Abstract
In response to nutrient deprivation and environmental insults, bacteria conjoin two copies of non-translating 70S ribosomes that form the translationally inactive 100S dimer. This widespread phenomenon is believed to prevent ribosome turnover and serves as a reservoir that, when conditions become favorable, allows the hibernating ribosomes to be disassembled and recycled for translation. New structural studies have revealed two distinct mechanisms for dimerizing 70S ribosomes, but the molecular basis of the disassembly process is still in its infancy. Many details regarding the sequence of dimerization-dissociation events with respect to the binding and departure of the hibernation factor and its antagonizing disassembly factor remain unclear.
Similar content being viewed by others
Introduction
The bacterial 100S ribosome is a homodimeric particle of 70S complexes that are individually composed of 30S and 50S ribosomal subunits. The 70S pair dimerizes end-to-end via the joining of the 30S–30S ribosomal subunits (Fig. 1). The 100S ribosome was first discovered in Escherichia coli in the late 1950s (Huxley and Zubay 1960; Tissieres and Watson 1958). It was originally thought to undergo passive aggregation of the 70S ribosome until recently, when its physiological significance and the active participants of 70S dimerization became widely appreciated (Yoshida and Wada 2014). While the 70S complexes can either be translationally silent or competent upon binding to mRNA templates, 100S ribosomes are devoid of translational activity and often accumulate during the late stationary growth phase; hence, the inactive 70S and 100S complexes were dubbed “hibernating ribosomes”.
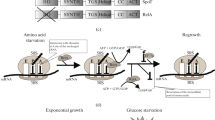
A simplified series of events on the biogenesis of the S. aureus 100S ribosome. The post-termination vacant ribosomes and/-or newly synthesized ribosomes could serve as the precursors of the 70S dimerization. The IF3 initiation factor prevents subunit joining but is unable to saturate all of the 30S subunits due to low cellular concentrations. The general stress response sigma factor B (SigB) activates hpflong expression. S. aureus HPFlong is a basic protein made of 190 amino acids that consists of the N-terminal domain (NTD) and C-terminal domain (CTD) connected by an unstructured region. It is unclear whether the HPFlong binds to the 70S ribosome as a monomer or a dimer. The CTD-HPFlong dimerization provides the primary binding platform, while the uS2-h26 and CTD-h40 interactions play secondary roles. The hibernating ribosomes enable cells to survive under various harsh environments. The recycling factor pair (EF-G and RRF) and the GTPase HflX presumably dissociate the 100S ribosomes into 70S or 30S/50S subunits
Ribosome hibernation is critical for bacterial adaptation to various environments. 100S ribosome-proficient cells exhibit a greater survival rate both in vitro and inside animal hosts by 2–3 orders of magnitude. Under standard laboratory conditions, the stability of the E. coli 100S ribosome is reduced in the presence of low magnesium, high salt, extreme pH (Tissieres and Watson 1958), and nutrient replenishment in culture (Aiso et al. 2005; Wada et al. 1990). The dissociation process of the 100S ribosome is poorly understood, although it is believed to allow reentry of mature ribosomes into the active translation pool, thereby bypassing the need of synthesizing new ribosomes that are energetically costly. By contrast, dimerization of 70S ribosomes has been investigated further in several bacterial species. The mammalian equivalent of the bacterial 100S ribosome, a dimer of 80S monomers (110S complex), has been observed in nutrient-deprived cancer cells, but the dimerizing factor has yet to be identified (Krokowski et al. 2011). Most inactive ribosomes in eukaryotes exist as non-translating 80S monomers (van den Elzen et al. 2014). This review will focus on recent findings that have advanced our understanding of the mechanism of 70S dimerization and the diverse roles of the 100S ribosome in bacterial survival (Fig. 1). Finally, we also discuss outstanding questions that have emerged from recent structural, biochemical and genetic studies.
The many flavors of ribosome hibernation factors
In the γ-proteobacterium E. coli, the ribosome modulation factor (RMFEc) and hibernation promoting factor (HPFEc, formerly YhbH) cooperatively stimulate the dimerization of 70S monomers, first by RMFEc-induced formation of the 90S particles, followed by HPFEc-mediated stabilization of the dimer. The third protein, YfiAEc (also known as pY or RaiA), is a paralog of HPFEc that silences the 70S monomer and antagonizes 70S dimerization. YfiA inhibits protein biosynthesis by sterically occluding the binding of tRNAs to the decoding sites of the 30S subunit (Agafonov and Spirin 2004; Polikanov et al. 2012; Ueta et al. 2005; Vila-Sanjurjo et al. 2004). YfiA is absent outside of the γ-proteobacteria but is functionally homologous to PSRP1 in plant chloroplasts (Bieri et al. 2017; Sharma et al. 2010).
The majority of bacteria carry only one long form of HPF (HPFlong, ~ 200 amino acids long) that is twice the size of HPFEc. The bipartite HPFlong proteins consist of the translational silencing N-terminal domain (NTD) and a dimerizing C-terminal domain (CTD) linked by an unstructured region composed of 16–62 residues (Franken et al. 2017) (Figs. 1, 2). Intriguingly, the temporal abundance of the 100S ribosome varies significantly across species. The picture that has now emerged is that E. coli and Pseudomonas aeruginosa, which carry both RMFEc and HPFEc homologs, produce 100S ribosomes during the stationary phase (Ueta et al. 2013; Wada 1998; Williamson et al. 2012). By contrast, 100S ribosomes are constitutively produced in HPFlong-harboring bacteria, including Staphylococcus aureus, Bacillus subtilis, and Lactococcus lactis (with the exception of cyanobacteria), from the lag log phase through the late stationary phase (Davis et al. 2014; Ueta et al. 2010, 2013). The stability of the hpf mRNA and protein, leaky transcriptional activation of the hibernation factors, and levels of 100S disassembly factor(s) may account for the differences in temporal production (see below).
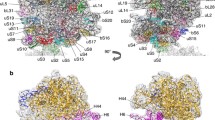
Overview of the native S. aureus 100S ribosome. The 100S particle consists of two 70S ribosomes, each consisting of a 50S (blue) and 30S (gold) subunit. The two 70S monomers form an interface via the 30S subunits and are tethered together by two S. aureus hibernation-promoting factor (SaHPF) molecules (magenta and green). a Relative to the top 70S ribosome, the second 70S ribosome is oriented by 180° rotations along the horizontal and vertical axes of the page. b Same view as a. The 100S particle has been sliced to better show the location and interaction of the two SaHPF molecules. Each SaHPF molecule is composed of an N-terminal domain (NTD; residues 1–95) and a C-terminal domain (CTD; residues 130–190) connected by a flexible linker. The CTD of each SaHPF molecule interact at the 30S/30S interface
Functional consequences of the loss of 100S ribosomes
Null mutants of rmf and hpf are phenotypically diverse from Proteobacteria to Fermicutes, suggesting the existence of a species-specific role of the 100S ribosome. An E. coli rmf Ec mutant is susceptible to nutrient limitation, acid and heat stress (El-Sharoud and Niven 2007; Niven 2004; Yamagishi et al. 1993). Listeria monocytogenes lacking hpf long are more than 20 times less virulent in a mouse model of infection and are more sensitive to killing with aminoglycosides (Kline et al. 2015; McKay and Portnoy 2015). Membrane damage may contribute to antibiotic sensitivity, similar to the loss of membrane integrity observed in a P. aeruginosa Δrmf mutant (Williamson et al. 2012). The S. aureus hpf long null or dimerization-defective strain has an extremely low survival rate in long-term culture (Basu and Yap 2016) and under acute heat exposure (Matzov et al. 2017). hpf knockouts of L. lactis, B. subtilis, and P. aeruginosa fail to resuscitate from starvation (Akanuma et al. 2016; Akiyama et al. 2017; Beckert et al. 2017; Puri et al. 2014). The Synechococcus elongatus Δhpf mutant undergoes translational derepression (Hood et al. 2016) analogous to a S. aureus Δhpf mutant (Basu and Yap 2016). Bacteria lacking the 100S ribosome are prone to cell death, a feature that is accompanied by massive ribosome degradation (Akiyama et al. 2017; Basu and Yap 2016; Shcherbakova et al. 2015). The causal relationship between ribosome integrity and the formation of the 100S ribosome is unclear. The structure of the 100S ribosome may be more resistant to RNase, and the binding of hibernation factors may compete with the RNase target sites. It is also possible that the expression of specific factors involving in the ribosome decay pathway (Redder 2016) is compromised in the rmf and hpf mutants.
In general, the phenotypes described above are manifested during slow growth, such as in aging and dormant cells, and intracellular growth inside the host cells, which are normally characterized by metabolic arrest and translational dormancy that restrict energy consumption (Dai et al. 2016). Consistent with this observation, the levels of HPFlong or RMF are substantially elevated in a murine pneumonia model (Michalik et al. 2017), in starved cells (Aiso et al. 2005; Sanchuki et al. 2017), in biofilms (Williamson et al. 2012), and in non-replicating persisters (Tkachenko et al. 2017). Moreover, HPFlong reduces translational efficiency of a subset of genes in vivo (Basu and Yap 2016; Hood et al. 2016) and in cell-free translation assays (Basu and Yap 2016; Ueta et al. 2008, 2013), presumably because 70S dimerization titrates functional ribosomes away from translational initiation. Paradoxically, deficiency of HPFlong may be favorable under certain antibiotic stress conditions, as reported in Synechocystis sp. and Streptomyces venezuelae (Galmozzi et al. 2016; Jones et al. 2014).
Mechanistic differences of 70S dimerization
Four recent cryo-EM maps of the HPFlong-bound 100S ribosome have revealed a surprising mechanism of 70S dimerization in three fermicutes (S. aureus, B. subtilis and L. lactis) that is fundamentally distinct from that of the E. coli counterpart. In all three species, the translation-inactivating NTD of HPFlong adopts a βαβββα fold and occupies the tRNA- and mRNA-binding sites of the 30S subunit that is virtually superimposable on the HPFEc. In all cases, the CTD-HPFlong on one copy of the 70S monomer slightly extends outside of the solvent-accessible face of the 30S subunit and directly interacts with another CTD-HPFlong that is tethered on the opposite copy of the 70S monomer (Figs. 1, 2). Aside from the primary CTD–CTD contact in S. aureus, secondary interactions between the ribosomal protein uS2 and rRNA h26 and CTD-h40 further stabilize the dimer interface (Matzov et al. 2017). Similar interactions have been observed in L. lactis (Franken et al. 2017). HPFlong in S. aureus also induces a 5° swiveling of the 30S head domain, but this movement was not seen in other cryo-EM maps. Notably, the uS2-h26 and CTD-h40 interactions are absent in the second S. aureus cryo-EM model reported by Khusainov et al. and, instead, are replaced by an h26-h26 interaction between the two 30S subunits (Khusainov et al. 2017). The discrepancies may be attributed to different approaches in the preparation of 100S ribosomes. One was purified directly from cell culture whereas the other was reconstituted in vitro using a recombinant HPFlong protein. In Bacillus subtilis, the secondary contacts are established between the uS2-bS18 and uS2-h26 pairs on the opposing 30S subunits (Beckert et al. 2017). Despite these differences, a convergent rule emerges from these studies is that the physical CTD–CTD interaction is crucial for HPFlong-induced dimerization.
By contrast, E. coli RMFEc allosterically promotes 70S dimerization without any contact between the two RMFEc molecules. Instead, RMFEc induces conformational changes in the 30S subunit and, in turn, widens the dimer interface to accommodate interactions that comprise of rRNA h39, and the uS2, uS3, and uS5 ribosomal proteins (Kato et al. 2010; Ortiz et al. 2010; Polikanov et al. 2012). RMFEc is mapped to a site that blocks the binding of the 30S subunit to the mRNA Shine–Dalgarno (SD) sequence distantly away from where the CTD-HPFlong binds. The differences in the 30S binding mode affect the overall dimeric architectures. A comparison of various 3D maps of 100S ribosomes demonstrates that the orientation of the native S. aureus 100S ribosome (Matzov et al. 2017) deviates from that of E. coli, B. subtilis, and S. aureus (Khusainov et al. 2017) by approximately 40°, 24°, and 11° counterclockwise rotations, respectively (Fig. 3).
Comparison of multiple 100S particle orientations with respect to the S. aureus 100S ribosome. The 100S particle structure from S. aureus (PDB 5NG8) is colored and oriented as shown in Fig. 2. Cryo-EM maps for E. coli (a EMD-5174), B. subtilis (b EMD-3664) and a second structure determination for S. aureus (c EMD-3638) were downloaded from the Electron Microscopy DataBank (EMDB). For each map, atomic coordinate files were obtained from the RCSB. No corresponding model was available for the E. coli cryo-EM map. Therefore, the 5DFE structure was used instead. Deposited coordinate files for 5NJT and 5ND8 were used for B. subtilis and S. aureus, respectively. With the exception of 5NG8, 100S particles were generated from the 70S ribosome coordinates. For each structure, a second 70S monomer was generated and manually docked (translated and rotated) into their corresponding electron density maps using PyMOL v1.8.6 (Schrödinger). The overall goodness of fit was qualitatively assessed by visual inspection. In the case of E. coli, the electron density map contained only the first 70S monomer and a portion of the density for the second 30S subunit. The partial density for the second 30S subunit was used to align and orient the entire second 70S monomer relative to the first. Following construction of the complete 100S structures, each structure was superpositioned onto 5NG8 using the top 70S ribosome and by maintaining the relative orientation of the second 70S ribosome as defined by the cryo-EM maps prior to superpositioning. Using the bottom 70S ribosome from 5NG8 as a reference (blue and gold), the relative orientation for E. coli, B. subtilis and S. aureus (all in grey), represent approximate 40, 24 and 11 degree counterclockwise rotations, respectively, along the plane of the page
Disassembly of the 100S ribosome
Dissociation of the 100S ribosome into 70S or 30S and 50S subunits may provide renewable ribosomes for restarting translation. This process likely involves an active mechanism to dislodge hibernation factors from the ribosome. Recent biochemical and genetic studies have demonstrated that an evolutionarily conserved GTPase known as HflX disassembles both the S. aureus vacant 70S and 100S ribosomes, but only the dissociation of the 100S ribosome requires GTP hydrolysis (Basu and Yap 2017). Escherichia coli HflX binds to the peptidyltransferase center of the 50S subunit (Zhang et al. 2015). Thus, the S. aureus HflX may bind to similar regions and split the 100S ribosome by disrupting the intersubunit bridges of the 70S ribosome, given that breaking just one of the twelve intersubunit bridges (uS13-bL31 B1b bridge) could collapse subunit joining and reduce the formation of 100S (Ueta et al. 2017). HflX is likely not the only disassembly candidate because an ΔhflX knockout exhibits a modest phenotype (Basu and Yap 2017), and the E. coli ribosome recycling factor (RRF), in conjunction with EF-G, is able to partially rescue the growth defect of an E. coli ΔhflX mutant (Zhang et al. 2015). Other canonical translation factors such as IF3, in addition to RRF and EF-G, have been implicated in counteracting the formation of the E. coli 100S complex and the hibernating 70S ribosome in chloroplasts (Sharma et al. 2010; Yoshida et al. 2009).
Regulation of RMF and HPF
Small signaling molecules positively control the levels of hibernation factors under specific stress conditions. Escherichia coli rmf Ec is a member of the polyamine and cAMP modulons (Shimada et al. 2013; Tkachenko et al. 2017). The stationary phase-specific stringent response alarmore (p)ppGpp up-regulates the transcription of E. coli rmf Ec and hpf long of B. subtilis and S. elongates. (Hood et al. 2016; Tagami et al. 2012; Terui et al. 2010). Although ppGpp does not appear to directly influence the expression level of HflX GTPase, ppGpp binds and inhibits the 100S splitting activity of HflX (Basu and Yap 2017; Corrigan et al. 2016). The intracellular ppGpp concentration is inversely correlated with GTP synthesis. This raises the possibility that ppGpp-GTP homeostasis may play an intricate role in regulating the expression and function of HPF and HflX. Collectively, the transcription of rmf and hpf are sensitive to environmental stimuli. For instance, nitrogen- and carbon-starvation increase rmf Ec expression whereas thermal stress or darkness strongly induces hpf long transcription.
In Fermicutes and cyanobacteria, hpf long is the direct target of a stress-responsive alternate sigma factor SigB whose highly conserved binding motif is found at the 5′-UTR of the hpf operon. B. subtilis hpf long is also under positive control of the sporulation regulator SigH (Akanuma et al. 2016; Basu and Yap 2017; Galmozzi et al. 2016; Kline et al. 2015). Deletion of sigB in S. aureus does not completely abolish the synthesis of HPFlong. Additional putative binding motifs of housekeeping SigA (or σ70) and global regulator CodY are detected upstream of the hpf long (Majerczyk et al. 2010; Waters et al. 2016). The additive effect of multiple regulatory activators on S. aureus hpf long provides an explanation for the constitutive production of hpf long and the 100S ribosome (Davis et al. 2014; Majerczyk et al. 2010; Ueta et al. 2010; Waters et al. 2016). Alternately, S. aureus hpf long may be ill regulated due to the absence of a cognate repressor or long-lived transcript and protein. The turnover of S. aureus HPFlong is relatively slow even when the ribosome concentration drops substantially after 4 days in culture (Basu and Yap 2016). Furthermore, the mRNA structure may prolong the transcript half-life and, in turn, determines the biosynthesis of the hibernation factors (Redder 2016). Due to the 5′-UTR hairpin structure, the rmf Ec mRNA has an unusually long half-life during the early exponential phase (24 min versus the average E. coli transcripts of 1–2 min) and 120 min during the stationary phase (Aiso et al. 2005).
Outlook
The formation of the 100S ribosome is one of the most important survival strategies in bacteria to help cope with various environmental stressors. Recent structural and genetic studies have opened new avenues of research to investigate structure–function relationships and the biological role of the 100S ribosome. Despite several recent advances, many outstanding questions remain to be addressed to obtain a more complete picture of ribosome hibernation (Fig. 1):
-
1.
At which stage of translation does dimerization occur? Surprisingly, overexpression of HPFlong does not significantly retard cell growth, and an hpf null mutant only modestly increases global translation (Basu and Yap 2017). This finding raises the possibility that ribosomes are usually produced in excess during exponential growth and that the translation capacity is not significantly compromised when 20–40% of the ribosomes are converted to the silent 100S ribosome. Thus, the newly synthesized free ribosomes that have yet to engage in translation may be the targets of HPFlong. Another possibility is that post-termination 70S ribosomes do not undergo subunit splitting (Chen et al. 2017; Orelle et al. 2015; Yamamoto et al. 2016) and instead serve as precursors for dimerization. The latter may occur more frequently during transition from log phase to stationary phase when the ribosomes disproportionally outnumber mRNA molecules.
-
2.
The precise role of dimerization in unclear. Bacteria have evolved two disparate mechanisms of 70S dimerization, yet they share an overlapping dimer interface. This finding suggests that these regions are protected and may be vulnerable to nucleolytic attacks and thermal insults. Alternately, these regions may be a hotspot of unintended molecular interactions in the crowded cytoplasm. Bacterial transcription and translation are coupled, and RNA polymerase (RNAP) may bind to the 70S dimer interface (Fan et al. 2017; Kohler et al. 2017). Thus, dimerization may simply prevent spurious interactions with the RNAP.
-
3.
The mechanistic details of dimerization and translation inactivation are poorly defined. Truncated CTD-HPFlong variants self-dimerize in solution and are sufficient to induce the formation of the 100S ribosome in vivo, albeit less effectively than the full-length HPFlong (Beckert et al. 2017; Matzov et al. 2017; Puri et al. 2014). 70S dimerization may be initiated through one of two pathways (or both). First, a free HPF-dimer binds to one copy of the 70S monomer and seizes the second 70S monomer. Second, each free HPF monomer binds to individual 70S monomers, and dimerization occurs when the CTDs make contacts with each other. Discerning these scenarios will require time-resolved kinetic studies of 70S dimerization coupled with monitoring the oligomeric state of HPFlong. Along the same line, it is unclear whether monomeric HPFlong and dimeric HPFlong on a single 70S monomer equally achieve translational repression compared to the mature 100S complex.
-
4.
How is the 100S ribosome remobilized into the active translation pool? Does the RRF/EF-G pair play a more prominent role than HflX in the disassembly of the 100S ribosome? Future kinetic studies are needed to delineate the precise sequence of 100S disassembly and the hierarchical order of IF3, RRF/EF-G, and HflX as well as to monitor the fates of HflX and HPF upon dissociation. Furthermore, HPFlong appears to be abundant over a long period of time. Therefore, it is unclear whether proteolysis occurs following the detachment of HPFlong from the ribosome or whether an unknown factor sequesters free HPFlong away from re-dimerizing the 70S ribosomes.
References
Agafonov DE, Spirin AS (2004) The ribosome-associated inhibitor A reduces translation errors. Biochem Biophys Res Commun 320:354–358
Aiso T, Yoshida H, Wada A, Ohki R (2005) Modulation of mRNA stability participates in stationary-phase-specific expression of ribosome modulation factor. J Bacteriol 187:1951–1958
Akanuma G, Kazo Y, Tagami K, Hiraoka H, Yano K, Suzuki S, Hanai R, Nanamiya H, Kato-Yamada Y, Kawamura F (2016) Ribosome dimerization is essential for the efficient regrowth of Bacillus subtilis. Microbiology 162:448–458
Akiyama T, Williamson KS, Schaefer R, Pratt S, Chang CB, Franklin MJ (2017) Resuscitation of Pseudomonas aeruginosa from dormancy requires hibernation promoting factor (PA4463) for ribosome preservation. Proc Natl Acad Sci USA 114:3204–3209
Basu A, Yap MN (2016) Ribosome hibernation factor promotes Staphylococcal survival and differentially represses translation. Nucleic Acids Res 44:4881–4893
Basu A, Yap MN (2017) Disassembly of the Staphylococcus aureus hibernating 100S ribosome by an evolutionarily conserved GTPase. Proc Natl Acad Sci USA 114:E8165–E8173
Beckert B, Abdelshahid M, Schafer H, Steinchen W, Arenz S, Berninghausen O, Beckmann R, Bange G, Turgay K, Wilson DN (2017) Structure of the Bacillus subtilis hibernating 100S ribosome reveals the basis for 70S dimerization. EMBO J 36:2061–2072
Bieri P, Leibundgut M, Saurer M, Boehringer D, Ban N (2017) The complete structure of the chloroplast 70S ribosome in complex with translation factor pY. EMBO J 36:475–486
Chen Y, Kaji A, Kaji H, Cooperman BS (2017) The kinetic mechanism of bacterial ribosome recycling. Nucleic Acids Res 45:10,168–10,177
Corrigan RM, Bellows LE, Wood A, Grundling A (2016) ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc Natl Acad Sci USA 113:E1710–E1719
Dai X, Zhu M, Warren M, Balakrishnan R, Patsalo V, Okano H, Williamson JR, Fredrick K, Wang YP, Hwa T (2016) Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol 2:16231
Davis AR, Gohara DW, Yap MN (2014) Sequence selectivity of macrolide-induced translational attenuation. Proc Natl Acad Sci USA 111:15379–15384
El-Sharoud WM, Niven GW (2007) The influence of ribosome modulation factor on the survival of stationary-phase Escherichia coli during acid stress. Microbiology 153:247–253
Fan H, Conn AB, Williams PB, Diggs S, Hahm J, Gamper HB Jr, Hou YM, O’Leary SE, Wang Y, Blaha GM (2017) Transcription-translation coupling: direct interactions of RNA polymerase with ribosomes and ribosomal subunits. Nucleic Acids Res 45:11043–11055
Franken LE, Oostergetel GT, Pijning T, Puri P, Arkhipova V, Boekema EJ, Poolman B, Guskov A (2017) A general mechanism of ribosome dimerization revealed by single-particle cryo-electron microscopy. Nat Commun 8:722
Galmozzi CV, Florencio FJ, Muro-Pastor MI (2016) The Cyanobacterial ribosomal-associated protein LrtA Is involved in post-stress survival in Synechocystis sp. PCC 6803. PLoS One 11:e0159346
Hood RD, Higgins SA, Flamholz A, Nichols RJ, Savage DF (2016) The stringent response regulates adaptation to darkness in the cyanobacterium Synechococcus elongatus. Proc Natl Acad Sci USA 113:E4867–E4876
Huxley HE, Zubay G (1960) Electron microscope observations on the structure of microsomal particles from Escherichia coli. J Mol Biol 2:10–18
Jones SE, Leong V, Ortega J, Elliot MA (2014) Development, antibiotic production, and ribosome assembly in Streptomyces venezuelae are impacted by RNase J and RNase III deletion. J Bacteriol 196:4253–4267
Kato T, Yoshida H, Miyata T, Maki Y, Wada A, Namba K (2010) Structure of the 100S ribosome in the hibernation stage revealed by electron cryomicroscopy. Structure 18:719–724
Khusainov I, Vicens Q, Ayupov R, Usachev K, Myasnikov A, Simonetti A, Validov S, Kieffer B, Yusupova G, Yusupov M, Hashem Y (2017) Structures and dynamics of hibernating ribosomes from Staphylococcus aureus mediated by intermolecular interactions of HPF. EMBO J 36:2073–2087
Kline BC, McKay SL, Tang WW, Portnoy DA (2015) The Listeria monocytogenes hibernation-promoting factor is required for the formation of 100S ribosomes, optimal fitness, and pathogenesis. J Bacteriol 197:581–591
Kohler R, Mooney RA, Mills DJ, Landick R, Cramer P (2017) Architecture of a transcribing-translating expressome. Science 356:194–197
Krokowski D, Gaccioli F, Majumder M, Mullins MR, Yuan CL, Papadopoulou B, Merrick WC, Komar AA, Taylor D, Hatzoglou M (2011) Characterization of hibernating ribosomes in mammalian cells. Cell Cycle 10:2691–2702
Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, Bodi K, Sonenshein AL (2010) Direct targets of CodY in Staphylococcus aureus. J Bacteriol 192:2861–2877
Matzov D, Aibara S, Basu A, Zimmerman E, Bashan A, Yap MNF, Amunts A, Yonath A (2017) The cryo-EM structure of hibernating 100S ribosome dimer from pathogenic Staphylococcus aureus. Nat Commun 8:723
McKay SL, Portnoy DA (2015) Ribosome hibernation facilitates tolerance of stationary-phase bacteria to aminoglycosides. Antimicrob Agents Chemother 59:6992–6999
Michalik S, Depke M, Murr A, Gesell Salazar M, Kusebauch U, Sun Z, Meyer TC, Surmann K, Pfortner H, Hildebrandt P, Weiss S, Palma Medina LM, Gutjahr M, Hammer E, Becher D, Pribyl T, Hammerschmidt S, Deutsch EW, Bader SL, Hecker M, Moritz RL, Mader U, Volker U, Schmidt F (2017) A global Staphylococcus aureus proteome resource applied to the in vivo characterization of host-pathogen interactions. Sci Rep 7:9718
Niven GW (2004) Ribosome modulation factor protects Escherichia coli during heat stress, but this may not be dependent on ribosome dimerisation. Arch Microbiol 182:60–66
Orelle C, Carlson ED, Szal T, Florin T, Jewett MC, Mankin AS (2015) Protein synthesis by ribosomes with tethered subunits. Nature 524:119–124
Ortiz JO, Brandt F, Matias VR, Sennels L, Rappsilber J, Scheres SH, Eibauer M, Hartl FU, Baumeister W (2010) Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ. J Cell Biol 190:613–621
Polikanov YS, Blaha GM, Steitz TA (2012) How hibernation factors RMF, HPF, and YfiA turn off protein synthesis. Science 336:915–918
Puri P, Eckhardt TH, Franken LE, Fusetti F, Stuart MC, Boekema EJ, Kuipers OP, Kok J, Poolman B (2014) Lactococcus lactis YfiA is necessary and sufficient for ribosome dimerization. Mol Microbiol 91:394–407
Redder P (2016) How does sub-cellular localization affect the fate of bacterial mRNA? Curr Genet 62:687–690
Sanchuki HB, Gravina F, Rodrigues TE, Gerhardt EC, Pedrosa FO, Souza EM, Raittz RT, Valdameri G, de Souza GA, Huergo LF (2017) Dynamics of the Escherichia coli proteome in response to nitrogen starvation and entry into the stationary phase. Biochim Biophys Acta 1865:344–352
Sharma MR, Donhofer A, Barat C, Marquez V, Datta PP, Fucini P, Wilson DN, Agrawal RK (2010) PSRP1 is not a ribosomal protein, but a ribosome-binding factor that is recycled by the ribosome-recycling factor (RRF) and elongation factor G (EF-G). J Biol Chem 285:4006–4014
Shcherbakova K, Nakayama H, Shimamoto N (2015) Role of 100S ribosomes in bacterial decay period. Genes Cells 20:789–801
Shimada T, Yoshida H, Ishihama A (2013) Involvement of cyclic AMP receptor protein in regulation of the rmf gene encoding the ribosome modulation factor in Escherichia coli. J Bacteriol 195:2212–2219
Tagami K, Nanamiya H, Kazo Y, Maehashi M, Suzuki S, Namba E, Hoshiya M, Hanai R, Tozawa Y, Morimoto T, Ogasawara N, Kageyama Y, Ara K, Ozaki K, Yoshida M, Kuroiwa H, Kuroiwa T, Ohashi Y, Kawamura F (2012) Expression of a small (p)ppGpp synthetase, YwaC, in the (p)ppGpp(0) mutant of Bacillus subtilis triggers YvyD-dependent dimerization of ribosome. Microbiologyopen 1:115–134
Terui Y, Tabei Y, Akiyama M, Higashi K, Tomitori H, Yamamoto K, Ishihama A, Igarashi K, Kashiwagi K (2010) Ribosome modulation factor, an important protein for cell viability encoded by the polyamine modulon. J Biol Chem 285:28698–286707
Tissieres A, Watson JD (1958) Ribonucleoprotein particles from Escherichia coli. Nature 182:778–780
Tkachenko AG, Kashevarova NM, Tyuleneva EA, Shumkov MS (2017) Stationary-phase genes upregulated by polyamines are responsible for the formation of Escherichia coli persister cells tolerant to netilmicin. FEMS Microbiol Lett 364: https://doi.org/10.1093/femsle/fnx1084
Ueta M, Yoshida H, Wada C, Baba T, Mori H, Wada A (2005) Ribosome binding proteins YhbH and YfiA have opposite functions during 100S formation in the stationary phase of Escherichia coli. Genes Cells 10:1103–1112
Ueta M, Ohniwa RL, Yoshida H, Maki Y, Wada C, Wada A (2008) Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J Biochem 143:425–433
Ueta M, Wada C, Wada A (2010) Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 15:43–58
Ueta M, Wada C, Daifuku T, Sako Y, Bessho Y, Kitamura A, Ohniwa RL, Morikawa K, Yoshida H, Kato T, Miyata T, Namba K, Wada A (2013) Conservation of two distinct types of 100S ribosome in bacteria. Genes Cells 18:554–574
Ueta M, Wada C, Bessho Y, Maeda M, Wada A (2017) Ribosomal protein L31 in Escherichia coli contributes to ribosome subunit association and translation, whereas short L31 cleaved by protease 7 reduces both activities. Genes Cells 22:452–471
van den Elzen AM, Schuller A, Green R, Seraphin B (2014) Dom34-Hbs1 mediated dissociation of inactive 80S ribosomes promotes restart of translation after stress. EMBO J 33:265–276
Vila-Sanjurjo A, Schuwirth BS, Hau CW, Cate JH (2004) Structural basis for the control of translation initiation during stress. Nat Struct Mol Biol 11:1054–1059
Wada A (1998) Growth phase coupled modulation of Escherichia coli ribosomes. Genes Cells 3:203–208
Wada A, Yamazaki Y, Fujita N, Ishihama A (1990) Structure and probable genetic location of a “ribosome modulation factor” associated with 100S ribosomes in stationary-phase Escherichia coli cells. Proc Natl Acad Sci USA 87:2657–2661
Waters NR, Samuels DJ, Behera RK, Livny J, Rhee KY, Sadykov MR, Brinsmade SR (2016) A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol Microbiol 101:495–514
Williamson KS, Richards LA, Perez-Osorio AC, Pitts B, McInnerney K, Stewart PS, Franklin MJ (2012) Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J Bacteriol 194:2062–2073
Yamagishi M, Matsushima H, Wada A, Sakagami M, Fujita N, Ishihama A (1993) Regulation of the Escherichia coli rmf gene encoding the ribosome modulation factor: growth phase- and growth rate-dependent control. EMBO J 12:625–630
Yamamoto H, Wittek D, Gupta R, Qin B, Ueda T, Krause R, Yamamoto K, Albrecht R, Pech M, Nierhaus KH (2016) 70S-scanning initiation is a novel and frequent initiation mode of ribosomal translation in bacteria. Proc Natl Acad Sci USA 113:E1180–E1189
Yoshida H, Wada A (2014) The 100S ribosome: ribosomal hibernation induced by stress. Wiley Interdiscip Rev RNA 5:723–732
Yoshida H, Ueta M, Maki Y, Sakai A, Wada A (2009) Activities of Escherichia coli ribosomes in IF3 and RMF change to prepare 100S ribosome formation on entering the stationary growth phase. Genes Cells 14:271–280
Zhang Y, Mandava CS, Cao W, Li X, Zhang D, Li N, Zhang X, Qin Y, Mi K, Lei J, Sanyal S, Gao N (2015) HflX is a ribosome-splitting factor rescuing stalled ribosomes under stress conditions. Nat Struct Mol Biol 22:906–913
Acknowledgements
This work was supported by the National Institutes of Health grant GM121359 (to MNY) and in part by the Edward Mallinckrodt Jr. Foundation and the Pew Charitable Trusts. We apologize to authors whose work could not be cited due to space constraints.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gohara, D.W., Yap, MN.F. Survival of the drowsiest: the hibernating 100S ribosome in bacterial stress management. Curr Genet 64, 753–760 (2018). https://doi.org/10.1007/s00294-017-0796-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-017-0796-2