Abstract
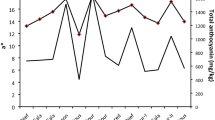
Changes in agronomic characters and the profile of various endogenous phytohormones during tuber development were studied in Dioscorea opposite (Chinese yam) cv. Guihuai 16. Tuber development exhibited a sigmoidal growth pattern according to the changes in tuber agronomic characters. The growth cycle of yam tuber could be divided into three stages: initiation stage, enlargement stage, and maturation stage. Moreover, the enlargement stage could be separated into three phases—slow growth phase, rapid growth phase, and late growth phase. Endogenous changes in phytohormones were associated with developmental changes in the tubers. The pulses of bioactive gibberellins (such as GA3 and GA4) were measured in tubers. The highest contents of GA3 and GA4 were reached 90 days after field planting, corresponding to the beginning of the rapid growth phase of tuber enlargement. Changes in trans-zeatin (tZ), jasmonic acid (JA), indole-3-acetic acid (IAA), and abscisic acid (ABA) levels were also observed, and seemed to be related to tuber enlargement at different phases. Continuous increases in JA and tZ contents accompanied tuber enlargement. Transient pulses of both IAA and ABA contents were also observed at the start of tuber rapid growth. Additionally, a second peak level of IAA was detected at the tuber maturation stage. These results suggest GAs play a key role at the beginning of the tuber rapid growth stage, and there is a close relationship between whole tuber enlargement and the contents of JA and tZ. Moreover, it is suggested that IAA and ABA also may be linked to the beginning of tuber rapid growth, and IAA also seems to be correlated to late tuber maturation.


Similar content being viewed by others
References
Abdala G, Castro G, Guinazu MM, Tizio R, Miersch O (1996) Occurrence of jasmonic acid in organs of Solanum tuberosum L. and its effect on tuberisation. Plant Growth Regul 19:139–143
Aighewi BA, Asiedu R, Maroya N, Balogun M (2015) Improved propagation methods to raise the productivity of yam (Dioscorea rotundata Poir.). Food Secur 7:823–834
Aksenova NP, Konstantinova TN, Golyanovskaya SA, Sergeeva LI, Romanov GA (2012) Hormonal regulation of tuber formation in potato plants. Russ J Plant Physiol 59(4):451–466
Alhassan AY, Mantell AH (1991) Manipulation of cultural factors to increase microtuber size and frequency in shoot cultures of food yam Dioscorea alata L. cv. Oriental Libson. In: Ofori F, Hahn SK (eds) Proceedings of the ninth symposium of the international society for tropical root crops. International Society for Horticultural Science, Accra, pp 342–348
Ammirato PV (1976) Hormonal control of tuber formation in cultured axillary buds of Dioscorea bulbifera and D. alata. Plant Physiol 57(suppl):66
Ammirato PV (1982) Growth and morphogenesis in cultures of the monocot yam Dioscorea. In: Fujiwara A (ed) Plant tissue culture. Maruzen, Tokyo, pp 169–170
Balcke GU, Handrick V, Bergau N, Fichtner M, Henning A, Stellmach H, Tissier A, Hause B, Frolov A (2012) An UHPLC–MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 8:47–58
Balogun MO (2005) Development of microtuber production and dormancy control protocols for yams (Dioscorea spp.) germplasm conservation. PhD Thesis, University of Ibadan, Nigeria, p 23
Bazabakana R, Wattiez R, Baucher M, Diallo B, Jaziri M (2003) Effect of jasmonic acid on developmental morphology during in vitro tuberization of Dioscorea alata (L.). Plant Growth Regul 40:229–237
Burkhill IH (1960) The oceanography and the evolution of the Dioscoreaceae, the family of the yams. Bot J Linn Soc 56:319–420
Chang KJ, Shiwachi H, Hayashi M (1995) Ecophysiological studies on growth and enlargement of tubers in yams (Dioscorea spp) II. Detection of effects of plant growth regulators on growth and enlargement of microtubers of yams. Jpn J Trop Agric 32(2):69–75
Chen FQ, Fu Y, Wang DL, Gao X, Wang L (2007) The effect of plant growth regulators and sucrose on the micropropagation and microtuberization of Dioscorea nipponica Makino. J Plant Growth Regul 26:38–45
Chen SW, Shiwachi H, Sanada A, Toyohara H (2010) Theobroxide and day-length effects on the growth of yam (Dioscorea spp). J ISSAAS 16(1):22–30
Craufurd PQ, Summerfield RJ, Asiedu R, Prasad PVV (2001) Dormancy in yams. Exp Agric 37:147–181
Ewing EE, Struik PC (2010) Tuber formation in potato: induction, initiation and growth. In: Janik J (ed) Horticultural reviews, vol 14. Wiley, Oxford, pp 65–82
Girardin O, Farah Z, Nindjin C, Otokore D, Dao D, Zaoungrana P, Stamp P, Escher F (1998a) The effects of cultural and chemical treatments on yam storage in Cote d’ivoire. Post Harvest Syst Newsl 3:3–4
Girardin O, Nindjin C, Farah Z, Escher F, Stamp P, Otokore D (1998b) Effect of storage system and sprout removal on post-harvest yam (Dioscorea spp.) fresh weight losses. J Agric Sci 130:329–336
GirardinO Nindjin C, Farah Z, Escher F, Stamp P, Otokore D (1998) Use of gibberellic acid to prolong dormancy and reduce losses during traditional storage of yams. J Sci Food Agric 77:172–178
Gukasyan IA, Golyanovskaya SA, Grishunina EV, Konstantinova TN, Aksenova NP, Romanov GA (2005) Effect of Rol transgenes, IAA, and kinetin on starch content and the size of starch granules in tubers of in vitro potato plants. Russ J Plant Physiol 52:809–813
Hannapel DJ, Chen H, Rosin FM, Banerjee AK, Davies PJ (2004) Molecular controls of tuberization. Am J Potato Res 81:263–274
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530
Ile IE, Crauford PQ, Battey NH, Asiedu R (2006) Phases of dormancy in yam tubers (Dioscorea rotundata). Ann Bot 97:497–504
Jasik J, de Klerk GJ (2006) Effects of methyl jasmonate on morphology and dormancy development in lily bulblets regenerated in vitro. J Plant Growth Regul 25:45–50
Jasik J, Mantell SH (2000) Effects of jasmonic acid and its methylesther on in vitro microtuberisation of three food yam (Dioscrorea) species. Plant Cell Rep 19:863–867
Javier A, Cela MJ, Bezerra LA, Arrom L, Juvany M, ller MM, Munne´-Bosch S (2014) Application of a rapid and sensitive method for hormonal and vitamin E profiling reveals crucial regulatory mechanisms in flower senescence and fruit ripening. J Plant Growth Regul 33:34–43
Kikuno H, Onjo M, Kusigemati K, Hayashi M (2002a) A relationship between the initiation of tuber enlargement and endogenous plant hormones in water yam (Discorea alata L.). Jpn J Trop Agric 46(1):39–46
Kikuno H, Onjo M, Kusigemati K, Hayashi M (2002b) A relationship between the initiation of tuber enlargement and changes in the content of endogenous jasmonic acid in water yam (Discorea alata L.). Jpn J Trop Agric 46(2):109–113
Kim SK, Lee SC, Choi HJ, Kim KU, Lee IJ (2003a) Bulbil formation and yield responses of Chinese yam to application of gibberellic acid, mepiquat chloride and trinexapac-ethyl. J Agron Crop Sci 189:255–260
Kim SK, Lee SC, Kim KM, Lee BH, Lee IJ (2003b) Possible residual effects of gibberellic acid and gibberellin biosynthesis inhibitors on sprouting, early bulbil formation and tuber yield in Chinese yam. J Agronomy Crop Sci 189:428–432
Kim SK, Lee SC, Shin DH, Jang SW, Nam JW, Park TS, Lee IJ (2003c) Quantification of endogenous gibberellins in leaves and tubers of Chinese yam, Dioscorea opposite Thunb. cv. Tsukune during tuber enlargement. Plant Growth Regu 39:125–130
Kim SK, Kim JT, Jang SW, Lee SC, Lee BH, Lee IJ (2005a) Exogenous effect of gibberellins and jasmonate on tuber enlargement of Dioscorea opposita. Agron Res 3:39–44
Kim SK, Shon TK, Park Y, Lee SC, Kim HY, Sohn EY, Jang SW, Choo YS, Kim KU, Lee IJ (2005b) Endogenous gibberellins in bulbils of Chinese yam during growth and storage. Plant Prod Sci 8(2):181–185
Kim SK, Choi HJ, Lee IJ, Kim HY (2010) Effect of combined indole acetic acid and mepiquat chloride onendogenous gibberellins and tuber growth in Chinese yam (Dioscorea opposite Thunb.). J Crop Sci Biotechnol 13(1):29–32
Koda Y (2006) Possible involvement of jasmonates in various morphogenic events. Physiol Plant 4:639–646
Koda Y, Kikuta Y (1991) Possible involvement of jasmonic acid in tuberisation of yam plants. Plant Cell Physiol 32:629–633
Koda Y, Okazawa Y (1983) Characteristic changes in levels of endogenous plant hormones in relation to the onset of potato tuberisation. Jpn J Crop Sci 52:592–597
Koda Y, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T (1998) Potato tuber-inducting activities of jasmonic acid and related compounds. Phytochemistry 30:1435–1438
Kuroha T, Kato H, Asami T, Yoshida S, Kamada H, Satoh S (2002) A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J Exp Bot 53:2193–2200
Liang RF, Li CZ, Zhang J, He LF, Wei BH, Gan XQ, He HY (2011) Changes of matter accumulation and relative enzymatic activity during yam tuber development. Acta Agron Sin 37(5):903–910
Malkawi A, Jensen BL, Langille AR (2007) Plant hormones isolated from ‘‘Katahdin’’ potato plant tissues and the influence of photoperiod and temperature on their levels in relation to tuber induction. J Plant Growth Regul 26:308–317
Matsumoto R, Kikuno H, Shiwachi H, Toyohara H, Takebayashi Y, Jikumaru Y, Kamiya Y (2013) Growth of vine cuttings and fluctuations of concentrations of endogenous plant hormones in water yam (Dioscorea alata L.). Trop Agric Dev 57(1):23–30
Ng SYC, Mantell SH (1996) Final report of ODA project R4886 (H) on technologies for germplasm conservation and distribution of pathogen-free Dioscorea yams to national root crop research programs, Wye College, IITA, SARI, p 73
Njoku E, Nwoke FIO, Okonkwo SNC, Oyolu C (1984) Pattern of growth and development in Dioscorea rotundata Poir. Trop Agric 61:17–19
Novàk O, Tarkowski P, Tarkowska D, Dolezal K, Lenobel R, Strnad M (2003) Quantitative analysis of cytokinins in plants by liquid chromatography-single-quadrupole mass spectrometry. Anal Chim Acta 480:207–218
Nováková L, Vlèková H (2009) A review of current trends and advances in modern bio-analytical methods: chromatography and sample preparation. Anal Chim Acta 656:8–35
Onwueme IC (1978) The tropical tuber crops -Yams, Cassava, Sweet potato and Coco yams. Wiley, Chichester, p 223
Ovono PO, Kevers C, Dommes J (2010) Tuber formation and development of Dioscorea cayenensis–Dioscorea rotundata complex in vitro effect of polyamines. In Vitro Cell Dev Biol 46:81–88
Pan X, Welti R, Wang X (2008) Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography tandem mass spectrometry. Phytochemistry 69:1773–1781
Prat S (2004) Hormonal and daylength control of potato tuberisation. In: Davies PJ (ed) Plant hormones, biosynthesis, signal transduction, action. Kluwer Academic Publishers, The Netherlands, pp 538–560
Ravnikar M, Zel J, Plaper I, Spacapan A (1993) Jasmonic acid stimulates shoot and bulb formation of garlic in vitro. J Plant Growth Regul 12:73–77
Rodríguez-Falcón M, Bou J, Prat S (2006) Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu Rev Plant Biol 57:151–180
Roumeliotis E, Visser RGF, Bachem CWB (2012) A crosstalk of auxin and GA during tuber development. Plant Signal Behav 7(10):1360–1363
Santos I, Salema R (2000) Promotion by jasmonic acid bulb formation in shoot cultures of Narcissus triandus. J Plant Growth Regul 12:133–138
Sarkar D (2008) The signal transduction pathways controlling in planta tuberization in potato: an emerging synthesis. Plant Cell Rep 27:1–8
Shiwachi H, Chang KJ, Hatashi M (1995) Ecological and morphological characterization andgeneral evaluation of the introduced yams (Dioscorea alata L.). Bull Fac Agric Kagoshima Univ 45:1–17
Shiwachi H, Onjo M, Hayashi M (1999) Comparison of ecological characters of water yam (Dioscore alata L.), Chinese yam (D. opposite Thunb) and Jinen-jo (D. japonica Thunb). Jpn J Trop Agric 43:149–156
Shiwachi H, Onjo M, Hayashi M (2000) Photoperiodic response of water yam (Dioscore alata L.), Chinese yam (D. opposite Thunb) and Jinen-jo (D. japonica Thunb). Jpn J Trop Agric 44:107–114
Shiwachi H, Ayankanmi T, Asiedu R (2002) Effect of day length on the development of tubers in yams (Dioscorea spp.). Trop Sci 42:162–170
Sun Q, Zhou G, Cai Y, Fan Y, Zhu X, Liu Y, He X, Shen J, Jiang H, Hu D, Pan Z, Xiang L, He G, Dong D, Yang J (2012) Transcriptome analysis of stem development in the tumourous stem mustard Brassica juncea var. tumida Tsen et Lee by RNA sequencing. BMC Plant Biol 12:53
Suttle JC (2000) The role of endogenous hormones in potato tuber dormancy. In: Viémont JD, Crabbe J (eds) Dormancy in plants from whole plant behaviour to cellular control. CABI Publishing, Wallingford, pp 211–226
Svačinová J, Novák O, Plačková L, Lenobel R, Holík J, Strnad M, Doležal K (2012) A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: pipette tip solid-phase extraction. Plant Methods 8:17–31
Tamogami S, Kodama O (1998) Quantification of amino acid conjugates of jasmonic acid in rice leaves by high-performance liquid chromatography–turboionspray tandem mass spectrometry. J Chromatogr A 822:310–315
Tanno N, Yokota T, Abe M, Okagami N (1992) Identification of endogenous gibberellins in dormant bulbils of Chinese yam Dioscorea opposite. Plant Physiol 100:1823–1826
Trouslot MF (1982) Croissance et tuberisation chez quatre cultivars du complex Dioscorea cayenensis–D.rotundata. In: Miege J, Lyonga N (eds) Yams=Ignames. Clarendon Press, Oxford, pp 118–146
Wareing PF, Jennings AMV (1980) The hormonal control of tuberization in potato. In: Skoog F (ed) Plant growth substances. Springer, New York, pp 293–300
Wu N, Clausen AM (2007) Fundamental and practical aspects of ultrahigh pressure liquid chromatography for fast separations. J Sep Sci 30:1167–1182
Xu X, Lammeren AAM, Vreugdenhil D (1998) The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584
Yamazaki H, Nishijima T, Koshioka M, Miura H (2002) Gibberellins do not act against abscisic acid in the regulation of bulb dormancy of Allium wakegi. Plant Growth Regul 36:223–229
Yoshida Υ, Kanahama K (1999) Effect of photoperiod and temperature on the development of spikes and new tubers in chinese yam (Dioscorea opposite Thund. cv. Ichpimo). J Jpn Soc Hortic Sci 68:124–129
Yoshida Y, Takahashi H, Kanda H, Kanahama K (2002) Interactive effects of photoperiods and plant growth regulators on the development of tubers and flowering spikes in Chinese yam (Dioscorea oppostita) cv. nagaimo. J Jpn Soc Hortic Sci 71:752–757
Yoshida Y, Takahashi H, Kanda H, Kanahama K (2007) Effect of seed tuber weights on the development of tubers and flowering spikes in Japanese yams (Dioscorea japonica) grown under different photoperiods and with plant growth regulators. J Jpn Soc Hortic Sci 76(3):230–236
Yu H, Dhavale T, Yang S (2006) Molecular mechanisms of hormone functions in flowering. Floricult Ornam Biotechnol 1:25–32
Acknowledgements
The authors thank Weijian Cen for the hormone quantification carried out at the Plant Hormone Quantification Service. The authors are also grateful to Doctor Quanguang He for helpful advice regarding plant hormone extraction. This work has been supported by two research Projects from the National Natural Science Foundation of China (30760126), and the special funds for Agro-Scientific Research in the Public Interest (200903022).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, M., Luo, H., Wang, A. et al. Phytohormone Profiling During Tuber Development of Chinese Yam by Ultra-high performance Liquid Chromatography–Triple Quadrupole Tandem Mass Spectrometry. J Plant Growth Regul 36, 362–373 (2017). https://doi.org/10.1007/s00344-016-9644-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9644-8