Abstract
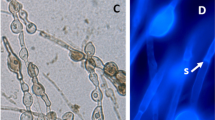
A system was developed to evaluate the effects of root growth of cotton seedlings on the inoculum dynamics ofGliocladium virens in nonsterile soil. In soil infested withG. virens, inoculum densities of the fungus increased when plants remained alive. After 30 days, shoots were excised and the roots allowed to deteriorate. During this portion of the experiment (30–60 days) soil inoculum densities ofG. virens declined. In infested soil without a seedling, inoculum densities remained constant throughout the duration of the experiments. Colonization of roots byG. virens was found to increase throughout the duration of the experiments. At 60 daysG. virens was recovered from approximately 60% of the root pieces (1-cm) sampled. The percentage of primary, secondary, or tertiary roots colonized was different (P = 0.01), but the total colonization of roots at three depths (0–10, 10–20, and 20–30 cm) was not different (P = 0.64). In noninfested soil, colonization of roots by indigenous propagules ofG. virens was never greater than 3%.
Similar content being viewed by others
References
Beagle-Ristaino JE, Papavizas GC (1985) Survival and proliferation of propagules ofTrichoderma spp. andGliocladium virens in soil and in plant rhizosphere. Phytopathology 75:729–732
Cook RJ, Baker KF (1983) The nature and practice of biological control of plant pathogens. APS Press, St. Paul, MN
Freund RJ, Littell RC, Spector PC (1986) SAS system for linear model. SAS Institute Inc, Cary, NC
Hardman JM, Pike DJ, Dick MW (1989) Short-term retrievability ofPythium propagules in simulated soil environments. Mycol Res 93:199–207
Howell CR (1987) Relevance of mycoparasitism in the biological control ofRhizoctonia solani byGliocladium virens. Phytopathology 77:992–994
Howell CR, Stipanovic RD (1983) Gliovirin, a new antibiotic fromGliocladium vixens, and its role in the biological control ofPythium ultimum. Can J Microbiol 29:321–324
Huisman OC (1988) Colonization of field-grown cotton roots by pathogenic and saprophytic soilborne fungi. Phytopathology 78:716–722
Huisman OC (1988) Seasonal colonization of roots of field-grown cotton byVerticillium dahliae andV. tricorpus. Phytopathology 78:708–716
Kamal M, Weinhold AR (1967) Virulence ofRhizoctonia solani as influenced by age of inoculum in soil. Can J Bot 45:1761–1765
Lewis JA, Papavizas GC (1984) A new approach to stimulate population proliferation ofTrichoderma species and other potential biocontrol fungi introduced into natural soils. Phytopathology 74:1240–1244
Lewis JA, Papavizas GC (1984) Chlamydospore formation byTrichoderma spp. in natural substrates. Can J Microbiol 30:1–7
Lewis JA, Papavizas GC (1985) Effect of mycelial preparations ofTrichoderma andGliocladium on populations ofRhizoctonia solani and the incidence of damping-off. Phytopathology 75:812–817
Lumsden RD, Carter JP, Whipps JM, Lynch JM (1990) Comparison of biomass and viable propagule measurements in the antagonism ofTrichoderma harzianum againstPythium ultimum. Soil Boil Biochem 22:187–194
Newman EI, Watson A (1977) Microbial abundance in the rhizosphere: A computer model. Plant Soil 48:17–56
Park Y, Stack JP, Kenerley CM (1992) Selective isolation and enumeration ofG. virens andG. roseum from soil. Plant Dis 76:230–235
Park Y, Stack JP, Kenerley CM (1991) Production of gliotoxin byGliocladium virens as a function of source and concentration of carbon and nitrogen. Mycol Res 95:1242–1248
Ray AA, Sall JP, Saffer M (1982) SAS user's guide: Statistics. SAS Institute Inc, Cary, NC
Tennant D (1975) A test for a modified line intersect method of estimating root length. J Ecol 63:995–1001
Tinker PB (1986) Conditions in the rhizosphere in relation in microbial development. In: Lugtenberg B (ed) Recognition in microbe-plant symbiotic and pathogenic interactions. Springer-Verlag, Berlin Heidelberg New York, pp 413–421
Author information
Authors and Affiliations
Additional information
Offprint requests to: C. M. Kenerley.
Rights and permissions
About this article
Cite this article
Park, YH., Kenerley, C.M. & Stack, J.P. Inoculum dynamics ofGliocladium virens associated with roots of cotton seedlings. Microb Ecol 23, 169–179 (1992). https://doi.org/10.1007/BF00172638
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00172638