Abstract
We present a biologically inspired actuator exhibiting a novel pumping action. The design of the 'artificial heartbeat' actuator is inspired by physical principles derived from the structure and function of the human heart. The actuator employs NiTi artificial muscles and is powered by electrical energy generated by microbial fuel cells (MFCs). We describe the design and fabrication of the actuator and report the results of tests conducted to characterize its performance. This is the first artificial muscle-driven pump to be powered by MFCs fed on human urine. Results are presented in terms of the peak pumping pressure generated by the actuator, as well as for the volume of fluid transferred, when the actuator was powered by energy stored in a capacitor bank, which was charged by 24 MFCs fed on urine. The results demonstrate the potential for the artificial heartbeat actuator to be employed as a fluid circulation pump in future generations of MFC-powered robots ('EcoBots') that extract energy from organic waste. We also envisage that the actuator could in the future form part of a bio-robotic artwork or 'bio-automaton' that could help increase public awareness of research in robotics, bio-energy and biologically inspired design.
Export citation and abstract BibTeX RIS
1. Introduction
The heart is one of the most elegant mechanisms in nature and has long been a source of fascination across the scientific and artistic disciplines. For example, the renaissance artist and polymath Leonardo da Vinci studied the anatomy of the heart of an ox. His drawings describe an experiment devised to investigate the fluid dynamics of the aortic track, and also include designs for an artificial heart valve [1]. In this paper we present an interdisciplinary investigation that brings together research in design, 3D printing, smart materials and energetically autonomous robotics. The investigation focusses on the development and testing of a biologically inspired pump, based on physical principles derived from the structure and function of the heart.
The development of artificial hearts and heart assist devices for the treatment of patients suffering from cardiac failure has been the subject of research in medical and engineering disciplines for a number of decades [2, 3]. However, the goal of research described in the present paper was not to create a prosthetic device for use in human or animal patients. The principal aim of the present study was to develop a biologically inspired pump for future generations of energetically autonomous robots ('EcoBots') which generate low-level electrical energy from waste organic material [4]. EcoBots employ microbial fuel cells (MFCs) to generate the energy needed for their function, from a liquid organic feedstock, and can perform useful tasks, which include locomotion, environmental sensing and wireless communication with a remote base station. Conventional electric motor-driven pumps are currently used to deliver liquid feedstock (e.g. waste water or human urine) to the anode chambers of EcoBot's MFCs and to supply fresh water to hydrate the fuel cells' cathode electrodes.
A goal of research in the field of artificial muscle materials is to replace conventional actuators (e.g. electric motors, pneumatic or hydraulic actuators) which are complex and can be prone to mechanical failure with 'smart' materials which are capable of muscle-like movement and behaviour [5]. The artificial muscle-driven pump which is the subject of the present paper could potentially replace the electric motor-driven pumps currently employed onboard the EcoBots for fluid circulation.
Further to this, we envisage that the biologically driven pump could in future form part of an engaging bio-robotic artwork or 'bio-automaton' which could help raise public awareness of biologically inspired engineering and design.
2. Biological inspiration
According to Gray [6] the human heart is a hollow muscular organ which pumps blood through the circulatory system of the body by means of regular rhythmic contractions. These cyclic muscular contractions push blood out of the heart and into the arteries of the circulatory system. Following each contraction, the heart relaxes and blood flows from the superior vena cava and inferior vena cava into the heart in readiness for the next cycle. A system of one-way valves regulates the flow of blood as it passes through the chambers of the heart and out into the circulatory system of the body. The design of the prototype pump shown in figure 1 was inspired by the physical principles described above.
Figure 1. (a) Schematic view of the artificial heartbeat pump; (b) the artificial muscles contract, compressing the soft region of the pump body and forcing fluid out of the top of the pump; (c) artificial muscles relax, the soft region re-expands and fresh fluid is drawn into the pump.
Download figure:
Standard image High-resolution imageThe pump has a hollow body which is filled with fluid (figure 1(a)). The body of the pump has a soft, flexible region that is compressed by the action of a pair of shape memory alloy 'artificial muscle' fibres. The fibres contract when heated by electric current. As they contract, they compress the soft region of the pump body, forcing fluid upwards, to be ejected out of the top of the pump (figure 1(b)). When the electric current is removed, the artificial muscle fibres cool and relax. The soft region re-expands, and fresh fluid is drawn into the body of the pump in readiness for the next cycle of actuation (figure 1(c)). One-way ball valves are employed to regulate the flow of fluid through the pump.
Having outlined the functional principles of the biologically inspired pump, we will now introduce the key technologies to be exploited in the design and prototyping of the device. We will then describe in detail the design and fabrication of the prototype pump and report results of tests carried out to characterize its performance. We will conclude by discussing the results and identifying implications for future research.
3. Microbial fuel cells and energetically autonomous robotics
Microbial fuel cells (MFCs) are bio-electrochemical transducers that exploit the metabolic action of live micro-organisms to convert bio-chemical energy into electricity. The origins of the technology may be traced back to Luigi Galvani's famous 18th century work on 'animal electricity', when he demonstrated for the very first time the flow of electrons through biological material by passing electrical current through frog legs [7]. The first practical demonstration of a working MFC was provided by Michael C Potter at the University of Durham in 1911, using the live micro-organisms Saccharomyces cerevisiae (baker's yeast) [8].
MFCs make use of the ability of live microbes to digest organic feedstock within an electrochemical cell. MFCs are commonly split into two half-cells–-an anode half-cell and a cathode half-cell–-which are separated by an ion-selective membrane. The anode half-cell contains micro-organisms in the form of a biofilm, which are fed on an organic feedstock. As they digest the organic feedstock, the microbes produce electrons which are transferred to the anode electrode. The anode electrode connects to an external circuit, allowing electrons to flow from the anode to the cathode. In the anode half-cell, protons leak into the liquid and pass through the ion-selective membrane to reach the cathode half-cell. At the cathode, the incoming electrons and protons react with an oxidizing agent, completing the reactions and closing the circuit.
A series of robots–-known as ecological robots or EcoBots-–have been developed to exploit the electrical energy generated by MFCs. So far, the organic feedstock that has been used successfully to power these robots includes food waste such as rotten fruit and vegetables, dead flies, waste water, sewage sludge and human urine. Electrical energy generated by MFCs is stored in a capacitor bank and when sufficient energy has been accumulated, it is used to perform useful work. EcoBot-III (figure 2) was the first robot to fully demonstrate energy autonomy by feeding itself and discharging waste, and by performing tasks including locomotion, environmental sensing, information processing and wireless data communication [4].
Figure 2. The EcoBot-III robot is powered by electricity generated by MFCs, which are fed on dead flies and sewage sludge. EcoBot-III can perform environmental as well as internal sensing (e.g. ambient temperature and MFC health) and locomotion, moving backward and forward on rails, as well as feeding itself and discharging waste.
Download figure:
Standard image High-resolution imageThe present generation of EcoBots employ conventional dc electric motors and pumps for performing the functions of locomotion and fluid circulation. In future generations of EcoBots, there is the potential that these conventional actuators will be replaced by 'smart' materials which are capable of muscle-like behaviour.
4. Artificial muscle materials
'Smart' materials that exhibit movement or changes in size or shape when stimulated have been described as 'artificial muscles'. In previous studies, artificial muscle materials have been actuated successfully using electricity generated by MFCs as stimulus. These include dielectric elastomer actuators (DEAs), ionic polymer metal composites (IPMCs) and shape memory alloys (SMAs). We will now introduce the basic operating principles of these different classes of artificial muscle materials.
DEAs consist of a soft elastomeric membrane which is sandwiched between a pair of stretchable electrodes. When a relatively high voltage (typically in the order of kV) is applied across the electrodes, electrostatic forces cause the elastomeric membrane to compress in thickness and expand in area. When the voltage is removed, the DEA returns to its original shape. Whilst the voltage needed to actuate DEAs is relatively high, the electric current required is low. This means that it is feasible to step up the low-level electrical output generated by MFCs to the higher voltage that is required to actuate DEAs. IPMCs comprise a flexible, ion-permeable polymer that is sandwiched between a pair of flexible electrodes. When a voltage is applied across the electrodes, the migration of cations within the ion-permeable polymer causes expansion on one side of the IPMC, resulting in a bending action. In comparison with DEAs, IPMCs require a much lower voltage to operate [5, 9, 10]. An extensive survey of artificial muscle technologies is presented in [11]. The survey describes the physical principles, capabilities and limitations of a range of artificial muscle technologies including DEAs, IPMCs, SMAs and others, such as ferroelectric polymers, conducting polymers, liquid crystal elastomers and carbon nanotubes.
SMAs are metals which can be programmed by a heat treatment process to 'remember' a desired shape. Once the shape has been programmed, and when the SMA is below its transition temperature, it can easily be deformed into a different shape. Then, when heated above the transition temperature, a change in the crystal structure within the SMA causes it to recover its programmed shape. This can be brought about either by a change in ambient temperature or by Joule heating, when an electric current is passed through the material to raise its temperature. SMA wires, fibres and helical springs can be stretched easily at room temperature, and will recover their shape when heated above the transition temperature, generating significant force [12].
In [13] it is reported that both DEA and IPMC actuators can be powered by electricity generated by MFCs. A working artificial sphincter is demonstrated which is actuated by a DEA and which could function as a valve to discharge waste from a fuel cell. Also demonstrated are IPMC-based stirrer and cilia mechanisms, which could be used to retain organic matter and microbes in suspension within the MFC, and to effect fluid circulation. In [14] a biologically inspired tubular pumping unit is described which has been developed for use as part of an artificial digestive system within future MFC-powered robots. This DEA-actuated pump expands and contracts radially and includes one-way valves to regulate fluid flow. It is intended to function as part of a chain of units within a peristaltic pumping system. Operating at 3.4 kV, pressures of up to 4.9 mbar were achieved by the pumping unit, and flow rates of greater than 40 µl s−1 were demonstrated for horizontal fluid pumping. A maximum pressure stroke of 6.4 mbar was obtained for an actuation voltage of 4.4 kV. With appropriate dc–dc voltage conversion, the pumping unit could be powered by energy stored in a capacitor bank that has been charged by MFCs.
SMA artificial muscles have been employed in a number of studies to develop prototype cardiac assist devices [15–17]. These devices are intended to support the natural contraction of the human heart in cases of chronic heart disease and heart failure. The prototype devices typically comprise a contractile band or patch which is to be attached to the exterior of the heart. They are intended to contract in unison with the heart, to assist in its pumping action. The cardiac assist devices do not include valves, since the valves of the patient's heart continue to be used to regulate the flow of blood through the heart. It is noted that the number of SMA fibres used in the cardiac assist devices is typically greater than in the 'artificial heartbeat' actuator which is the subject of the present investigation, and the electrical power requirements of the cardiac assist devices are also greater than could feasibly be provided by the EcoBot MFC stack.
Prior to undertaking the research described within the present paper, the first and last authors developed an earlier 'artificial heartbeat' actuator. This earlier device exploited pneumatic pressure from CO2 gas produced by live yeast to inflate an elastomer diaphragm [18, 19]. Movement of the diaphragm is regulated by a SMA-actuated valve, which is powered by electricity generated by MFCs. When the diaphragm is fully inflated, the valve opens to release pressure, allowing the diaphragm to return to its state of rest in readiness for the next cycle of actuation. The diaphragm exhibits a pulsating motion, with a peak pressure of approximately 21 millibars for each actuation. A 0.408 F capacitor bank was charged to 2.5 V by a stack of 48 MFCs, and this provided sufficient energy for twelve consecutive actuation cycles. This earlier device has yet to be demonstrated pumping liquid. Yet with further development, this novel, biologically driven actuator has the potential to be exploited as a diaphragm pump, for example, for the circulation of liquid feedstock and water in future MFC-powered robots. This could be a particularly appealing avenue for future research, since live yeast can be employed within an MFC as demonstrated by Potter in 1911 [8] and can also provide a means to generate pneumatic pressure for actuation.
In the fabrication of the earlier 'artificial heartbeat' actuator described above, 3D printing was employed to create the rigid structural and mechanical components, and to make moulds in which soft silicone elastomer membrane was cast to create the soft diaphragm. 3D printing is increasingly being exploited in the fabrication of robots and smart devices. We will now introduce 3D printing within the context of robotics.
5. 3D printable robots
3D printing, also known as additive layer manufacturing, is a computer-controlled fabrication process that enables physical objects to be fabricated directly from computer-aided design data. Objects can be made by 3D printing without the need for part-specific tooling. Furthermore 3D printing can be employed to create complex shapes that would be costly or impractical to make by conventional workshop-based fabrication techniques. In [20] Daigle proposes the concept of 'printable robots', envisaging the construction of complete robots by 3D inkjet printing, including 3D printable structures and mechanisms, printable electronics, batteries, and electroactive polymer-based artificial muscles.
In [21] photopolymer inkjet 3D printing (Stratasys, US/Objet Geometries, Israel) was employed to fabricate DEAs for future use in soft robotics. Materials testing carried out as part of this study identified that the 3D printed elastomer (Tango Plus, Objet Geometries) showed strong viscoelastic behaviour, indicating that its performance would not compare favourably with other elastomeric materials. Nevertheless, the study identified that despite this limitation, the 3D printable elastomer did enable the proof-of-concept actuator to be successfully fabricated and tested. 3D printing was employed extensively in the fabrication of EcoBot-III (2010), where it was used to create key structural and mechanical components of the robot, and the individual anode and cathode chambers of the 48 MFCs, which provide the robot with power [4].
Within the present study, 3D printing has been employed in the fabrication of the prototype artificial heartbeat actuator. We will now describe in detail the design and prototyping of the actuator and present results of tests carried out to characterize its performance.
6. Materials and methods
The design of the artificial heartbeat actuator is illustrated in figure 3. The actuator comprises a hollow body with a soft, compressible region, a lid with inlet and outlet ports, and one-way valves, which regulate the flow of fluid into and out of the body of the actuator. The valves employ precision-ground rubber balls (Minivalve International BV, Netherlands), and soft elastomeric valve seats help to ensure an effective seal when the valves are closed. A pair of NiTi SMA fibres (Biometal Fiber BMF100, Toki Corporation, Japan) are utilized as artificial muscles, each having an active length of 30 mm. The NiTi fibres have crimped ends and are attached by screws and threaded inserts to the body and lid sections of the actuator. A compression spring is disposed between the lid and body sections of the actuator. This spring helps return the actuator to its rest position once power is removed from the NiTi artificial muscle fibres. A silicone rubber tube (internal diameter 3.2 mm) is attached to the outlet port.
Figure 3. (a) Artificial heartbeat actuator. (b) Artificial heartbeat actuator. Prototype, 3D printed body and lid made from Objet Fullcure 720 rigid resin, paint finish. Compressible region made from silicone cast in 3D printed moulds.
Download figure:
Standard image High-resolution imageThe internal volume of the prototype device is approximately 24.5 ml and its weight is approximately 95 g. The internal diameter at the inlet and outlet ports is 3.2 mm. The wall thickness of the soft, compressible region is 1 mm. The wall thickness of the remainder of the body of the actuator varies from 1.5 to 8 mm. The compression spring has a spring constant of 0.42 N mm−1.
To fabricate the prototype artificial heartbeat actuator, photopolymer jetting (Stratasys, US/Objet Geometries, Israel) was employed. The hollow body and lid of the actuator were created in Objet Fullcure 720 rigid 3D printing resin. The same 3D printing process and material was used to fabricate a mould into which a silicone elastomer (RTV 139 with 5% w/w C148 catalyst, Alchemie, Warwick) was cast to create the soft, compressible region. The soft valve seats were moulded in the same way. In a second version of the prototype actuator, the hollow body section and compressible region were fabricated as a single component by 3D printing, using the soft, rubber-like 3D printing material, TangoPlus (Stratasys, US/Objet Geometries, Israel). Creating soft elastomer components directly by 3D printing in this way has the advantage of eliminating the mould-making and casting stages from the prototyping process. In the TangoPlus version, a rigid insert was incorporated in the body section of the actuator to provide localized stiffening. This was necessary to ensure that the force generated by the NiTi artificial muscles is effectively transmitted through the body of the actuator rather than merely causing localized flexing. The shore hardness of the TangoPlus material is in a similar range to the RTV139 silicone elastomer [22, 23]. Tests could therefore be carried out to compare the pumping performance of the actuator with the compressible region made from silicone with the version made from TangoPlus.
Following assembly, the body of the actuator was manually filled with water using a syringe. This step is necessary in order to remove air from the body of the actuator. Once the actuator has been filled with water it is ready to operate. The actuator operates as follows: electric current flows through the NiTi artificial muscle fibres; this raises the temperature of the NiTi fibres by Joule heating, causing them to contract in length by approximately 4% [24]. The contraction of the NiTi fibres compresses the soft region, causing water to be pushed though the body of the actuator and ejected through the outlet tube (figure 4(a)). When the electric current is removed, the artificial muscles cool and relax. The compressible region re-expands, and the actuator returns to its rest position. As the compressible region re-expands, water from the reservoir is drawn though the inlet valve and into the body of the actuator, in readiness for the next cycle of actuation (figure 4(b)).
Figure 4. Artificial heartbeat actuator (a) pressure pulse (systole) and (b) relaxation and refilling (diastole).
Download figure:
Standard image High-resolution image7. Material properties
To investigate the elastic properties of the silicone and TangoPlus materials used for the compressible region of the actuator, uniaxial tensile testing was undertaken on specimens of both materials. Testing was carried out using an Instron tensile testing machine fitted with a 10 N load cell. The test length of each specimen was 25 mm and the cross-sectional area was 2 mm × 6 mm. Specimens were extended to 110% strain and back at a rate of 500 mm min−1. Four specimens of each material were tested. Typical stress–strain curves for RTV 139 silicone and TangoPlus specimens are shown in (figure 5).
Figure 5. Engineering stress–strain curves for RTV 139 silicone and TangoPlus elastomers.
Download figure:
Standard image High-resolution imageIt can be clearly seen from the graphs that the hysteresis between loading and unloading curves for TangoPlus is significantly larger than for silicone. This indicates a greater viscoelastic response from the TangoPlus material than from the silicone specimen. This finding is in agreement with [21] who reported strong viscoelastic behaviour for TangoPlus.
Approximate values for the Young's modulus of silicone and TangoPlus specimens are recorded in table 1. These values are based on the gradient of the tangent to the stress–strain curve at 50% strain. The TangoPlus material has a slightly higher Young's modulus than silicone at 50% strain. However, it can be seen from the graphs in figure 5 that at higher strains, the curve for silicone steepens. The graphs indicate that for strains greater than approx. 65%, the Young's modulus of silicone overtakes that of TangoPlus.
Table 1. Young's modulus at 50% strain for RTV 139 silicone and TangoPlus elastomers.
| Specimen | Young's modulus (N mm−2) |
|---|---|
| Silicone 1 | 0.319 |
| Silicone 3 | 0.335 |
| Silicone 3 | 0.320 |
| Silicone 4 | 0.341 |
| Silicone mean | 0.329 |
| TangoPlus 1 | 0.376 |
| TangoPlus 2 | 0.376 |
| TangoPlus 3 | 0.361 |
| TangoPlus 4 | 0.367 |
| TangoPlus mean | 0.370 |
8. Finite element analysis
In order to estimate the change in volume that occurs in the pump when the NiTi fibres contract, finite element analysis was used to model the deformation in the compressible region of the pump body. A non-linear displacement control analysis was undertaken using the software Solidworks Simulation (Dassault Systemes, France). The software's standard linear rubber material model was modified to include the mean Young's modulus value for silicone from the preceding tensile tests.
The finite element model of the compressible region is shown in figure 6. In the model, the upper face of the compressible region was fixed, whilst the lower face was displaced upwards by a distance of 1.2 mm. This value represents a contraction of 4% in the 30 mm long NiTi fibres and is the practical contraction stated by the manufacturer [24].
Figure 6. (a) Finite element analysis displacement plot showing deformation in the compressible region of the artificial heartbeat actuator, (b) un-deformed cross-section and (c) deformed cross-section.
Download figure:
Standard image High-resolution imageThe cross-section of the compressible region in its un-deformed state is shown in figure 6(b). The deformed shape is shown in figure 6(c). The deformed shape was exported as a solid body and used to calculate the change in volume of the pump, which was found to be approximately 2.1 ml. This value corresponds to approximately 8.6% of the internal volume of the pump and represents the volume of fluid which could be displaced by the pump in an idealized scenario.
It should be noted that this is a highly simplistic analysis since it neglects such critical factors as hydrodynamic losses and inertial effects, which would clearly have a significant impact on the performance of a real pump. A more comprehensive analysis would need to include dynamic fluid–structure interaction and non-linear material properties. Such an analysis would be an extremely complex task, and is considered to be beyond the scope of the 'proof of concept' investigation described within this paper. Nevertheless, an analysis of this kind would be extremely valuable, since it would enable the design of the pump to be fine-tuned and optimized. It will therefore be vital for future research, following on from the present investigation.
9. Drive signal
In the prototype actuator, the NiTi artificial muscle fibres are powered by a rectangular wave drive signal, the 'on' and 'off' times of which had to be determined. This was carried out using the prototype actuator with the compressible region made from silicone, as this was the first version to be prototyped and tested. Power to the NiTi artificial muscles was controlled by a relay which was operated via a programmable microcontroller, and the drive signal voltage of 3.2 V was provided by a bench power supply. Pressure at the outlet port of the pump was measured using a digital manometer (GMSD 350 MR, Greisinger Electronic GmbH). Using the digital manometer connected to a data logger, the magnitude of pressure pulses generated by the pump were recorded for 'on' times of between 0.1 and 0.7 s, as shown in figure 7 and table 2.
Figure 7. Pressure pulse and drive signal 'on' time.
Download figure:
Standard image High-resolution imageTable 2. Pressure pulse and drive signal 'on' time.
| Drive signal 'on' time (s) | Pressure reading (mbar) |
|---|---|
| 0.1 | 13.09 |
| 0.3 | 32.29 |
| 0.5 | 34.74 |
| 0.7 | 34.98 |
For an 'on' time of 0.1 s, pressure pulses of approximately 13 mbar were recorded. The pressure pulses increased significantly for 'on' times of 0.3 s and 0.5 s, where pulses of 32.29 and 34.74 mbar were recorded. However, increasing 'on' times above 0.5 s (e.g. to 0.7 s) did not significantly increase the pressure pulse generated by the pump. Meanwhile an 'off' time of 3 s was found to allow sufficient time for the NiTi actuators to cool and relax in readiness for the next actuation. The 'on' and 'off' times of the drive signal were therefore set at 0.5 s and 3 s, respectively.
10. Comparison between the finite element analysis and the physical prototype
In the finite element analysis that was presented in section 8, the volume change in the pump body was found to be 2.1 ml. Tests were carried out to compare the volume change predicted by the finite element analysis with the behaviour of the physical prototype. The tests were performed using the version of the prototype actuator with compressible region made from silicone. Electrical power to the prototype actuator was provided by a bench power supply, with a relay and programmable microcontroller switching power to the NiTi artificial muscles. The drive signal voltage was 3.2 V and the 'on' and 'off' times were 0.5 and 3 s.
The body of the actuator was first filled with water, then the apparatus was arranged as shown in figure 8 with the outlet tube extending to a height of 80 mm above the inlet water level. The actuator performed 30 consecutive actuations, after which the water in the collection beaker was measured to determine the volume which had been pumped. The results for five trials of 30 actuations are recorded in table 3.
Figure 8. Pumping trial apparatus. The outlet tube is 80 mm higher than the water level of the inlet reservoir.
Download figure:
Standard image High-resolution imageTable 3. Volume of water pumped in 30 consecutive actuations.
| Trial no. | Volume pumped in 30 actuations (ml) |
|---|---|
| 1 | 31.0 |
| 2 | 31.2 |
| 3 | 31.9 |
| 4 | 29.6 |
| 5 | 29.4 |
| Mean volume | 30.62 |
From the data presented in table 3, the mean volume of water to be pumped in 30 actuations was calculated to be 30.62 ml. Therefore the mean volume of water to be pumped in a single actuation was calculated to be 1.02 ml. This compares with the volume change of 2.1 ml predicted by the finite element analysis. As stated previously, the finite element analysis represented an idealized scenario which neglected hydrodynamic losses and inertial effects.
In addition, the contraction of the NiTi fibres on the physical prototype was measured to be within the range of 0.9 to 1.1 mm. This is less than the value of 1.2 mm which was used in the finite element model. It was also observed that as well as deforming vertically, the compressible region bulges outwards slightly upon actuation. Taking these factors into consideration, it is not surprising that the volume pumped by the physical prototype is substantially lower than the change in volume that was predicted by the finite element model.
11. Further pumping tests
In order to gain a greater understanding of the performance of the artificial heartbeat actuator, to compare versions of the actuator with compressible regions made from silicone and TangoPlus, and to consider its suitability for use as a pump on the EcoBot platform, a further series of tests was conducted.
The first of these tests measured and compared the magnitude of the pressure pulses generated by the two versions of the prototype actuator. A piezoresistive pressure sensor (model MPXV5004, Freescale Semiconductor Inc., USA) was connected to the outlet port of the actuator. The output voltage from the pressure sensor was measured and recorded using a purpose-built data logger and control circuit, built using the Arduino microcontroller prototyping platform (Smart Projects, Italy). The power source for the actuator was a 5 F capacitor bank, which is representative of the capacitor bank which would be charged by the EcoBot MFC stack. For this test, the capacitor bank was charged to 3.2 V using a bench power supply and was then connected in series via a relay to the NiTi artificial muscles. The control circuit and data logger enabled electrical power to the artificial muscles to be switched on and off using the relay, whilst simultaneously recording the pressure sensor reading and the voltage of the capacitor bank. As with previous tests, the 'on' time of the drive signal was 0.5 s and the 'off' time was 3 s. The test successfully measured the pressure pulses generated by the two versions of the artificial heartbeat actuator and showed how the magnitude of the pulses is reduced as the capacitor bank discharges.
The purpose of the second test was to investigate and compare the performance of the prototype actuators in pumping water. The apparatus was arranged once again as shown in figure 8 with the outlet tube extending to a height of 80 mm above the inlet water level. The actuators were powered by a 5 F capacitor bank which was charged to 3.5 V using a bench power supply. For this and future tests the electrical power to the actuator was controlled by a self-contained timing circuit based on a low-power 555 timer (TS555CN, S. T. Microelectronics, Switzerland). A MOSFET (FDD306P, Fairchild Semiconductor, USA) at the output of the 555 timer switches power to the artificial muscles. The electrical charge stored in the capacitor bank was used to power both the timing circuit and the actuator itself. Again, the 'on' time was 0.5 s and the 'off' time was 3 s. In these tests, each version of the actuator was allowed to operate until the charge in the capacitor had dropped below the level at which it could provide sufficient power to pump water through the outlet tube. Following each test, the water in the collection beaker was measured in order to compare the volume pumped by each actuator.
The third test investigated the capability of MFCs to provide the electrical power needed to operate the prototype actuator. In this test a stack of 24 MFCs onboard the new EcoBot IV robot currently under development (individual MFC size 6.25 ml) was fed on human urine donated by healthy volunteers (figure 9). Urine was identified as an appropriate feedstock because it is an abundant waste product, and it has recently been shown to be converted directly into electricity by MFCs [25]. Also, as a liquid, urine could potentially be pumped by the artificial heartbeat actuator, leading to the future possibility of the actuator pumping urine as fuel for an MFC-powered robot. The time taken for the stack of 24 MFCs to charge the 5 F capacitor bank from 0 to 3.5 V was recorded. This represents the initial 'start-up time' that is required to charge the capacitor bank when it is first connected to the MFC stack. A capacitor bank which had been charged by the MCF stack was used to power the artificial heartbeat actuator. Following a successful series of actuations, the time taken for the MFC stack to recharge the capacitor bank to 3.5 V was recorded. This represents the 'recharge time' i.e. the time that is needed to recharge the capacitor bank between bursts of actuator activity.
12. Results and discussion
12.1. Pressure pulse test
The results of tests to investigate the magnitude of pressure pulses generated by the prototype actuators are shown in the graphs in figures 10–12. Figures 10 and 11 show the drive voltage signal and pressure pulses generated by the actuator with the compressible region made from silicone. Figure 12 shows results for the actuator with soft body and compressible region made from the TangoPlus material. On the graphs, the blue (dashed) curve indicates the drive voltage signal from the capacitor bank, whilst the red curve (solid) indicates the corresponding pressure pulses generated by the actuator. The drive voltage decays as the capacitor bank discharges with each actuation. This is emphasized in the inset graph in figure 10. The voltage level at the end of the first pulse is approximately the starting voltage of the subsequent pulse, subject to the 'bounce-back' effect of super capacitors (as exemplified by the spike at the start of each of the pulses). The size of the pressure pulses falls as the capacitor bank discharges with each actuation.
Figure 9. The 24 small-scale microbial fuels cells, which provided electrical power to charge the 5 F capacitor bank. Each MFC has a volume of 6.25 ml.
Download figure:
Standard image High-resolution imageFigure 10. Actuator with compressible region made from silicone. Drive voltage signal and pressure pulses, for ten successive actuations.
Download figure:
Standard image High-resolution imageFigure 11. Actuator with compressible region made from silicone. Drive voltage signal and pressure pulses, for 30 successive actuations.
Download figure:
Standard image High-resolution imageFigure 12. Actuator with soft body and compressible region made from TangoPlus. Drive voltage signal and pressure pulses, for 30 successive actuations.
Download figure:
Standard image High-resolution imageTable 4 presents values for the magnitude of the pressure pulses generated by the two versions of the actuator. For the actuator with compressible region made from cast silicone, the peak pressure pulse of 34.40 mbar (gauge pressure) occurred on the first actuation. By the tenth actuation, the magnitude of the pressure pulse generated by the actuator had fallen to 29.07 mbar and by 30 actuations, it had dropped to 20.74 mbar. For the version of the actuator with a soft body and compressible region made from TangoPlus, lower values of pressure were recorded. For the first actuation, the peak pressure pulse was 18.94 mbar. The pressure pulse at the tenth actuation measured 16.81 mbar and at the 30th actuation, 14.03 mbar.
Table 4. Magnitude of pressure pulse for 1st, 10th and 30th actuations.
| Material | Actuation no. | Pressure (mbar) | Pressure (percent) |
|---|---|---|---|
| Silicone | 1 | 34.40 | 100% |
| 10 | 29.07 | 84.5% | |
| 30 | 20.74 | 60.3% | |
| TangoPlus | 1 | 18.94 | 100% |
| 10 | 16.81 | 88.8% | |
| 30 | 14.03 | 74.1% |
12.2. Water pumping test
In the water pumping test, the capacitor bank was charged to 3.5 V using a bench power supply. This was found to provide enough electrical energy for at least 32 consecutive actuations, after which the voltage of the capacitor bank had dropped to approximately 2.3 V. The amount of water transferred in 32 actuations was measured for each actuator. The prototype actuator with the compressible region made from silicone transferred 26.4 ml of water. The prototype actuator with a soft body and compressible region made from TangoPlus transferred 16.6 ml of water.
12.3. MFC charging test
The remaining tests were performed with the prototype actuator with the compressible region made from silicone, since that version had performed best in the previous two tests. After feeding each of the 24 MFCs with 2 ml of fresh urine, the initial charge from 0 to 3.5 V took approximately 698 min (less than 12 h). The actuator then performed 33 consecutive actuations, after which the voltage across the capacitor bank had dropped to approximately 2.3 V. The volume of water transferred in 33 actuations was 27 ml. The capacitor bank was then recharged. It took between 110 and 203 min (between 2 and 3 h approx.) to recharge the capacitor bank from 2.3 to 3.5 V in readiness for the next actuator activity.
12.4. Efficiency calculations
In the previous section, the artificial heartbeat performed 33 actuations, pumping a total 27 ml of water to a height of 80 mm. After 33 actuations, the voltage across the 5 F capacitor dropped from 3.5 to approximately 2.3 V.
The total electrical energy expended in 33 actuations can be calculated as follows:
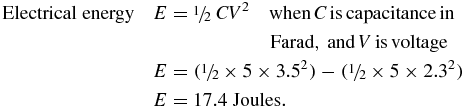
The work done raising 27 ml of water through to a height of 80 mm:

The efficiency of the artificial heartbeat actuator is therefore:

The value of 0.11% appears very low. However we can compare the efficiency of the artificial heartbeat actuator with that of a conventional, electric motor-driven impeller pump (TCS Micropumps M200S) used recently by the EcoBot research team for fluid circulation in MFC systems.
For a direct comparison with the artificial heartbeat actuator, the impeller pump was also powered by the 5 F capacitor bank, which was charged to 3.5 V. Water was pumped to a height of 80 mm. The impeller pump was allowed to run for 22 s, which was the time taken for the voltage across the capacitor to drop from 3.5 V to approximately 2.3 V. In this time, the impeller pump was found to transfer 175.5 ml of water.
In the case of the impeller pump, the work done was therefore equal to

The efficiency of the impeller pump is

The conventional electric motor-driven impeller pump is found to be more efficient than the artificial heartbeat actuator, by a factor of approximately 7.2.
It is not surprising that the electric motor-driven pump is significantly more efficient than the artificial heartbeat actuator. Electric motor-driven pumps have been developed and refined over decades. The artificial heartbeat actuator is the first 'proof-of-concept' prototype of its kind, and as yet no effort has been made to investigate how it might be improved to give greater efficiency. Gains in efficiency may result from improvements in the hydrodynamic performance of the artificial heartbeat actuator–-in particular, by avoiding abrupt changes in cross-section in the internal cavity of the actuator. In the present design, hydrodynamic losses are likely to be due to frictional losses in fluid flow through the actuator, and the abrupt change in cross-section between the body of the pump and the outlet port. A smoother transition between the pump body and the outlet port may help to reduce such losses.
Also, reducing the weight of the lower section of the actuator would decrease the loading on the artificial muscle fibres. This could be achieved simply by reducing the wall thickness of this component, which is currently rather thick.
12.5. Repeatability
In order to investigate repeatability in the performance of the prototype artificial heartbeat actuator, a series of three further pumping trials were undertaken. As before, energy was provided by the 5 F capacitor bank charged to 3.5 V. These are shown in table 5 alongside the results for the previous two trials.
Table 5. Repeatability in volume of fluid pumped.
| Trial | Number of actuations | Volume transferred (ml) |
|---|---|---|
| 1 | 32 | 26.4 |
| 2 | 33 | 27.0 |
| 3 | 33 | 28.7 |
| 4 | 32 | 27.5 |
| 5 | 32 | 27.1 |
| Mean | 27.34 |
As can be seen from table 5, there is some variation in the volume of fluid pumped by the artificial heartbeat actuator. In order to provide a robust assessment of the reliability and durability of the actuator, a more extensive longitudinal study is needed. Regarding the service life of the NiTi artificial muscles, the manufacturer does provide data for a service life of more than 300 million cycles. This is, however, for specific operating conditions [24]. It will therefore be necessary to undertake an investigation of service life for the particular operating conditions of the artificial heartbeat actuator. This will be a focus for future research.
12.6. Urine pumping demonstration
In a further demonstration, urine was used as the liquid to be pumped by the artificial heartbeat actuator. The body of the actuator with the compressible region made from silicone was prefilled with urine, and the inlet reservoir was also filled with urine, as shown in figure 13. The capacitor bank was charged to 3.5 V, after which the actuator performed 32 actuations, transferring approximately 26 ml urine. As in previous tests, the pumping height was 80 mm above the level of the inlet reservoir.
Figure 13. Artificial heartbeat actuator pumping urine.
Download figure:
Standard image High-resolution imageIn tests carried out to compare the pumping performance of the two versions of the actuator, the TangoPlus version underperformed compared to the silicone version, both in terms of the magnitude of pressure pulses generated by the actuator and the volume of fluid that was transferred. This difference in performance is likely to be due to greater viscoelastic damping in the TangoPlus material resulting in a reduction in the magnitude of the pressure pulses generated by the actuator. This possibility is in agreement with the materials testing undertaken within this study and the results reported in [21], where it was found that TangoPlus showed strong viscoelastic behaviour. To investigate this aspect further, a more extensive study of the dynamic response of the TangoPlus material in comparison to other elastomers e.g. silicones, acrylates and polyurethanes would be desirable. A reduction in actuator performance resulting from viscoelastic effects would clearly be critical for low-energy applications such as the EcoBot, and so this aspect should be taken into account in any future developments.
A stack of 24 MFCs charged the 5 F capacitor bank which was used to power the prototype actuator. The initial charge from 0 to 3.5 V took less than 12 h. For the actuator with silicone compressible region, the 5 F capacitor bank provided enough energy to transfer 27 ml of water. The time taken to recharge the capacitor bank was then between 2 and 3 h (mean recharge time: 2 h 25 min).
In order to assess the suitability of the prototype artificial heartbeat actuator for potential use on a future EcoBot platform, it is necessary to identify the daily fluid pumping requirements for an MFC stack. The EcoBot generation currently under development (EcoBot IV) has a stack of 24 MFCs, which require 4 ml of water per MFC per day for cathode hydration. The total volume of water that is required to be pumped per day is therefore 96 ml. This could be pumped by the prototype actuator with silicone compressible region in four charging cycles. The feeding that is required to maintain a healthy MFC stack would be approximately 2 ml of urine per MFC per day. The total volume of urine required by the stack of 24 MFCs is therefore 48 ml. This could be pumped by the actuator in two charging cycles. Assuming an average recharge time of 2 h 25 min for charging the 5 F capacitor bank from 2.3 to 3.5 V, the total charge time required would be 14 h 30 min per day, which is perfectly aligned with EcoBot IV's maintenance regime. The artificial heartbeat has the capability to pump water or urine to a height of 80 mm, which is more than enough to satisfy the feeding and hydration requirements of EcoBot IV.
A problem encountered with the conventional electric motor-driven pumps employed on the existing EcoBot is the tendency for the pump to block due to the presence of small particulates sometimes found in the fuel (e.g. in wastewater) or through precipitation, especially in the case of urine. In the design of the artificial heartbeat actuator, steps have been taken to help overcome this problem. Firstly, at the narrowest point, the internal orifice size of the prototype actuator is larger than that of the existing electric motor-driven pumps. Secondly, the design allows for any particulates or precipitants to settle to the bottom of the body section of the actuator, without impeding flow. If needed, a drain plug could easily be incorporated in the body section of the actuator to allow any sediment to be periodically removed. The natural precipitation from urine is struvite which is a valuable resource, since it mainly consists of magnesium, ammonium and phosphate [26]. The artificial heartbeat actuator can thus not only facilitate fluid circulation onboard EcoBot without blockages, but it could also assist in the recovery of useful minerals.
13. Conclusion
We have described the design and prototyping of a novel artificial muscle-driven pump and presented results of a series of tests undertaken to characterize its performance. We have also demonstrated that this 'artificial heartbeat' actuator can be powered by electricity generated by MFCs fed on human urine. The prototype actuator is intended for future use as a pump for fluid circulation onboard an MFC-powered robot ('EcoBot').
We speculate that in future, a urine-powered EcoBot could be employed as an environmental sensing platform within an urban setting. The EcoBot could harvest energy from waste collected from urinals at public lavatories. The energy harvested could be used by the EcoBot to perform sensing tasks such as monitoring air quality and pollution levels, and a number of such EcoBots could form a distributed mobile sensor network within a future city environment.
Further work is required to improve the efficiency of the artificial heartbeat actuator and to investigate the long-term reliability of the pump. We will also explore the scalability of the design, to establish whether its performance may be improved by increasing or decreasing the size and capacity of the pump. Whilst at present the efficiency of the artificial heartbeat actuator is low, it could have other advantages over the conventional electric motor-driven pumps employed on the EcoBot platform. For example, it may be less prone to blockages. Providing efficiency can be improved and reliability demonstrated, a pump of this type could find future use in industrial, scientific or medical applications, such as coolant circulation or drug delivery, where silent actuation may be an advantage. Future research will consider the possibility of a more compact, 'all soft' version which could serve as a wearable drug delivery pump for patients requiring medication on the move.
We further envisage that the artificial heartbeat actuator could form part of a bio-robotic artwork or 'bio-automaton' which could help to increase public awareness of research in biologically inspired robotics and energetically autonomous systems. The EcoBot series of robots has already attracted significant public interest and media attention [27, 28]. We can imagine that the demonstration of a novel, cyborg-like machine with a biologically driven artificial heartbeat could achieve a similar level of impact, potentially attracting the interest of a new generation of scientists, artists and engineers.
Acknowledgments
The team's research into smart artificial muscle materials and 3D printing has been funded by an Early Career Grant from the University of the West of England. The research into urine as a feedstock for microbial fuel cells is funded by the UK Engineering and Physical Sciences Research Council (EPSRC) grant numbers EP/I004653/1 and EP/L002132/1 and the Bill and Melinda Gates Foundation grant number OPP1044458.












