Abstract
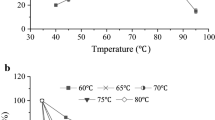
Industrially, the use of high temperatures (40–60°C) in the l-malate production process could result in rapid inactivation of the mesophilic fumarases, warranting constant replenishment of the biocatalyst. Thus, a thermostable fumarase C that is active and stable at high temperatures would be ideal. Biochemical studies using recombinant fumarase C from thermophilic Streptomyces thermovulgaris (stFUMC) indicated that it was optimally active at 50°C and highly stable even after 24 h of incubation at 40°C. The same gene from mesophilic Streptomyces coelicolor (scfumC) was also cloned and expressed as soluble proteins for comparison in thermal properties of both enzymes. In contrast to stFUMC, scFUMC exhibited a lower temperature optima of 30°C and was rapidly denatured at 50°C. The specific activity of stFUMC was also higher than that of scFUMC by 20-fold. After primary sequence comparison, three hydrophilic amino acid residues, R163, E170 and S347, were forged into the thermolabile scFUMC either singly or in combination for the investigation of their contributions in the thermal properties of the mutant enzymes. Of the mutants studied, the A347S scFUMC mutant resulted in the highest increase in optimum temperature of 10°C and a fourfold enhancement in specific activity. G163R/G170E and G163R/G170E/A347S scFUMC mutants are more thermostable than wild-type scFUMC. These findings support stFUMC as a highly efficient, thermostable fumarase C with industrial potential and suggest that R163, E170 and S347 are involved in the enhancement of thermal properties in fumarase C.



Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bressler E, Pines O, Goldberg I, Braun S (2002) Conversion of fumaric acid to L-malic by sol-gel immobilized Saccharomyces cerevisiae in a supported liquid membrane bioreactor. Biotechnol Prog 18(3):445–450
Chakravarty S, Varadarajan R (2002) Elucidation of factors responsible for enhanced thermal stability of proteins: a structural genomics based study. Biochemistry 41(25):8152–8161
Flint DH, Emptage MH, Guest JR (1992) Fumarase A from Escherichia coli: purification and characterization as an iron–sulfur cluster containing enzyme. Biochemistry 31(42):10331–10337
Giorno L, Drioli E, Carvoli G, Cassano A, Donato L (2001) Study of an enzyme membrane reactor with immobilized fumarase for production of L-malic acid. Biotechnol Bioeng 72(1):77–84
Goh LL, Barkham T, Sim TS (2005) Molecular cloning and functional characterization of fumarases C in Neisseria species. Antonie van Leeuwenhoek 87(3):205–213
Gromiha MM, Oobatake M, Sarai A (1999) Important amino acid properties of enhanced thermostability from mesophilic to thermophilic proteins. Biophys Chem 82(1):51–67
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces—a laboratory manual. The John Innes Foundation, Norwich
Kanarek L, Marler E, Bradshaw RA, Fellows RE, Hill RL (1964) The subunits of fumarase. J Biol Chem 239:4207–4211
Kobayashi K, Yamanishi T, Tuboi S (1981) Physicochemical, catalytic, and immunochemical properties of fumarases crystallized separately from mitochondrial and cytosolic fractions of rat liver. J Biochem (Tokyo) 89(6):1923–1931
Kumar S, Tsai CJ, Nussinov R (2000) Factors enhancing protein thermostability. Protein Eng 13(3):179–191
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Li WF, Zhou XX, Lu P (2005) Structural features of thermozymes. Biotechnol Adv 23(4):271–281
Loladze VV, Ibarra-Molero B, Sanchez-Ruiz JM, Makhatadze GI (1999) Engineering a thermostable protein via optimization of charge–charge interactions on the protein surface. Biochemistry 38(50):16419–16423
Mizobata T, Fujioka T, Yamasaki F, Hidaka M, Nagai J, Kawata Y (1998) Purification and characterization of a thermostable Class II fumarase from Thermus thermophilus. Arch Biochem Biophys 355(1):49–55
O’Hare MC, Doonan S (1985) Purification and structural comparisons of the cytosolic and mitochondrial isoenzymes of fumarase from pig liver. Biochim Biophys Acta 827:127–134
Pack SP, Yoo YJ (2004) Protein thermostability: structure-based difference of amino acid between thermophilic and mesophilic proteins. J Biotechnol 111(3):269–277
Puchegger S, Redl B, Stöffler G (1990) Purification and properties of a thermostable fumarate hydratase from the archaebacterium Sulfolobus solfataricus. J Gen Microbiol 136(8):1537–1541
Sacchettini JC, Meininger T, Roderick S, Banaszak LJ (1986) Purification, crystallization, and preliminary X-ray data for porcine fumarase. J Biol Chem 261(32):15183–15185
Song JK, Rhee JS (2000) Simultaneous enhancement of thermostability and catalytic activity of phospholipase A1 by evolutionary molecular engineering. Appl Environ Microbiol 66(3):890–894
Strickler SS, Gribenko AV, Gribenko AV, Keiffer TR, Tomlinson J, Reihle T, Loladze VV, Makhatadze GI (2006) Protein stability and surface electrostatics: a charged relationship. Biochemistry 45(9):2761–2766
Takata I, Tosa T (1993) Production of L-malic acid. Bioprocess Technol 16:53–65
Terasawa M, Nara T, Yukawa H, Yamagata H, Satoo Y (1990) Method of preparing L-malic acid. US Patent 4,912,043
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Vogt G, Woell S, Argos P (1997) Protein thermal stability, hydrogen bonds, and ion pairs. J Mol Biol 269(4):631–643
Weaver T, Banaszak L (1996) Crystallographic studies of the catalytic and a second site in fumarase C from Escherichia coli. Biochemistry 35(44):13955–13965
Weaver T, Lees M, Banaszak L (1997) Mutations of fumarase that distinguish between the active site and a nearby dicarboxylic acid binding site. Protein Sci 6(4):834–842
Acknowledgements
We would like to express our greatest gratitude to Dr. Doreen Tan Sian Hoong for reading and editing this manuscript and her valuable comments, which aided us in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, W., Chan, M., Goh, LL. et al. Molecular basis for thermal properties of Streptomyces thermovulgaris fumarase C hinge at hydrophilic amino acids R163, E170 and S347. Appl Microbiol Biotechnol 75, 329–335 (2007). https://doi.org/10.1007/s00253-006-0822-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0822-7