Abstract
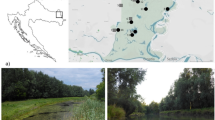
The aim of this study was to determine the species composition, biodiversity and, relative abundance of epiphytic algae and their relationship with environmental variables on three different macrophytes (Nymphaea alba, Ceratophyllum demersum, Typha latifolia ) at Acarlar Floodplain Forest (AFF). Epiphytic algae were gathered monthly by collecting aquatic plants between November 2011 and October 2012, except in winter when there were no plants. In this study, 67 taxa on N. alba, 66 taxa on C. demersum and 66 taxa on T. latifolia were identified as epiphytic algae. The mean value of species richness was 17, that of diversity was 1.5 and that of evenness was 0.54 for epiphytic algae on N. alba, 17, 1.1, and 0.39 on C. demersum, and 18, 1.64, and 0.56 on T. latifolia, respectively. Oscillatoria sp. and Komvophoron crassum (Vozzen) Anagnostidis and Komárek were the most abundant and consistent epiphytic algal species, occurring in high abundance on all macrophytes. Results show that species composition of epiphytic algae was different, but diversity values were similar on all the macrophytes. The hydrological pulse is one of the most important factors determining the physical and chemical environment of the epiphytic algal community. However, substrate type also affected the colonization by F. capucina, O. sancta, P. catenata, and L. truncicola more than the epiphytic algal seasonality.
Similar content being viewed by others
References
Albay M, Akcaalan R. 2003. Comparative study of periphyton colonisation on common reed (Phragmites australis) and artificial substrate in a shallow lake, Manyas, Turkey. Hydrobiologia, 506(1): 531–540.
Antoine S E, Benson-Evans K. 1985. The epipelic algal flora of the River Wye System, Wales, UK 1. Productivity and total biomass dynamics. Internationale Revue der gesamten. Hydrobiologie und Hydrographie, 70(4): 575–589.
Barinova S S, Anissimova O V, Nevo E, Jarygin M M, Wasser S P. 2004. Diversity and ecology of algae from the Nahal Qishon river, northern Israel. Plant Biosystems. 138(3): 245–259.
Biggs B J F, Goring D G, Nikora V I. 1998. Subsidy and stress responses of stream periphyton to gradients in water velocity as a function of community growth form. Journal of Phycology, 34: 598–607.
Blindow I. 1987. The composition and density of epiphyton on several species of submerged macrophytes-the neutral substrate hypothesis tested. Aquatic Botany, 29(2): 157–168.
Bobat A. 2004. Zebra mussel and fouling problems in the Euphrates basin. Turkish Journal of Zoology, 28: 161–177.
Borchardt M A. 1996. Nutrients. In: Stevenson R J, Bothwell M L, Lowe R L eds. Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego. p.183–227.
Cattaneo A, Kalff J. 1978. Seasonal changes in the epiphyte community of natural and artificial macrophytes in Lake Memphremagog (Que. and Vt.). Hydrobiologia, 60(2): 135–144.
Claps M C. 1991. Diatom communities on aquatic macrophytes of pampasic lotic environments (Argentina). Acta Hydrobiologica, 33: 195–208.
Comte K, Fayolle S, Roux M. 2005. Quantitative and qualitative variability of epiphytic algae on one Apiaceae (Apium nodiflorum L.) in a karstic river (Southeast of France). Hydrobiologia, 543(1): 37–53.
de Emiliani M O G. 1993. Seasonal succession of phytoplankton in a lake of the Paraná River floodplain, Argentina. Hydrobiologia, 264(2): 101–114.
Dunn A E, Dobberfuhl D R, Casamatta D A. 2008. A survey of algal Epiphytes from Vallisneria americana Michx. (Hydrocharitaceae) in the lower St. Johns River, Florida. Southeastern Naturalist, 7(2): 229–244.
Elber F, Schanz F. 1990. Algae, other than diatoms, affecting density, species richness and diversity of diatom communities in rivers. Archiv für Hydrobiologie, 119: 1–14.
Eminson D, Moss B. 1980. The composition and ecology of periphyton communities in freshwaters. British Phycological Journal, 15(4): 429–446.
Fahmy T. 2013. XLSTAT, Version 2013. Addinsoft, Paris.
Fonseca I A, Rodrigues L. 2007. Periphytic Cyanobacteria in different environments from the upper Paraná River floodplain, Brazil. Acta Limnol. Bras., 19(1): 53–65.
Goldsborough L G, Robinson G C. 1996. Patterns in wetlands. In: Stevenson R J, Bothwell M L, Lowe R L eds. Algal Ecology: Freshwater Benthic Ecosystems. Academic, London. p.78–117.
Gottlieb A, Richards J, Gaiser E. 2005. Effects of desiccation duration on the community structure and nutrient retention of short and long-hydroperiod Everglades periphyton mats. Aquatic Botany, 82: 99–112.
Gross E M, Feldbaum C, Graf A. 2003. Epiphyte biomass and elemental composition on submersed macrophytes in shallow eutrophic lakes. Hydrobiologia, 506–509: 559–565.
Guiry M D, Guiry G M. 2013. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed on 22 July 2013.
Hasler P, Poulickova A. 2010. Diversity, taxonomy and autecology of autochtonous epipelic cyanobacteria of the genus Komvophoron (Borziaceae, Oscillatoriales): a study on populations from the Czech Republic and British Isles. Biologia, 65(1): 7–16.
Havens K E, East T L, Meeker R H, Davis W P, Steinman A D. 1996. Phytoplankton and periphyton responses to in situ experimental nutrient enrichment in a shallow subtropical lake. Journal of Plankton Research, 18(4): 551–566.
Hill W. 1996. Effects of light. In: Stevenson R J, Bothwell M L, Lowe R L eds. Algal Ecology: Freshwater Benthic Ecosystems. Academic Press, San Diego. p.121–148.
Huber-Pestalozzi G. 1941. Das Phytoplankton des Süßwassers. (Die Binnengewässer, Band XVI). Teil 2. (i) Chrysophyceen, Farblose Flagellaten Heterokonten. E. Schweizerbart’sche Verlag-sbuchhandlung, Stuttgart.
Huber-Pestalozzi G. 1950. 3 Teil. Cryoptophyceen,, Chloromonadien, Peridineen. In: Thienemann A ed. Das Phytoplankton des Süsswassers. Die Binnengewasser, E. Schweizerbart’sche Verlagsbuchhhandlung, Stuttgart.
Huber-Pestalozzi G. 1961. Das Phytoplankton des Süßwassers. (Die Binnengewässer, Band XVI). Teil 5. Chlorophyceae, Ordnung: Volvocales. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Huber-Pestalozzi G. 1962. Das phytoplankton des süsswassers systematik und biologie, 1. Teil, Blaualgen. E. Schweizerbarth’sche Verlagsbuchhandlung (Nagele u. Obermiller), Stuttgart.
Huber-Pestalozzi G. 1969. Das phytoplankton des süsswassers systematik und biologie, 4. Teil, Euglenophycean. E. Schweizerbarth’sche Verlagsbuchhandlung (Nagele u. Obermiller) Stuttgart.
Huber-Pestalozzi G. 1982. Das phytoplankton des süsswassers systematik und biologie, 8. Teil, 1.Halffe Conjugatophyceae Zygnematales und Desmidiales (excl. Zygnemataceae). E. Schweizerbarth’sche Verlagsbuchhandlung (Nagele u. Obermiller), Stuttgart.
Huber-Pestalozzi G. 1983. Das phytoplankton des süsswassers systematik und biologie, 7. Teil, 1. Halffe Chlorophyceae (Grünalgen) Ordnung: Chlorococcales. E. Schweizerbarth’sche Verlagsbuchhandlung (Nagele u. Obermiller), Stuttgart.
Irvine R L, Jackson L J. 2006. Spatial variance of nutrient limitation of periphyton in montane, headwater streams (McLeod River, Alberta, Canada). Aquatic Ecology, 40: 337–348.
John D M, Whitto B A, Brook A J. 2003. The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. The Natural History Museum and the British Phycological Society, Cambridge University Press, Cambridge.
Junk W J, Bayley P B, Sparks R. 1989. The flood pulse concept in river-floodplain systems. In: Podge J P ed. Proceedings of the International Large River Sympsoium. Canadians Special Publication of Fisheries and Aquatic Sciences, Ohawa. p.110–127.
Kawecka B, Eloranta P. 1994. Zarys ekologii glonow wodsłodkich i środowisk ldowych (Outline of the ecology of freshwater and terrestrial algae). PWN, Warszawa. Kıvrak E. 2006. Seasonal and long term changes of the phytoplankton in the Lake Tortum in relation to environmental factors, Erzurum, Turkey. Biologia, 61: 339–345.
Komarek J, Anagnostidis K. 2008. Cyanoprokaryota, 2. Teil/Part 2: Oscillatoriales, Süswasser Flora von Mitteleuropa (Freshwater Flora of Central Europe).
Krammer K, Lange-Bertalot H. 1986. Bacillariophyceae: Naviculaceae. In: Ettl H, Gerloff I, Heynıg H, Mollenhauer D eds. Süsswasserflora Von Mitteleuropa. Stuttgart: G. Fischer.
Krammer K, Lange-Bertalot H. 1991a. Bacillariophyceae. 3. Centrales, Fragilariaceae, Eunoticeae. In: Süβwasserflora von Mitteleuropa, 2/3, Gustav Fischer Verlag, Stuttgart, New York. 577p.
Krammer K, Lange-Bertalot H. 1991b. Bacillariophyceae. 4. Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. In: Süβwasserflora von Mitteleuropa, 2/4, Gustav Fischer Verlag, Stuttgart, New York. 437p.
Krammer K, Lange-Bertalot H. 1999. Bacillariophyceae. 2. Epithemiaceae, Surirellaceae. In: Süβwasserflora von Mitteleuropa, 2/2, Gustav Fischer Verlag, Stuttgart, New York. 596p.
McCormick P V, Shuford R B E, John G. Backus J G, Kennedy W C. 1998. Spatial and seasonal patterns of periphyton biomass and productivity in the northern Everglades, Florida, U.S.A. Hydrobiologia, 362(1–3): 185–210.
Messyasz B, Kuczy’nska-Kippen N, Nagengast B. 2009. The epiphytic communities of various ecological types of aquatic vegetation of five pastoral ponds. Biologia, 64(1): 88–96.
Millie D F, Lowe R L. 1983. Studies on Lake Erie’s littoral algae; Host specificity and temporal periodicity of epiphytic diatoms. Hydrobiologia, 99: 7–18.
Mosisch T D, Bunn S E, Davies P M, Marshall C J. 1999. Effects of shade and nutrient manipulation on periphyton growth in a subtropical stream. Aquatic Botany, 64(2): 167–177.
Najafpour S, Alkarkhi A F M, Kadir M O A, Najafpour G D. 2008. Evaluation of spatial and temporal variation in river water quality. Int. J. Environ. Res., 2(4): 349–358.
Padisák J, Crossetti L O, Naselli-Flores L. 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia, 621(1): 1–19.
Pomazkina G, Kravtsova L, Sorokovikova E. 2012. Structure of epiphyton communities on Lake Baikal submerged macrophytes. Limnological Review, 12(1): 19–27.
Rodríguez P, Tell G, Pizarro H. 2011. Epiphytic algal biodiversity in humic shallow lakes from the Lower Paraná River Basin (Argentina). Wetlands, 31(1): 53–63.
Rott E, Pfister P. 1988. Natural epilithic algal communities in fast-flowing mountain streams and rivers and some maninduced changes. Verhandlungen Internationale Vereinigung fur Theoretische und angewandte Limonologie, 23: 1 320–1 324.
Round F E, Crawford R M, Mann D G. 1990. The Diatoms: Morphology and Biology of the Genera. Cambridge Universty Press, Cambridge.
Round F E. 1953. An investigation of two benthic algal communities in Malharm Tarn, Yorkshire. Journal of Ecology, 41: 97–174.
Schallenberg M, Burns C. 2004. Effects of sediment resuspension on phytoplankton production: teasing apart the influences of light, nutrients and algal entrainment. Freshwater Biology, 49: 143–159.
Schramm H L, Jirka K J. 1989. Epiphytic invertebrates as a food resource for bluegills in Florida lakes. Transactions of the American Fishery Society, 118: 416–426.
Shannon C E, Weaver W. 1963. The Mathematical Theory of Communication. University of Illinois Press, Urbana. p.210–360.
Sims P A. 1996. An Atlas of British Diatoms. Biopress Ltd., London. p.601.
State Meteorological Works. 2013. Official Statistics (Statistical data belongs to provinces and districts). Bulletin of Meteorology. Department of Water Affairs and Forestry.
Strickland J D H, Parsons T R. 1972. A Practical Handbook of Seawater Analysis, 2nd Edition. Bull. Fish. Res. Bd Can. p.167.
Technicon Industrial Methods. 1977a. Nitrate and Nitrite in Water and Wastewater. No. 158-71, W/A.
Technicon Industrial Methods. 1977b. Phosphate and Silicate Analysis in Water and Seawater. No. 253-280 E. Application note, U.K. p.5–7.
Ter Braak C J F, Smilauer P. 2002. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canoinical Community Ordination (Version 4.5). Ithaca (NY, USA): Microcomputer Power. p.500.
Triest L, Lung’ayia H, Ndiritu G, Beyene A. 2012. Epilithic diatoms as indicators in tropical African rivers (Lake Victoria catchment). Hydrobiologia, 695: 343–360.
Tuchman M, Blinn D W. 1979. Comparison of attached algal communities on natural and artificial substrata along a thermal gradient. British Phycological Journal, 14(3): 243–254.
Ustaoğlu B. 2012. Spatiotemporal analysis of land cover change patterns in western part of the Sakarya River Delta and its surroundings in Turkey. Enery Education Science and Tecnology Part A: Energy Science and Research, 29: 721–730.
Utermöhl H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton Methodik. Mitteilung Internationale Vereinigung fuer Theoretische und Amgewandte. Limnologie, 9: 1–38.
Uzun A, Tabur M A, Ayvaz Y. 2008. Birds of lake acarlar and environmental problems. Ekoloji, 17: 1–14.
van Donk E, van de Bund W J. 2002. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquatic Bot., 72: 261–274.
Van Landingham S L. 1982. Guide to the identification, environmental requirments and pollution tolerance of freshwater blue-green algae (Cyanophyta). US Environmental Protection Agency Environmental Monitoring Series 600/3-83-072.
Watson S B, McCauley E, Downing J A. 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol. Oceanogr., 42: 486–495.
Wium-Andersen S, Anthoni U, Christophersen C. 1982. Allelopathic effects on phytoplankton by substances isolated from aquatic macrophytes (Charales ). Oikos, 39: 187–190.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Sakarya University Research Foundation (No. 2012-02-20-012)
Rights and permissions
About this article
Cite this article
Tunca, H., Ongun Sevindik, T., Bal, D.N. et al. Community structure of epiphytic algae on three different macrophytes at Acarlar floodplain forest (northern Turkey). Chin. J. Ocean. Limnol. 32, 845–857 (2014). https://doi.org/10.1007/s00343-014-3205-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-014-3205-4