques and in situ hybridization. Southern blotting or telomere restriction fragment analysis (TRF) is the traditional method and still considered the gold standard [22]. The telomeres are represented as smears, and the weight of the smear is representative for the average telomere length. The main disadvantage of this technique is the relatively high amounts of DNA which is required. This technique is therefore not feasible for determining telomere length in single cells, or for different chromosomes, or when DNA availability is limited. The real-time PCR-based method is relatively fast and only requires small amounts of genomic DNA. This technique is based on modified PCR primers to avoid primer-dimer amplification as much as possible [13]. The final measure will be a ratio telomere quantity divided by a reference gene quantity (T/S ratio) which is a relative measure, perfectly valid within a given population (as it will correctly rank subjects) but more difficult to compare between populations. The most recent advantage is the development of a multiplex assay in which both the telomere and the reference gene is targeted in a single well [14]. The PCR technique suffers the same disadvantage as the TRF method when considering single cell or specific chromosome analysis. The quantitative PCR technique has been widely used and accepted to estimate telomere length in large cohort studies [10, 15, 86, 93]. A specific modification of the previous techniques is single telomere length analysis, which uses Southern blotting techniques to separate PCR-amplified products, by combining specific primers and probes for the telomeres and the subtelomeric regions to measure telomere length per chromosome [5]. This technique is at the moment thought to be the most accurate telomere measurement, but it is a labor-intensive and technically challenging technique that can only be used for chromosomes from which the subtelomeric region is known. In situ hybridization techniques make it possible to visualize the telomeres in single cells. Quantitative fluorescence in situ hybridization (Q-FISH) uses a (CCCTAA)3 peptide nucleic acid probe to visualize the telomeres. In metaphase spreads, the telomeres are visible at the end of the chromosomes and they can be quantified also in single chromosomes [52]. An important variation on this technique is the flow fluorescence in situ hybridization, or Flow-FISH. By combining Q-FISH hybridization and flow cytometry analysis, it is possible to measure average telomere length in interphase cells in combination with standard flow cytometry antibodies to select the cell population of interest [71].
Telomere maintenance
Telomerase, a ribonucleoprotein complex that is composed of RNA and protein components, can elongate the telomere sequence in mammals and yeast by binding to the open end of the G-strand. Telomerase is highly expressed during embryonic development but its expression is suppressed within a few weeks after birth in most somatic cells. Highly proliferative cells maintain high levels of telomerase, like stem cells, progenitor cells, lymphocytes, skin keratinocytes, and cancer cells [27]. The major components of the active telomerase complex are telomerase reverse transcriptase (TERT), a telomerase RNA component (TERC, that is complementary to the telomere sequence) and dyskerin, which is a protein that binds to both TERT and TERC and increases stability of the complex [19, 32]. Elongation of the telomeres in mammals and yeast not depending on telomerase is called alternative lengthening of telomeres (ALT). In human tumors, it was discovered that cells negative for telomerase could also elongate their telomeres by a recombination mechanism [12]. Recombination takes place by binding of the ALT-associated promyelocytic leukemia bodies to the telomeres. Telomere elongation occurs heterozygous in these cells and ALT can best be recognized by the presence of both short and long telomeres.
The TERC -/- mouse has increased our knowledge on the importance of telomerase in aging and in the potential role of telomerase and telomere shortening in the different diseases [101]. As always, it is difficult to translate data from knockout models directly to human pathology, but especially in premature aging diseases the TERC -/- mice show great overlap with human disease [7].
Telomere biology and cellular senescence
Primary cells in culture are not immortal. As Hayflick demonstrated in 1961, cells stop dividing after a certain number of passages and become sedative [38], a phenotype also known as replicative senescence. The senescent phenotype is accompanied by changes in morphology, gene expression, and proteins. Beta galactosidase staining is frequently used to identify senescent cells and is associated with changes in p53, p16, and p21 expression [26, 62, 78]. There are multiple stimuli that can induce senescence; telomere shortening, DNA damage, and induction of oncogenic or tumor suppressor signals [16, 47, 79]. Induction of cellular senescence is an important suppressor of tumorigenesis [79, 103]. Although telomere attrition might not be primarily involved in the acute induction of senescence [16], the cumulative burden of oxidative stress and the cumulative telomere attrition might increase to likelihood of a cell to enter senescence [47]. Telomere attrition through replication and accumulation of DNA damage can result in an increase of senescent cells in different tissues and organs eventually resulting in decreased function and pathology. Telomere shortening has been implicated as one of the major mechanisms of replicative senescence [97]. The end-replication problem accounts for a loss of ∼100 bp telomere length at each population doubling. On average cells are estimated to reach senescence after ∼ 50 population doublings. This is a bit earlier than predicted by the end-replication problem alone. It is likely that the state of the telomere and the presence of the proteins involved in forming the shelterin complex are important cofactors associated with the induction of senescence [46]. For example, there is ample evidence that disruption of the telomere binding proteins results in early senescence. In primary human fibroblasts, TRF2 inhibition induces a p53- and retinoblastoma-dependent senescent phenotype [46, 94]. Likewise, inhibiting POT1 by RNA interference led to the disappearance of the telomeres single-stranded overhangs and induced apoptosis, chromosomal instability, and senescence [104].
Telomere biology in stem cells
Stem cells and progenitor cells have an important role in maintaining tissue homeostasis by replenishing (senescent, apoptotic) cells and repairing damage that occurs throughout life. Exhaustion of the stem cell or progenitor cell pool has been considered an important factor in the aging process of an organism [67]. One of the hallmarks of stem cells is their telomerase activity and stable length of their telomeres [63, 87]. Stem cells reside in different compartments throughout the body. In mice it has been shown that there exists a large difference in telomere length among the different compartments. The longest telomeres have been observed in skin, small intestine, cornea, testis, and brain compartments [28]. Although it seems that stem cells have stable telomeres by its increased telomerase activity, it does not make them invulnerable to telomere erosion. Clonal expansion after damage or in a disease state could induce telomere erosion that ultimately could induce senescence and an exhaustion of the stem cell pool. This hypothesis is supported by data from bone marrow-derived cells exhibiting a decreased migratory capacity and significant telomere shortening in patients with coronary artery disease [80]. The best characterized stem cells are the hematopoietic stem cells (HSC) which continuously replenish the hematopoietic cell lineages. HSC have been reported to have shorter telomeres compared to fetal liver and cord blood derived cells [96]. Recent data have also suggested a reduction of telomere length of HSC during aging [99].
Telomere biology and premature aging
Some human disorders associated with shorter telomere length originate from defective telomerase function or mutations in the DNA repair system. For example, Dyskeratosis congenita (DC) is a human premature aging syndrome linked to mutations in the telomerase complex resulting in decreased telomerase stability and shorter telomeres [57]. Patients with DC develop numerous different pathologies, including short stature, hypogonadism, infertility, bone marrow failure, skin defects, hematopoietic defects, and premature death. In addition, these patients have an increased susceptibility to develop malignancies. Another human disease example that involves a telomerase mutation is aplastic anemia. Subjects with aplastic anemia experience accelerated telomere shortening and die young [51]. Some diseases originating from mutations in genes of the DNA repair system also result in a phenotype characterized by accelerated telomere shortening and premature aging. Example genes include the Ataxia telangiectasia (ATM), Werner syndrome, Bloom syndrome, and Fanconi anemia genes. Most of these DNA repair genes also have a role in telomere biology. Mouse knockout models for these proteins do not always result in the same characteristics as the human disease. It has been suggested that the remarkable longer telomere length in mice might provide an explanation for these discrepancies. Combining DNA repair KO mice for Werner, Bloom, and ATM syndrome with the TERC -/- mice indeed resulted into a phenotype with characteristics that more closely resembled the expected pathology in humans [7].
Telomeres and aging associated diseases
The debate on how telomere biology affects life span is ongoing, but a link between telomere length and mortality has been established. In addition, numerous associations between aging-associated diseases and telomere length have been reported [15]. Telomere length could be considered as a biological parameter that intertwines replicative history and exposure to environmental stress. Human life span is highly dependent on the development of aging associated diseases especially cancer and cardiovascular disease.
Cancer
Tumorigenesis is a major factor influencing life expectancy in long-lived species. Shorter telomere length is also a risk factor for the development of cancer [102]. Progressive shortening of the telomeres will lead to activation of the DNA damage response [34, 102]. In the normal situation this will result in activation of ataxia telangiectasia mutated (ATM) and ataxia telangiectasia- and Rad3-related (ATR), and the associated downstream factors including CHK1, CHK2, and phosphorylation of p53. In the setting of a competent p53 pathway, senescence or apoptosis will be initiated and tumorigenesis inhibited [25]. However, when the p53 pathway is inadequate tumorigenesis is no longer inhibited in the presence of telomere dysfunction [25, 34, 69]. In addition, 80 to 90% of all tumors express telomerase or have a form of alternative telomere lengthening [77]. Clinical data revealed that telomere length (measured in lymphocytes) is shorter in subjects with different types of cancer, including cancers of the head and neck, breast, bladder, prostate, lung, and kidney [102].
Cardiovascular risk factors
Next to cancer, cardiovascular disease and its risk factors are the major contributors to the population’s disease burden during aging. For example, the presence of diabetes has been linked to reduced telomere length [1, 44, 75]. In the Framingham Heart Study, even a subclinical presence of insulin resistance was associated with reduced telomere length [24]. Hypertension and the responsiveness to angiotensin are related to outcome in humans [88, 92]. The number of genetic variants influencing the development of hypertension is only small [61]. However, a role for telomeres has been suggested, normotensive persons with short telomeres were more susceptible to develop hypertension and hypertensive subjects with short telomeres were more susceptible to develop atherosclerosis [105]. Interestingly, even subclinical activation of the renin-angiotensin system (RAS) has been associated with shorter telomeres [24, 95]. A final example is cigarette smoking, a strong risk factor for the development of coronary heart disease [2]. Smoking negatively affects telomere length [54, 58, 84], possibly due to mechanisms involving oxidative stress [98].
Atherosclerosis
Endothelial dysfunction is recognized as one of the earliest events of atherogenesis [3] and is associated with classical risk factors or risk markers including cholesterol and inflammatory markers [85, 91], which can be modified by pharmacological treatment [92]. The endothelial and smooth muscle cells in the vessel, which are most susceptible to develop atherosclerosis are highly proliferative and are subjected to stress by increasing mean arterial pressure, increased cholesterol, and increased oxidative stress. This results in an increased susceptibility for senescence [45, 53]. Indeed senescent-positive endothelial cells can be found in almost any atherosclerotic plaque [56] and an association with shorter telomeres in atherosclerotic plaques has been established [64]. The first clinical study linking coronary artery disease to telomere length dates back to 2001 [72]. This ground breaking study suggested telomeres of circulating white blood cells to be approximately 300 bp shorter in patients with coronary artery disease compared to controls. The authors estimated that this telomere differences resembles an age difference of almost 9 years [72]. Further and larger scale studies confirmed these findings and extended it to premature atherosclerosis and ischemic heart failure [9, 10, 15, 93]. For example, the West of Scotland Primary Prevention Study (WOSCOPS) observed that subjects in the middle or lower tertile of telomere length were at greater risk to experience a clinical manifestation of coronary heart disease than persons with longer telomeres [10]. The WOSCOPS data also suggested that the use of statins was more beneficial for the patients with the shortest telomeres [10]. Apparently patients that are protected by longer telomeres addition of statin treatment did not result in additional protection. Interestingly, telomeres of offspring from subjects with coronary artery disease already have shorter telomeres compared to offspring from parents without atherosclerosis [11]. This might explain part of the heritability of coronary artery disease next to other genetic factors [73, 83].
Heart failure
Chronic heart failure (CHF) is the main cardiovascular discharge diagnosis in the United States [23]. In particular, after the necessity of hospital admission, CHF is associated with a high mortality rate [43]. Recent clinical trials have not added much to the prognosis, and the search for new strategies is intensive [49, 89, 90, 100]. Although in general, the incidence and prevalence of CHF steeply increases with aging, there exists a striking variability in the susceptibility, age of onset and pace of progression. This variability cannot completely be attributed to the presence of conventional risk factors and recent evidence is suggesting a role for telomere biology [74]. Endomyocardial biopsies from patients with heart failure have demonstrated that diseased hearts are characterized by shorter telomeres, increased cellular senescence, and cell death [18]. It has been estimated that telomere length is reduced by as much as 25% in failing hearts compared to nonfailing hearts [65]. Also, the telomere length in leukocytes of subjects with heart failure are significantly shorter compared to age and gender balanced controls [86]. In this study, the severity of heart failure symptoms was also associated with the degree of telomere shortening. Furthermore, cardiac function as measured by ejection fraction in general has been associated with telomere length [81]. One standard deviation of longer telomere length was associated with a 5% higher left ventricular ejection fraction. In these elderly subjects, telomere length alone accounted for 12% in the observed variability of ejection fraction. Renal function impairment relates to even worse outcome in patients with CHF. Shorter telomere length in CHF is also associated with decreased renal function, possibly due to drop-out of functional nephrons [93].
Conclusions and future perspectives
Telomere biology is involved in biological aging and disease processes. Experimental evidence suggests that telomere shortening, uncapping, and cellular senescence results in an “aging” phenotype [46]. The exhaustion of progenitor cells and the cumulating of senescent cells might explain the decline in organ function associated with aging. Shorter telomere length has been associated with several age associated diseases, including cancer, diabetes, atherosclerosis, and heart failure. To gain more insights in the role of telomere biology in the aging process of humans, we are still in need of large population based cohorts with telomere length and telomerase activity measurements at multiple time-points. If telomere biology can be proven to be causally involved in the development and progression of these age-associated diseases, it will pave the way for new therapeutic or preventive strategies. For example, telomerase or telomere length could be targeted in the emerging stem cell therapies for organ dysfunctions.
References
Adaikalakoteswari A, Balasubramanyam M, Mohan V (2005) Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med 22:1151–1156
Ambrose JA, Barua RS (2004) The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43:1731–1737
Asselbergs FW, van der Harst P, Jessurun GA, Tio RA, van Gilst WH (2005) Clinical impact of vasomotor function assessment and the role of ACE-inhibitors and statins. Vascul Pharmacol 42:125–140
Aubert G, Lansdorp PM (2008) Telomeres and aging. Physiol Rev 88:557–579
Baird DM, Rowson J, Wynford-Thomas D, Kipling D (2003) Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet 33:203–207
Blackburn EH, Gall JG (1978) A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol 120:33–53
Blasco MA (2005) Telomeres and human disease: ageing, cancer, and beyond. Nat Rev Genet 6:611–622
Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW (1997) Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25–34
Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ (2003) White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 23:842–846
Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland primary prevention study: a nested case-control study. Lancet 369:107–114
Brouilette SW, Whittaker A, Stevens SE, van der Harst P, Goodall AH, Samani NJ (2008) Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart 94:422–425
Bryan TM, Reddel RR (1997) Telomere dynamics and telomerase activity in in vitro immortalised human cells. Eur J Cancer 33:767–773
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47
Cawthon RM (2009) Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 37:e21
Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA (2003) Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361:393–395
Chen QM, Prowse KR, Tu VC, Purdom S, Linskens MH (2001) Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts. Exp Cell Res 265:294–303
Cherif H, Tarry JL, Ozanne SE, Hales CN (2003) Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res 31:1576–1583
Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P (2003) Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93:604–613
Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR (2007) Protein composition of catalytically active human telomerase from immortal cells. Science 315:1850–1853
d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426:194–198
de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110
de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE (1990) Structure and variability of human chromosome ends. Mol Cell Biol 10:518–527
DeFrances CJ, Cullen KA, Kozak LJ (2007) National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 13:1–209
Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A (2006) Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 5:325–330
Deng Y, Chan SS, Chang S (2008) Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8:450–458
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92:9363–9367
Flores I, Benetti R, Blasco MA (2006) Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol 18:254–260
Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA (2008) The longest telomeres: a general signature of adult stem cell compartments. Genes Dev 22:654–667
Gardner JP, Kimura M, Chai W, Durrani JF, Tchakmakjian L, Cao X, Lu X, Li G, Peppas AP, Skurnick J, Wright WE, Shay JW, Aviv A (2007) Telomere dynamics in macaques and humans. J Gerontol A Biol Sci Med Sci 62:367–374
Graakjaer J, Bischoff C, Korsholm L, Holstebroe S, Vach W, Bohr VA, Christensen K, Kolvraa S (2003) The pattern of chromosome-specific variations in telomere length in humans is determined by inherited, telomere-near factors and is maintained throughout life. Mech Ageing Dev 124:629–640
Greider CW, Blackburn EH (1985) Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405–413
Greider CW, Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51:887–898
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T (1999) Mammalian telomeres end in a large duplex loop. Cell 97:503–514
Hackett JA, Greider CW (2002) Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene 21:619–626
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Haussmann MF, Winkler DW, Huntington CE, Nisbet IC, Vleck CM (2007) Telomerase activity is maintained throughout the lifespan of long-lived birds. Exp Gerontol 42:610–618
Haussmann MF, Winkler DW, O'Reilly KM, Huntington CE, Nisbet IC, Vleck CM (2003) Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc Biol Sci 270:1387–1392
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Hemann MT, Greider CW (2000) Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res 28:4474–4478
Hemann MT, Strong MA, Hao LY, Greider CW (2001) The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107:67–77
Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A (2008) Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 7:451–458
Huzen J, van Veldhuisen DJ, van Gilst WH, van der Harst P (2008) Telomeres and biological ageing in cardiovascular disease. Ned Tijdschr Geneeskd 152:1265–1270
Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst WH, Lok DJ, Dunselman PH, Tijssen JG, Hillege HL, van Veldhuisen DJ (2008) Effect of moderate or intensive disease management program on outcome in patients with heart failure: coordinating study evaluating outcomes of advising and counseling in heart failure (COACH). Arch.Intern.Med 168:316–324
Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram JH, Aviv A (1998) Shortened telomere length in white blood cells of patients with IDDM. Diabetes 47:482–486
Johansson B (1984) Cellular senescence and atherosclerosis. Med Hypotheses 14:115–124
Karlseder J, Smogorzewska A, de Lange T (2002) Senescence induced by altered telomere state, not telomere loss. Science 295:2446–2449
Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117:2417–2426
Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M (1999) Estrogen activates telomerase. Cancer Res 59:5917–5921
Lipsic E, Westenbrink BD, van der Meer P, van der Harst P, Voors AA, van Veldhuisen DJ, Schoemaker RG, van Gilst WH (2008) Low-dose erythropoietin improves cardiac function in experimental heart failure without increasing haematocrit. Eur.J.Heart Fail 10:22–29
Lorite P, Carrillo JA, Palomeque T (2002) Conservation of (TTAGG)n telomeric sequences among ants (Hymenoptera, Formicidae). J Hered 93:282–285
Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ (2004) Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood 104:3936–3942
Martens UM, Zijlmans JM, Poon SS, Dragowska W, Yui J, Chavez EA, Ward RK, Lansdorp PM (1998) Short telomeres on human chromosome 17p. Nat Genet 18:76–80
Martin GM, Sprague CA (1972) Clonal senescence and atherosclerosis. Lancet 2:1370–1371
McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I (2007) Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 16:815–819
Meyne J, Ratliff RL, Moyzis RK (1989) Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A 86:7049–7053
Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I (2002) Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105:1541–1544
Mitchell JR, Wood E, Collins K (1999) A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402:551–555
Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG (2006) Telomere shortening in smokers with and without COPD. Eur Respir J 27:525–528
Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR (1988) A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A 85:6622–6626
Muller HJ (1938) The remaking of chromosomes. Collecting Net 13:15
Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB (2009) Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41:666–676
Oeseburg H, Iusuf D, van der Harst P, van Gilst WH, Henning RH, Roks AJ (2009) Bradykinin protects against oxidative stress-induced endothelial cell senescence. Hypertension 53:417–422
Oeseburg H, Westenbrink BD, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P (2007) Can critically short telomeres cause functional exhaustion of progenitor cells in postinfarction heart failure? J Am Coll Cardiol 50:1911–1912
Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M (2004) Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol 24:546–550
Oh H, Wang SC, Prahash A, Sano M, Moravec CS, Taffet GE, Michael LH, Youker KA, Entman ML, Schneider MD (2003) Telomere attrition and Chk2 activation in human heart failure. Proc Natl Acad Sci U S A 100:5378–5383
Olovnikov AM (1971) Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR 201:1496–1499
Rando TA (2007) The immortal strand hypothesis: segregation and reconstruction. Cell 129:1239–1243
Richards EJ, Ausubel FM (1988) Isolation of a higher eukaryotic telomere from Arabidopsis thaliana. Cell 53:127–136
Rudolph KL, Millard M, Bosenberg MW, DePinho RA (2001) Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet 28:155–159
Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM (1999) Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190:157–167
Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM (1998) Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol 16:743–747
Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH (2001) Telomere shortening in atherosclerosis. Lancet 358:472–473
Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H (2007) Genomewide association analysis of coronary artery disease. N Engl J Med 357:443–453
Samani NJ, van der Harst P (2008) Biological ageing and cardiovascular disease. Heart 94:537–539
Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA (2006) Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 29:283–289
Shampay J, Szostak JW, Blackburn EH (1984) DNA sequences of telomeres maintained in yeast. Nature 310:154–157
Shay JW, Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33:787–791
Shelton DN, Chang E, Whittier PS, Choi D, Funk WD (1999) Microarray analysis of replicative senescence. Curr Biol 9:939–945
Sherr CJ, McCormick F (2002) The RB and p53 pathways in cancer. Cancer Cell 2:103–112
Spyridopoulos I, Erben Y, Brummendorf TH, Haendeler J, Dietz K, Seeger F, Kissel CK, Martin H, Hoffmann J, Assmus B, Zeiher AM, Dimmeler S (2008) Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol 28:968–974
Starr JM, McGurn B, Harris SE, Whalley LJ, Deary IJ, Shiels PG (2007) Association between telomere length and heart disease in a narrow age cohort of older people. Exp Gerontol 42:571–573
Takubo K, Izumiyama-Shimomura N, Honma N, Sawabe M, Arai T, Kato M, Oshimura M, Nakamura K (2002) Telomere lengths are characteristic in each human individual. Exp Gerontol 37:523–531
Tregouet DA, Konig IR, Erdmann J, Munteanu A, Braund PS, Hall AS, Grosshennig A, Linsel-Nitschke P, Perret C, DeSuremain M, Meitinger T, Wright BJ, Preuss M, Balmforth AJ, Ball SG, Meisinger C, Germain C, Evans A, Arveiler D, Luc G, Ruidavets JB, Morrison C, van der Harst P, Schreiber S, Neureuther K, Schafer A, Bugert P, El Mokhtari NE, Schrezenmeir J, Stark K, Rubin D, Wichmann HE, Hengstenberg C, Ouwehand W, Ziegler A, Tiret L, Thompson JR, Cambien F, Schunkert H, Samani NJ (2009) Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet 41:283–285
Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD (2005) Obesity, cigarette smoking, and telomere length in women. Lancet 366:662–664
van der Harst P, Asselbergs FW, Buikema H, Voors AA, van Veldhuisen DJ, van Gilst WH (2006) Effects of C-reactive protein and cholesterol on responsiveness in vitro of the internal thoracic artery to angiotensin II in patients having coronary artery bypass grafting. Am J Cardiol 98:751–753
van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ (2007) Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol 49:1459–1464
van der Harst P, van Veldhuisen DJ, Samani NJ (2008) Expanding the concept of telomere dysfunction in cardiovascular disease. Arterioscler Thromb Vasc Biol 28:807–808
van der Harst P, Volbeda M, Voors AA, Buikema H, Wassmann S, Bohm M, Nickenig G, van Gilst WH (2004) Vascular response to angiotensin II predicts long-term prognosis in patients undergoing coronary artery bypass grafting. Hypertension 44:930–934
van der Harst P, Voors AA, van Gilst WH, Bohm M, van Veldhuisen DJ (2006) Statins in the treatment of chronic heart failure: a systematic review. PLoS.Med 3:e333
van der Harst P, Voors AA, van Gilst WH, Bohm M, van Veldhuisen DJ (2006) Statins in the treatment of chronic heart failure: biological and clinical considerations. Cardiovasc Res 71:443–454
van der Harst P, Voors AA, Volbeda M, Buikema H, van Veldhuisen DJ, van Gilst WH (2006) Usefulness of preoperative C-reactive protein and soluble intercellular adhesion molecule-1 level for predicting future cardiovascular events after coronary artery bypass grafting. Am J Cardiol 97:1697–1701
van der Harst P, Wagenaar LJ, Buikema H, Voors AA, Plokker HW, Morshuis WJ, Six AJ, Boonstra PW, Nickenig G, Wassmann S, van Veldhuisen DJ, van Gilst WH (2005) Effect of intensive versus moderate lipid lowering on endothelial function and vascular responsiveness to angiotensin II in stable coronary artery disease. Am J Cardiol 96:1361–1364
van der Harst P, Wong LS, de Boer RA, Brouilette SW, van der Steege G, Voors AA, Hall AS, Samani NJ, Wikstrand J, van Gilst WH, van Veldhuisen DJ (2008) Possible association between telomere length and renal dysfunction in patients with chronic heart failure. Am J Cardiol 102:207–210
van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92:401–413
Vasan RS, Demissie S, Kimura M, Cupples LA, Rifai N, White C, Wang TJ, Gardner JP, Cao X, Benjamin EJ, Levy D, Aviv A (2008) Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation 117:1138–1144
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM (1994) Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A 91:9857–9860
Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB (1993) Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 52:661–667
Von Zglinicki T, Pilger R, Sitte N (2000) Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med 28:64–74
Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD (2009) Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One 4:e5846
Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, Koster J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG (2007) Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J 28:2018–2027
Wong LS, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P (2009) Telomere biology in cardiovascular disease: the TERC-/- mouse as a model for heart failure and ageing. Cardiovasc Res 81:244–252
Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR (2003) Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 95:1211–1218
Wynford-Thomas D (1999) Cellular senescence and cancer. J Pathol 187:100–111
Yang Q, Zheng YL, Harris CC (2005) POT1 and TRF2 cooperate to maintain telomeric integrity. Mol Cell Biol 25:1070–1080
Yang Z, Huang X, Jiang H, Zhang Y, Liu H, Qin C, Eisner GM, Jose P, Rudolph L, Ju Z (2009) Short telomeres and prognosis of hypertension in a Chinese population. Hypertension 53:639–645
Acknowledgements
This work was supported by Netherlands Heart Foundation (Grant 2006B140) and the Innovational Research Incentives Scheme program of the Netherlands Organization for Scientific Research (NWO VENI, grant 916.76.170 to P. van der Harst). P. van der Harst and R.A. de Boer are research fellows of the Netherlands Heart Foundation (grant 2006 T003 and 2007 T046, respectively) and the Interuniversitair Cardiologisch Instituut Nederland (ICIN).
Disclosures of interest
None
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Oeseburg, H., de Boer, R.A., van Gilst, W.H. et al. Telomere biology in healthy aging and disease. Pflugers Arch - Eur J Physiol 459, 259–268 (2010). https://doi.org/10.1007/s00424-009-0728-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0728-1