Overexpression of MdIAA9 confers high tolerance to osmotic stress in transgenic tobacco
- Published
- Accepted
- Received
- Academic Editor
- Ute Hoecker
- Subject Areas
- Ecology, Genomics, Plant Science
- Keywords
- Apple, Auxin, AUX/IAA gene family, Expression analysis, Abiotic stress
- Copyright
- © 2019 Huang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Overexpression of MdIAA9 confers high tolerance to osmotic stress in transgenic tobacco. PeerJ 7:e7935 https://doi.org/10.7717/peerj.7935
Abstract
Auxin is a plant hormone that takes part in a series of developmental and physiological processes. There are three major gene families that play a role in the early response of auxin and auxin/indole-3-acetic acid (Aux/IAA) is one of these. Although the genomic organization and function of Aux/IAA genes have been recognized in reference plants there have only been a few focused studies conducted with non-model crop plants, especially in the woody perennial species. We conducted a genomic census and expression analysis of Aux/IAA genes in the cultivated apple (Malus × domestica Borkh.). The Aux/IAA gene family of the apple genome was identified and analyzed in this study. Phylogenetic analysis showed that MdIAAs could be categorized into nine subfamilies and that these MdIAA proteins contained four whole or partially conserved domains of the MdIAA family. The spatio-specific expression profiles showed that most of the MdIAAs were preferentially expressed in specific tissues. Some of these genes were significantly induced by treatments with one or more abiotic stresses. The overexpression of MdIAA9 in tobacco (Nicotiana tabacum L.) plants significantly increased their tolerance to osmotic stresses. Our cumulative data supports the interactions between abiotic stresses and plant hormones and provides a theoretical basis for the mechanism of Aux/IAA and drought resistance in apples.
Introduction
Auxins participate in diverse processes in the development and physiology of plants, including their cell division and differentiation, organogenesis, plant geotropic and phototropic responses, embryogenesis, apical dominance, root development, lateral root formation, axillary bud and lateral branch formation, leaf morphology, and vascular differentiation (Woodward & Bartel, 2005; Ljung, 2013). Ultimately, these processes are governed by the expression of an assortment of auxin-responsive genes (De Smet & Jürgens, 2007). The most prevalent and predominantly studied auxin, indole-3-acetic acid (IAA), enhances the transcription of several classes of early genes, which includes small auxin-up RNA (SAUR), Gretchen Hagen3 (GH3), and auxin/indole-3-acetic acid (Aux/IAA) gene family members (Abel & Theologis, 1996). In the absence of auxin, Aux/IAA proteins act as transcriptional repressors at promoter sites of the auxin-responsive genes, at least in part by interfering with the activity of the auxin-response factor (ARF) transcriptional regulators. Once the auxin levels rise, the AUX/IAA proteins are degraded by poly ubiquitination and, subsequently, 26S proteasome, thus depressing ARF-mediated transcription (Tiwari et al., 2001).
Most Aux/IAA proteins have four conserved interaction domains (I–IV) (Hagen & Guilfoyle, 2002). Domain I contains an ERF-associated amphiphilic repression module that interacts with the TOPLESS (TPL) corepressor (Tiwari, Hagen & Guilfoyle, 2004; Oh et al., 2014). Domain II is associated with auxin transport inhibitor response 1; mutations occurring in this domain affect their interaction and results in a low auxin response (Gray et al., 2001). Domains III and IV are able to regulate hetero-dimerization with the other Aux/IAA family members, and interactions with ARF proteins (Ulmasov et al., 1997; Shen et al., 2010).
In 1982, the first Aux/IAA gene was identified in the soybean (Glycine max) (Walker & Key, 1982) and many more members of the Aux/IAA genes were subsequently reported by genome-wide analyses in Arabidopsis (Arabidopsis thaliana, 34 genes), maize (Zea mays L., 34 genes), rice (Oryza sativa L., 31 genes), cucumber (Cucumis sativus L., 27 genes), tomato (Solanum lycopersicon, 26 genes), Chinese hickory (Carya cathayensis Sarg., 22 genes), papaya (Carica papaya L., 18 genes) and Medicago (Medicago truncatula, 17 genes) (Liscum & Reed, 2002; Wu et al., 2012, 2014; Yuan et al., 2018; Wang et al., 2010b; Shen et al., 2014; Jain et al., 2006; Liu et al., 2017). In Arabidopsis, the function of several Aux/IAA genes have been characterized by their corresponding mutants. IAA7, IAA17, IAA19, and IAA28 are involved in determining the numbers of lateral roots, whereas IAA14 is necessary for the formation of the lateral roots (Fukaki et al., 2002). In tomatoes, SlIAA9 has been shown to play multiple roles in the development of the leaf shape and fruit (Wang et al., 2005), while SlIAA15 assists in the formation of the axillary buds and the epidermis (Deng et al., 2012). In potatoes (Solanum tuberosum L.), the down regulation of StIAA2 results in a greater plant height and petiole hyponasty (Kloosterman, Visser & Bachem, 2006). In rice, OsIAA6 is highly induced by drought stress and its overexpression (OE) in transgenic rice improves drought tolerance (Jung et al., 2015).
Apples (Malus × domestica Borkh.) are a fruit crop that is cultivated throughout the world, occupying an important economic position in the world’s fruit production (Dong et al., 2018a). Apple breeding focuses on improving the stress resistance of this crop as biological and abiotic stresses are key factors in the distribution and yield of apple trees (Mao et al., 2017). However, the GH3 gene family is currently the only one to have been investigated in the apple (Yuan et al., 2013). Previous studies have shown that specific Aux/IAA genes are involved in fruit development, maturation, and drought stress responses (Liu et al., 2017). However, MdIAAs have not been well studied under the background of abiotic stress response. Here, we have identified 34 Aux/IAA genes in the apple. Our goal was to document their chromosomal location, intron-exon structure, cis-element composition, and expression under development and stress. Additionally, we describe the corresponding protein domain architecture and phylogenetic relationships among the apple Aux/IAAs and those from other plants. This study also investigated the function of MdIAA9 by overexpressing MdIAA9 in tobacco. Our work provides a valuable resource for further investigating the functional mechanisms of MdIAAs in modulating the abiotic stress tolerance of the apple.
Materials and Methods
Plant materials and treatments
Tissue-specific expression was monitored in the young roots, stems, fully expanded leaves, flowers, and mature fruits from 5-year-old apple plants that had been treated with bud grafting. The apple plant was the combination of a scion (Malus domestica Golden Delicious) and a stock plant (Malus hupehensis). To vary the treatments, 1 year-old plants (Golden Delicious scions and Malus hupehensis rootstocks) were grown in pots (diameter: 300 mm, height: 320 mm) in a greenhouse. One seedling was grown per pot and 60 pots were used per treatment. In order to achieve drought stress, irrigation for the seedlings was discontinued when the plant height reached approximately 0.5 m. The 9th–12th leaves were collected from the base of the plants when irrigation had been withheld for 0, 2, 4, 6, 8, and 10 days. A 200 mM sodium chloride (NaCl) solution was used to irrigate plants using a salt stress treatment and the samples were collected at 0, 1, 5, 10, 15, and 20 days after irrigation. For chilling treatments, plants were put into phytotrons (24 h-constant illumination and photosynthetic photon flux of 270 μmol·m−2·s−1), the temperature was adjusted to 4 °C and the samples were collected at 0, 2, 4, 8, 12, and 24 h. For abscisic acid (ABA) and IAA treatments, plants were sprayed with 100 mM ABA or 10 µM IAA and the samples were collected at 0, 2, 4, 8, 12, and 24 h. The samples were frozen immediately in liquid nitrogen, then stored at −80 °C until RNA analysis occurred. The seedlings of the wild type (“NC89” tobacco) were used for genetic transformations. Transgenic plants and wild types were cultured in a growth chamber under a 16-h photoperiod at 23 °C. A total of 15 day old plants were grown on Murashige and Skoog (MS) agar medium supplemented with 0 or 200 mM of mannitol and left to grow for 13 day for the assay of osmotic stress. Seedling growth parameters, such as root lengths, fresh weights, and relative electrolyte leakage (REL), concentrations of chlorophyll, malondialdehyde (MDA), and proline were then measured. All of the experiments were repeated three times.
Identification of apple Aux/IAA genes
Annotated Aux/IAA open reading frame (ORF) translations from Arabidopsis were obtained by searching the TAIR website (TAIR10; http://www.arabidopsis.org/) (Dong et al., 2018a). A total of 34 Arabidopsis Aux/IAA sequences were used as queries in a homology search of annotated ORF translations from the Genome Database for Rosaceae (GDR; http://www.rosaceae.org/) (Tian et al., 2015) using the Basic Local Alignment Search Algorithm (https://www.rosaceae.org/blast). In addition, we searched the annotated apple ORF translations using a Hidden Markov Model-based approach (HMMER v3.0) and the Aux/IAA domain signature PF02309 as maintained by the Pfam database (http://pfam.xfam.org/family/PF02309). The presence of Aux/IAA domains was confirmed in each candidate MdIAA sequence using the Pfam database (http://pfam.sanger.ac.uk/search) and NCBI-Conserved Domain Search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) Shao et al. (2014) (Dong et al., 2018b).
Multiple sequence alignments, phylogenetic analysis, and exon/intron organization of MdAux/IAA proteins
Multiple sequence alignments were performed for 34 MdIAA protein sequences using DNAMAN 6.0.3.99 with default parameters (Zhou et al., 2017). Full-length protein sequences of Arabidopsis and rice were obtained from the NCBI protein database. The phylogenetic trees were estimated with the MEGA6 program (Dong et al., 2018b) using the neighbor-joining method (Saitou & Nei, 1987) with Poisson corrections and 1,000 replications for the bootstrap analysis. Genomic sequences and location data in apples were observed from the apple genome database (https://www.rosaceae.org/organism/Malus/x-domestica; Genome version 1.0). The structural features of these MdIAA genes which included exons/introns, exon numbers, and locations were obtained and shown using TBTools.
Cloning of MdIAAs and gene expression analysis
Total RNA was extracted from frozen samples using the cetyl trimethyl ammonium bromide method (Wang et al., 2017a). Two µg of total RNA was used for synthesizing the first-strand cDNA. MdIAA ORF sequences were obtained using RT-PCR, with fully expanded leaves from the Golden Delicious apple as an RNA source, in addition to 17 gene-specific primer pairs as listed in Table 1. For quantitative real time PCR (qRT-PCR) assays, gene-specific primers were designed and synthesized by Sangon Biotech (Shanghai, China). One μg of total RNA from each sample was used to perform reverse transcription and one μL of the product was used for PCR-amplification. All reactions contained 10 µL of SYBR® Premix Ex Taq™ (TaKaRa, Kusatsu, Japan), 0.4 µL of each specific primer, 8.2 µL of ddH2O2, and 1.0 µL of cDNA template, and the reactions were run by an iQ5 instrument (Bio-Rad, Hercules, CA, USA) (Dong et al., 2018a). The PCR conditions included an initial 95 °C for 3 min, then 40 cycles of 95 °C for 10 s, 58 °C for 30 s, and 72 °C for 15 s; this was followed by 72 °C for 3 min and then 81 cycles of 7 s each, increasing by an increment of 0.5 °C from 55 to 95 °C. Three biological replicates were conducted for each treatment and the values of ΔCt were calculated using the MdMDH gene or NtActin as the endogenous control (Wang et al., 2017b). The relative expression levels of MdIAA genes were obtained according to the 2−ΔΔCt method (Livak & Schmittgen, 2001) and the specificity of the amplifications was examined by dissociation curve analysis.
| Use | Primer name | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|---|
| Complete ORF amplification |
MdIAA4 | ATGGAGAGTGGAGGTTCT | CTAGGTTGGCTTACATCT |
| MdIAA5 | ATGTCTCCACCACTATTG | CTAGTTCCGGTTCTTGGA | |
| MdIAA8 | ATGTCTATATCTTTGGAG | CTAATTACTATTTTTGCAC | |
| MdIAA9 | ATGTCTCCGCCACTGCTT | CTAGTTCCTGTTCCTGCA | |
| MdIAA10 | ATGGCAATGTTTGCGCAG | TCAGAGCGGGCAGCAGCCT | |
| MdIAA14 | ATGGAAGGCAAGGCACAT | TCATACACCACACCCCAA | |
| MdIAA15 | ATGGTTAAGTTGTATAAT | TCAGATTCCAACTTTCAC | |
| MdIAA16 | ATGTTACCGGAAAATAAG | TCAGTGTGTGCTTGTGCA | |
| MdIAA20 | ATGGCAATGTTTGCGCAG | TCACGGCGTGCAGCAGCCT | |
| MdIAA21 | ATGGGGTTTGAAGAGACA | TCAGCTCCTGTTCTTGCAT | |
| MdIAA24 | ATGTCTAGGCCACTGGAGC | TTAGTTCCGGTTCTTGCAT | |
| MdIAA26 | ATGACGAGCATGCTTGGA | TCAGCTTCTGCCTTTGCAT | |
| MdIAA27 | ATGGCCACTGAGATGGAGG | TCAAAGTGCCATCTTATC | |
| MdIAA28 | ATGGAAGACCAGCTAAAT | TCAGAAACAAGTGCTCAA | |
| MdIAA30 | ATGGAAAGCAAGGCACATG | TCATACGCCACACCCCAAA | |
| MdIAA31 | ATGCAAACACAGACACAAG | TCAGCTTCTGCCCTTGC | |
| MdIAA33 | ATGTCACCGCCACTGCTT | CTAGTTCCTGTTCCTGCAC | |
| qRT-PCR | MdIAA4 | CGACACATGGTTCAGGTGGT | CCACAAGCATCCAGTCTCCA |
| MdIAA5 | TTCACGCTCTATGGACTGCATCTC | CTGACAAGTTCGAGACCGTGGAG | |
| MdIAA8 | CAAGATGGGAGCAGGGTGTC | GATAGCTGCCATAGGTTTTGAGAT | |
| MdIAA9 | GGAGCTCCAGGCAGAGAGAT | CAAGGAACATCGCCCACGAG | |
| MdIAA10 | CCAAGGATTCCGAGCAGAAC | CTTGGAAGGTGGAGGCTGAG | |
| MdIAA14 | AGGAAGAACAGCCTCCAGGTAA | AAGGTGCTCCATCCATGCTT | |
| MdIAA15 | CATGCTCCAGTTGTCGGGTG | GTGACTCCGAATCAGCTGCC | |
| MdIAA16 | GACGGACTGAACTACGATGAGACG | GTGCCTTAGCTGGAGCCTTATGAG | |
| MdIAA20 | AGACCACTGCAATCCGACATGAAC | TGTCTTGGTAGGTGGAGGCTGAG | |
| MdIAA21 | TTGGATGCTGGTTGGCGATGTG | GTCCAACAGCCTCCTTGCTCTTC | |
| MdIAA24 | CAGGGGCAAAAAGGGGAT | TGGCAGAGGAACAGAAGAAACC | |
| MdIAA26 | GTGGTACTTCTACTGTGGCTGAGC | ACAACTTGTGCCTTGGCTGGAG | |
| MdIAA27 | TGAAGGAGACAGGATGCTTGTTGG | TGCTGCTACGAAGACGAAGTGC | |
| MdIAA28 | ACGGCGACTGGATGCTTGTTG | ACAAGTGCTCAATCCTCTGGCTTC | |
| MdIAA30 | GAGCATGGATGGAGCACCTTACC | ATCTCCAACCAGCATCCAATCACC | |
| MdIAA31 | ACCAACACAACAACGCAACAAGAC | CGGAGGTTCAGATCACGCTCAAC | |
| MdIAA33 | AAGATGGTGACTGGATGCTTGTGG | TTCCTGTTCCTGCACTTCTCCATG | |
| MdMDH | CGTGATTGGGTACTTGGAAC | TGGCAAGTGACTGGGAATGA | |
| pBI-121 | MdIAA9 | ACGGGGGACTCTAGAGGATCCATGTCTCCGCCA | CGATCGGGGAAATTCGAGCTCCTAGTTCCTGTT |
| 35S | CGCACAATCCCACTATCCTT | ||
| qRT-MdIAA9 | GGAGCTCCAGGCAGAGAGAT | CAAGGAACATCGCCCACGAG | |
| NtActin | TCCAGGACAAGGAGGGTAT | CATCAACAACAGGCAACCTAG |
Note:
ORF, open reading frames.
Prediction of cis-acting elements in promoters
The upstream regions (1,500 bp upstream of the translational start codon) of the selected MdIAAs were used to identify putative cis-acting elements (Plant CARE database; http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Wang et al., 2017a).
Vector construction and plant transformation
In order to isolate the full-length cDNA of MdIAA9 used to construct the OE vectors, we conducted RT-PCR using fully expanded Golden Delicious apple leaves. The cDNA of MdIAA9 was cloned into pBI 121 vectors and was driven by a 35S promoter known as the cauliflower mosaic virus. For tobacco transformation, wild type (“NC89” tobacco) plants were transformed using the Agrobacterium tumefaciens EHA105-mediated leaf dip method (Xia et al., 2012). The PCR-positive plantlets were transplanted into soil for growing in the greenhouse. Seeds were screened with 50 mg L−1 kanamycin after the transgenic plants were harvested individually. Homozygous transgenic T3 plants were used in osmotic stress investigations.
Measurement of physiological indices
Relative electrolyte leakage was determined according to the method described by Tan et al. (2017). Chlorophyll concentrations and free proline were measured using the method of Dong et al. (2018a), and MDA levels were measured as described by Wei et al. (2018).
Statistical analysis
The experimental data obtained was analyzed using SPSS 20 software (SPSS, Inc., Chicago, IL, USA) and indicated by means ± standard deviation. Data was analyzed using One-way ANOVA and Tukey’s tests at a significance level of P < 0.05.
Results
Aux/IAA gene family members in apple
In order to identify AUX/IAA proteins in apples, 34 Arabidopsis Aux/IAA proteins were used to search the apple genome database by BLASTP. In addition, we carried out a Hidden Markov Model search based on the AUX/IAA domain signature. In all, 35 candidate genes were found. In order to further confirm and identify the conserved Aux/IAA domains, all candidate proteins were analyzed using Pfam and SMART databases. One gene, MD15G1305900, was not analyzed further because it contained an apparently incomplete reading frame. We obtained basic information for the remaining 34 Aux/IAA genes, which included gene names, locus IDs, intron numbers, ORF lengths, chromosome locations, and numbers of amino acids (Table 2). The ORF lengths of MdIAAs ranged from 498 base pairs (MdIAA19) to 1,158 base pairs (MdIAA34), with an average length of 789 base pairs. The size of MdIAA proteins were found to have a range of 165–385 amino acids. The corresponding molecular mass varied from 18.21 kD (MdIAA19) to 40.61 kD (MdIAA34).
| Gene | Locus | Gene position | Chr no. | ORF length | Amino acids | MW | PI | Number of exons |
|---|---|---|---|---|---|---|---|---|
| MdIAA1 | MD02G1027600 | 2109632 2111147 | 2 | 744 | 247 | 27.79 | 8.23 | 5 |
| MdIAA2 | MD15G1191800 | 15104026 15107317 | 15 | 957 | 318 | 33.29 | 8.19 | 5 |
| MdIAA3 | MD13G1222200 | 21514224 21516886 | 13 | 921 | 306 | 32.22 | 7.34 | 5 |
| MdIAA4 | MD10G1176400 | 26870201 26873035 | 10 | 888 | 295 | 31.09 | 6.78 | 5 |
| MdIAA5 | MD01G1045800 | 14633447 14638572 | 1 | 1,122 | 374 | 40.26 | 7.19 | 4 |
| MdIAA6 | MD17G1198300 | 23886616 23888516 | 17 | 618 | 205 | 22.40 | 7.56 | 5 |
| MdIAA7 | MD05G1205900 | 33515071 33516331 | 5 | 528 | 175 | 19.62 | 5.46 | 3 |
| MdIAA8 | MD02G1057200 | 4631406 4634380 | 2 | 960 | 319 | 33.22 | 8.07 | 2 |
| MdIAA9 | MD08G1207300 | 26957415 26962300 | 8 | 1,092 | 363 | 39.54 | 6.99 | 5 |
| MdIAA10 | MD12G1241700 | 31336165 31337389 | 12 | 594 | 197 | 22.27 | 6.59 | 4 |
| MdIAA11 | MD09G1208000 | 19937208 19940388 | 9 | 966 | 321 | 34.61 | 8.31 | 5 |
| MdIAA12 | MD16G1132500 | 10120494 10121822 | 16 | 603 | 200 | 22.32 | 8.63 | 4 |
| MdIAA13 | MD08G1111200 | 9774753 9777030 | 8 | 888 | 295 | 32.61 | 8.19 | 5 |
| MdIAA14 | MD16G1206500 | 19099027 19102542 | 16 | 576 | 191 | 21.38 | 8.19 | 3 |
| MdIAA15 | MD15G1090600 | 6282056 6284672 | 15 | 888 | 295 | 62.51 | 8.54 | 5 |
| MdIAA16 | MD09G1216100 | 21391959 21393811 | 9 | 615 | 204 | 22.62 | 7.08 | 4 |
| MdIAA17 | MD13G1137000 | 10537566 10539193 | 13 | 582 | 193 | 21.42 | 8.64 | 4 |
| MdIAA18 | MD10G1312300 | 39684595 39685128 | 10 | 534 | 177 | 19.96 | 6.84 | 1 |
| MdIAA19 | MD13G1253600 | 27267886 27268744 | 13 | 498 | 165 | 18.21 | 8.92 | 2 |
| MdIAA20 | MD04G1225000 | 30478283 30479519 | 4 | 591 | 196 | 22.13 | 5.62 | 2 |
| MdIAA21 | MD10G1192900 | 28909337 28912808 | 10 | 774 | 257 | 28.22 | 8.74 | 5 |
| MdIAA22 | MD05G1188800 | 31653742 31656640 | 5 | 897 | 298 | 31.01 | 2.68 | 5 |
| MdIAA23 | MD17G1198100 | 23798955 23803215 | 17 | 573 | 190 | 21.42 | 5.92 | 3 |
| MdIAA24 | MD17G1189100 | 22550880 22553660 | 17 | 996 | 331 | 35.96 | 8.30 | 5 |
| MdIAA25 | MD08G1151300 | 15483655 15485406 | 8 | 732 | 243 | 27.54 | 7.16 | 4 |
| MdIAA26 | MD16G1206700 | 19206017 19208495 | 16 | 735 | 244 | 26.75 | 7.88 | 5 |
| MdIAA27 | MD09G1202300 | 18823904 18826992 | 9 | 930 | 309 | 34.21 | 8.72 | 5 |
| MdIAA28 | MD10G1193000 | 28924800 28926049 | 10 | 522 | 173 | 19.29 | 7.80 | 3 |
| MdIAA29 | MD17G1183500 | 21576812 21580307 | 17 | 975 | 324 | 35.79 | 8.56 | 5 |
| MdIAA30 | MD13G1204700 | 18453283 18454792 | 13 | 573 | 190 | 21.34 | 8.86 | 3 |
| MdIAA31 | MD13G1205000 | 18556276 18559279 | 13 | 963 | 320 | 35.28 | 9.11 | 5 |
| MdIAA32 | MD15G1169100 | 13036569 13038091 | 15 | 753 | 250 | 28.09 | 7.39 | 4 |
| MdIAA33 | MD15G1391700 | 48773621 48776944 | 15 | 1,092 | 363 | 39.35 | 6.92 | 6 |
| MdIAA34 | MD16G1227100 | 23027760 23030866 | 16 | 1,158 | 385 | 40.61 | 8.82 | 5 |
Note:
ORF, opening reading frame; MW, molecular weight; PI, isoelectric point.
Phylogenetic relationships of Aux/IAA proteins
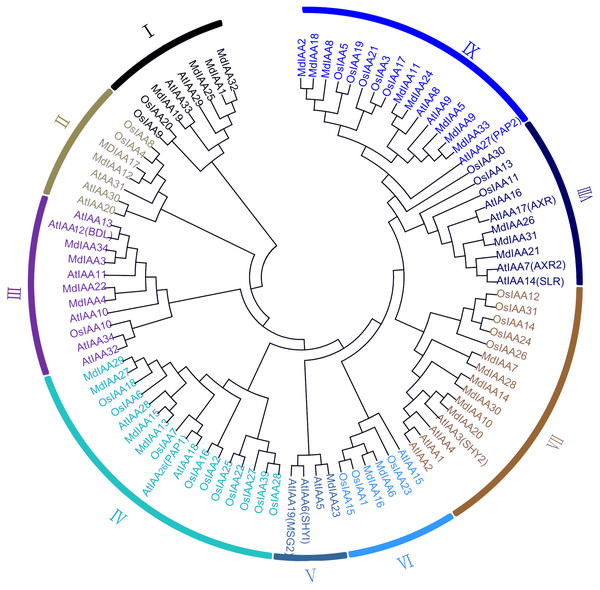
A phylogenetic tree was constructed which included 34 members in apple, 34 members in Arabidopsis, and 31 members in rice. According to their phylogenetic relationships, Aux/IAA members of the three species could be split into nine subgroups, which were numbered I–IX (Fig. 1). The number of MdIAAs in the nine subgroups ranged from four to 17. The 34 MdIAAs were distributed unevenly among the subgroups. The numbers of MdIAAs in Subgroups I, II, III, IV, V, VI, VII, VIII, and IX were 4, 2, 4, 4, 1, 2, 6, 3, and 8, respectively.
Figure 1: Phylogenetic analysis of 99 AUX/IAA proteins from apple, rice, Arabidopsis.
The phylogenetic tree was generated using MEGA 6.0 with the Neighbor-Joining (NJ) method with 1,000 bootstrap replicates. Aux/IAA members of the three species could be split into nine subgroups, which were numbered I–IX.Sequence alignment, gene structure, and conserved motifs of Aux/IAA proteins in apple
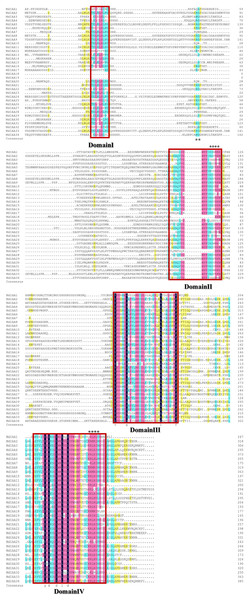
There were four conservative domains from a sequence alignment of the ORF translation of the Aux/IAA genes (Fig. 2). Nuclear localization signals were also found in most MdIAAs. Amino acids at five positions were absolutely conserved among all MdIAAs, but amino acids in 30 positions were strongly conserved (75–99%). The similarity between the other amino acids was <50%.
Figure 2: Amino acid sequence alignment and domain analysis of MdIAA family proteins.
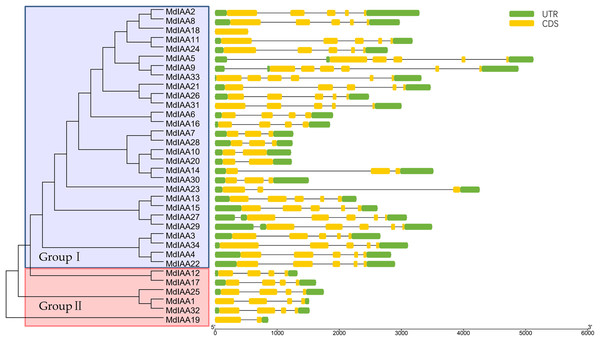
Multiple alignments were conducted for amino acid alignment and domain analysis among the MdIAA family proteins by using the DNAMAN program. The similar amino acids among the proteins were highlighted with different colors. Four domains of MdIAA proteins were marked with red frames. Nuclear localization signals (NLS) are indicated with black asterisks.To explore the MdIAA gene’s structural diversity in apples, we evaluated the conservation of the exon-intron organization. The MdIAA proteins were divided into groups I and II according to their phylogenetic relationships (Fig. 3). The gene structure analysis suggested that in group I, although MdIAA18 had one exon and the other two genes (MdIAA9 and MdIAA33) had six exons, most genes had four exons. In group II, only MdIAA19 contained two exons and the remaining genes contained four exons.
Figure 3: Phylogenetic relationships and structures for 34 MdIAAs.
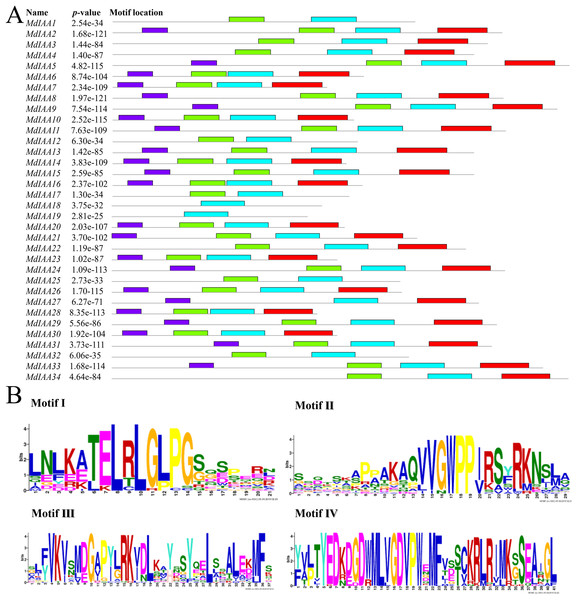
The phylogenetic tree for full-length amino acid sequences was constructed with MEGA software and the NJ method. Aux/IAA members of apple could be split into two subgroups, which were numbered I and II. The exons and introns are represented by green boxes and lines, respectively. The 5′- and 3′-untranslated regions (UTRs) are represented by yellow boxes.We identified common motifs using the MEME web server (http://meme-suite.org/tools/meme) (Fig. 4). The following parameter settings were used: size distribution, zero or one occurrence per sequence (zoops); motifs count, 4; and motif width, between six and 50 wide. In our research, the four motifs identified by MEME were consistent with the four classical domains. This identified four motifs, which corresponded to the four conserved domains found through sequence alignment above. All four motifs were apparent in 22 of the proteins, although only three motifs were found in seven additional proteins. Motif I was not contained in MdIAA3, MdIAA4, MdIAA22, and MdIAA34; Motif IV was not in MdIAA12 and MdIAA4; and Motif II was not in MdIAA27. Three MdIAAs, MdIAA1, MdIAA25, and MdIAA32, contained only Motif II and Motif III, and two other MdIAAs, MdIAA18 and MdIAA19, contained only Motif II. It is obvious that most of the domains and motif regions overlapped with each other. Additionally, several highly conserved key sequences, such as LxLxLx in Domain I and Motif I, and VGWPP in Domain II and Motif II, strongly suggests that these regions may be the key sites conferring the functions of MdIAAs.
Figure 4: The motif distribution of the MdIAA proteins.
(A) Shows 4 classical motifs I, II, III, and IV (labelled by different color blocks) of the Aux/IAA proteins, which were analyzed by the Multiple Expectation Maximization for Motif Elicitation (MEME) web server. (B) The degree of specific amino acids conservation in each motif of Aux/IAA varies with the height of each letter.Cloning of apple IAA genes
To confirm the coding sequence of MdIAAs as annotated in the reference genome and to further uncover the biological function of these MdIAAs, 17 MdIAA genes were cloned: MdIAA4, MdIAA5, MdIAA8, MdIAA9, MdIAA10, MdIAA14, MdIAA15, MdIAA16, MdIAA20, MdIAA21, MdIAA24, MdIAA26, MdIAA27, MdIAA28, MdIAA30, MdIAA31, and MdIAA33, respectively. As a result, all cloned sequences showed a high similarity to the predicted apple genome (Data S1). The sequences were submitted to NCBI GenBank.
Analysis of promoter cis-elements of apple IAA genes
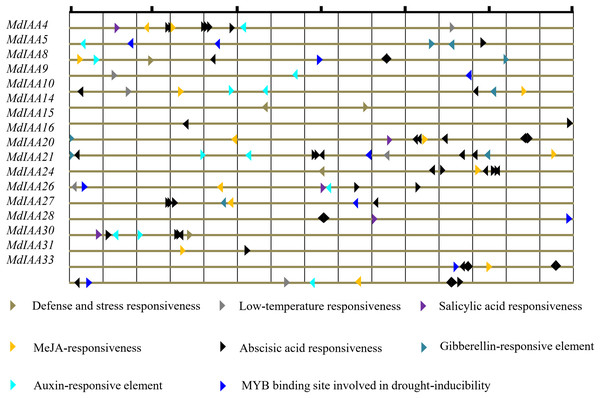
Promoter cis-regulatory elements are of great significance in regulating gene expression. In this study, the putative cis-elements within the MdIAA promoter (<1.5 kb upstream from the start site of putative translation) were identified through the PlantCARE database (Fig. 5) and different kinds of cis-elements related to stress and hormone responses were identified. Most MdIAA promoters contained ABRE elements, which usually participate in ABA related responses. Nine of the MdIAA genes contained a TGA-box, which is an auxin-responsive element. Six of the MdIAA genes contained gibberellins-responsive elements such as P-box, GARE-motif or TATC-box. A total of 11 of the MdIAA genes contained a G-box, CGTCA-motif, and/or TGACG-motif, which participate in JA/MeJA-responses. Five of the MdIAA genes contained a TCA-element, which is related to salicylic acid responsiveness. A large number of putative hormone-responsive elements suggested that these genes were involved in hormone signal transduction. Moreover, the promotors contained drought-inducibility elements (MBS), low-temperature response motifs (LTR), and defense and stress responsiveness elements (TC-rich repeats). The results indicated that MdIAA genes may be involved in defense signaling and biotic and abiotic stress in apple plants.
Figure 5: The main putative cis-elements within 1.5 kb upstream promoter regions of MdIAAs.
The different cis-elements are shown by using different icons and colors. The direction of the apex angle to the right indicates the cis-acting element in the (+) strand, and the direction of the apex angle indicates to the left the cis-acting element in the (−) strand.The spatio-specific profiles of MdIAA genes expression in various tissues
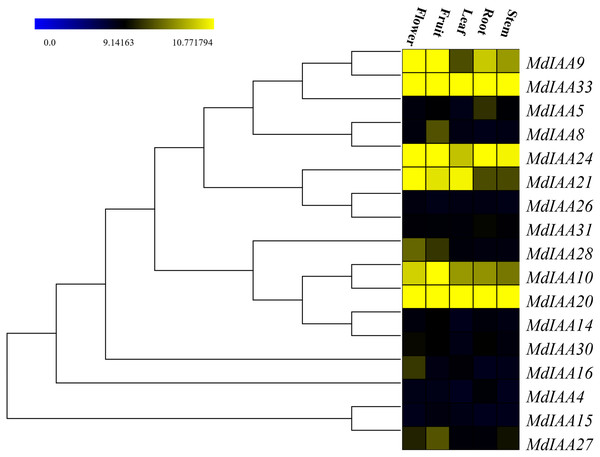
In order to determine the additional roles that MdIAAs play in growth and development, 17 selected MdIAA genes were examined in the roots, stems, leaves, flowers, and fruits using qRT-PCR. Some MdIAAs showed higher expressions in multiple tissues (Fig. 6). For instance, there were high levels of expression of MdIAA20 and MdIAA33 in all of the examined tissues. The expression of MdIAA20 was the highest in flowers and fruit while the expression of MdIAA33 was the highest in the roots, stems, and leaves. In addition, a higher transcriptional accumulation in the flowering and fruiting stages of MdIAA4, MdIAA9, and MdIAA24 were found, suggesting that they may play specific roles in the development of flowers and fruit. The mRNA abundances of the MdIAA family genes were higher in fruit than in other tissues. Furthermore, the expression of MdIAA genes in the stems was the lowest among all the tissues. These results indicated that MdIAA genes might play roles in fruit. Overall, the tissue-specific spatial differential expression of MdIAA suggests that these genes were specifically related to apple growth and development.
Figure 6: Expression profiles of MdIAA genes in various tissues.
Heat map of MdIAA genes expression in flowers, fruit, leaves, roots, and stems of apple plants generated by using TIGR MeV v4.8.1 software. Levels of down-regulated expression (green) or up-regulated expression (red) are shown on a log2 scale from the highest to the lowest expression for each MdIAA gene.Expression profiles of MdIAA genes following IAA treatment
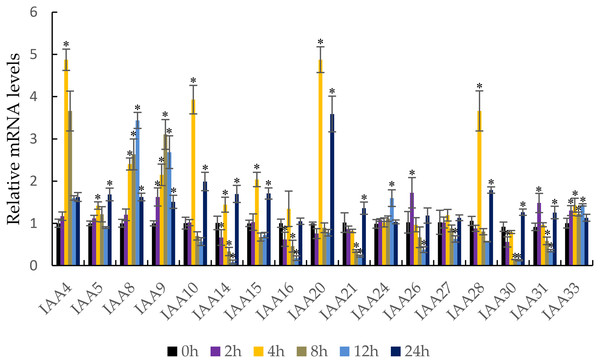
It is well known that auxin induces the strong expression of the Aux/IAA genes (Liu et al., 2017). qRT-PCR was performed to confirm the response of the MdIAA genes following IAA treatment. In this work, five testing time points (0, 2, 4, 8, 12, and 24 h) were selected. Under IAA treatment, the MdIAA family genes displayed various expression styles. The expressions of the majority of the MdIAA genes were up-regulated at 4 h as compared with controls (Fig. 7), but the expressions of most MdIAA genes were down-regulated at 8 and 12 h as compared with controls. No significant changes in gene expression were observed in other genes, such as MdIAA27, after IAA treatment. Taken together, this data indicated that most genes from the MdIAA family were auxin early-response genes.
Figure 7: The expression patterns of MdIAAs in apple under auxin treatments.
Samples were harvested at 0, 2, 4, 8, 12, and 24 h after foliar application. Error bars show standard deviations of three biological replicates. Asterisks indicate a significant difference (*P < 0.05) compared with the corresponding controls, based on one-way ANOVA and Tukey’s tests.The profiles of MdIAA gene expression following abiotic stresses and ABA spray
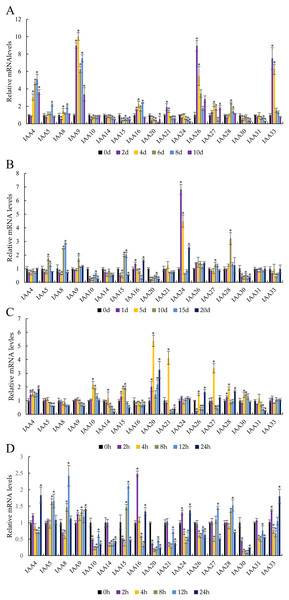
To investigate the effects of abiotic stresses and ABA treatment on MdIAA gene expression, the patterns of transcription were observed after drought, cold, NaCl, and ABA treatment (Fig. 8). Under drought stress, the expressions of MdIAA genes showed diverse responses at different time points (Fig. 8A). Among the 17 MdIAA selected genes, MdIAA4, MdIAA26, and MdIAA33 increased significantly. The expression of MdIAA4 was 5.15 times that of the control group at 8 days of drought treatment and the expression of MdIAA26 was 8.95 times that of the control group at 2 days and 5.50 times that at 4 days of drought treatment, respectively. The expression levels of MdIAA33 showed a trend similar to MdIAA26, being 7.50 times and 6.24 times that of the control group at 2 and 4 days, respectively. However, in this study, the expression levels of several MdIAA genes were significantly lower than those of the control group after drought treatment. For instance, the expression of MdIAA20 was only 12.7% of the control group at 8 d under drought treatment, while the expression of MdIAA24 was only 23.8% of the control group at 10 days under drought treatment.
Figure 8: Abiotic stress altered expression of selected IAA genes in apple leaves.
(A) Drought: samples were collected at 0, 2, 4, 6, 8, and 10 days after withdrawal irrigation. (B) NaCl treatment: samples were harvested at 0, 1, 5, 10, 15, and 20 days after irrigation with 200 mM NaCl solution. (C) Chilling challenge: samples were harvested at 0, 2, 4, 8, 12, and 24 h after exposure to 4 °C. (D) ABA (100 µM): samples were harvested at 0, 2, 4, 8, 12, and 24 h after foliar application. Error bars show standard deviations of three biological replicates. Asterisks indicate a significant difference (*P < 0.05) compared with the corresponding controls, based on one-way ANOVA and Tukey’s tests.Two genes showed an obvious response to the NaCl treatment (Fig. 8B). The expression of MdIAA24 was 6.82 and 4.49 times that of the control group at 1 and 5 days, respectively, but the expression of MdIAA28 was 3.22 times that of the control group at 15 days. However, some MdIAA gene expression was dramatically reduced after NaCl treatment. For example, the expression of MdIAA10 and MdIAA20 was only 14.1% and 17.7% of the control group, respectively.
In response to chilling at 4 °C, three MdIAA genes, MdIAA20, MdIAA21, and MdIAA27, increased significantly at 4 h of treatment (Fig. 8C). However, their expressions were then down-regulated over time. For example, at 8 h, MdIAA21 was only 10.82% of the control group. The other genes, such as MdIAA24 and MdIAA33, showed no alterations in expression under most conditions.
After ABA application (Fig. 8D), the expressions of MdIAA10, MdIAA14, MdIAA20, MdIAA21, and MdIAA30 decreased significantly. Among these genes, MdIAA30 changed most dramatically, and was only 7.05% of the control group at 8 h although some genes increased significantly during this time. For instance, the expression of MdIAA25 was 2.50 times that of the control group at 2 h whereas MdIAA8 was 2.42 times that of the control group at 12 h. Hence, these genes were designated as being ABA-responsive.
Overexpression of MdIAA9 enhanced plant growth of tobacco seedlings under osmotic stress
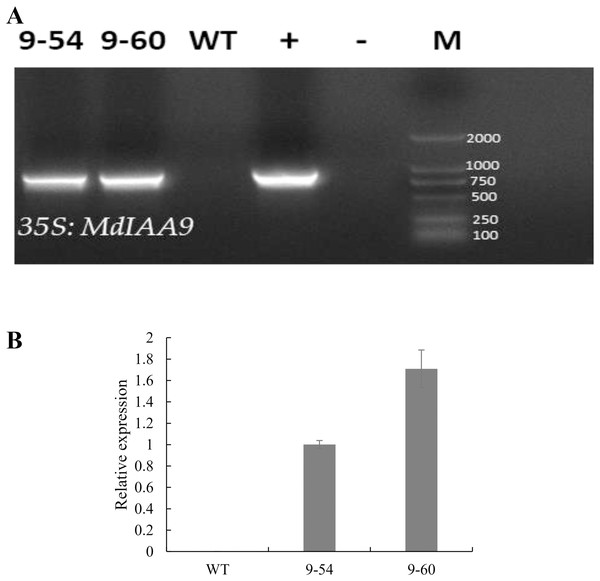
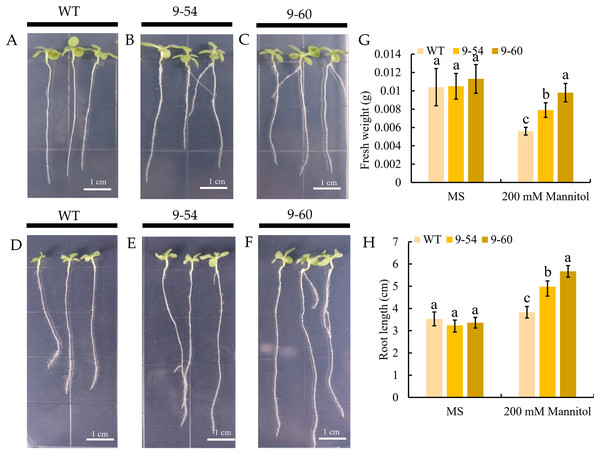
Under drought stress, MdIAA9 was significantly up-regulated, so we chose this gene to study its biological function in tobacco. Two transgenic lines were identified at the DNA level, using 35S promoter positive primers and the reverse primers of MdIAA9 (Table 2; Fig. 9). We examined the root length and fresh weights of transgenic and wild plants under osmotic stress. Our results showed that the root lengths and fresh weights of the transgenic plants were significantly higher than those of the wild types under osmotic stress. However, no significant difference was observed between the transgenic and wild plants under the control conditions (Fig. 10).
Figure 9: PCR identification and relative expression analysis of MdIAA9 in wild-type and transgenic tobacco lines.
(A) 35S and MdIAA9-R indicate that the primers used to identify the transgenic lines were designed according to the MdIAA9. M, DNA marker; −, negative control (H2O); +, positive control (plasmid DNA of 35S::MdIAA9 pBI121 vector). (B) Specific primers for MdIAA9 were used to detect relative expression levels of WT and transgenic tobacco lines.Figure 10: Comparative analysis of MdIAA9-overexpressing tobacco seedlings and wild type in response to mannitol treatment.
(A–C) Representative images of wild type and MdIAA9-overexpressing seedlings grown vertically in MS medium contained zero mM mannitol for 13 days. (D–F) Representative images of wild type and MdIAA9-overexpressing seedlings grown vertically in MS medium contained 200 mM mannitol for 13 days. Bars = 1 cm. (G) Fresh weights between WT and transgenic lines after exposure to osmotic stress for 13 days. (H) Comparison of primary root lengths between WT and transgenic lines after exposure to osmotic stress for 13 days. Error bars represent standard deviations from three biological replicates. For (G) and (H) different letters that follow the values indicate significant differences between treatments at P < 0.05, based on one-way ANOVA and Tukey’s tests.Analysis of physiological characteristics of MdIAA9-overexpressing tobacco seedlings and wild type to osmotic stress
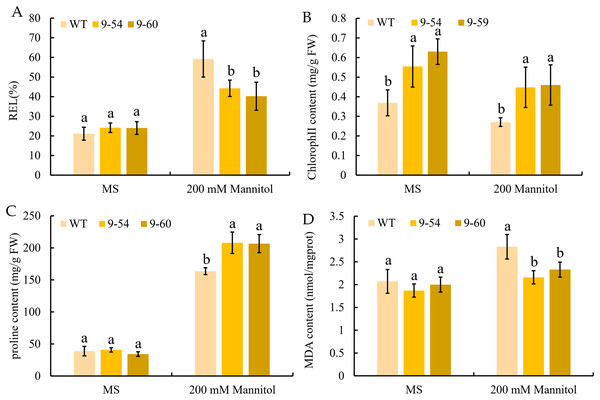
To further study the enhancement of osmotic stress tolerance mediated by MdIAA9, the REL, chlorophyll concentration, proline, and MDA concentrations were determined. These are important markers to measure the degree of osmotic stress injury. Under normal conditions, no significant difference was observed between the transgenic and wild plants. However, the REL values of the wild lines were significantly higher than the overexpressing tobacco seedlings under 200 mM mannitol conditions (Fig. 11A). The chlorophyll levels of wild and overexpressed plants were similar under both the osmotic stress and normal conditions. The quantities of proline in transgenic plants were significantly higher than the wild line under 200 mM mannitol, but no significant differences were revealed under normal conditions between the transgenic and wild plants (Figs. 11B and 11C). Furthermore, the MDA content of the transgenic plants was significantly lower than the wild plants under the 200 mM mannitol conditions, while no significant difference was observed in the transgenic lines and wild types under normal conditions (Fig. 8D). The results suggested that the OE of MdIAA9 in tobacco seedlings leads to a higher tolerance of osmotic stress.
Figure 11: Response to osmotic stress in transgenic and wild tobacco seedlings.
(A) relative electrolyte leakage (REL), (B) chlorophyll content, (C) proline content, and (D) MDA content in transgenic and WT seedlings after exposure to MS medium contained 0 or 200 mM mannitol for 13 days. Error bars represent standard deviations from three biological replicates. For (A–D), different letters following the values indicate significant differences between treatments at P < 0.05, based on one-way ANOVA and Tukey’s tests.Discussion
Indole-3-acetic acid, a key signaling molecule, plays a vital role in a series of processes during plant growth and development (Liu et al., 2015). Aux/IAA inhibits downstream gene expression through its interaction with ARFs (Liu et al., 2015; Yu et al., 2017; Kalluri et al., 2007). Plants are subjected to environmental stresses such as drought, salinity, and low temperature, adversely affecting their growth and development (Tan et al., 2014). Auxin-responsive genes are thought to participate in various responses related to environmental stress (Guo et al., 2018; Shani et al., 2017).
Many family members of Aux/IAA genes have been identified to date from different plant species such as Arabidopsis, tomato, rice, Medicago, and papaya (Yuan et al., 2018; Wang et al., 2010b; Shen et al., 2014; Wu et al., 2014; Jain et al., 2006). The characterization and expression analysis of the MdIAA genes help investigators to further understand the involvement of auxin in various types of stress. However, in the apple, there is little known about IAA genes or their expression. A total of 34 MdIAA genes were identified in this study, which are as many as have been identified in Arabidopsis.
We found that most of the MdIAA proteins possessed four classically conserved domains. The comparison of those conserved domains showed that 10 MdIAA genes (MdIAA1, MdIAA3, MdIAA4, MdIAA12, MdIAA17, MdIAA18, MdIAA19, MdIAA22, MdIAA25, and MdIAA34) lacked Domain I, which acts as a repressor in the signaling pathway of auxin. The MdIAA proteins contained Domain II (except for MdIAA18, MdIAA19, and MdIAA27), which was necessary for the protein degradation process mediated by auxin (Dharmasiri, Dharmasiri & Estelle, 2005). It is noteworthy that all MdIAA proteins contained Domain III and most of MdIAA proteins contained Domain IV. Our results suggested that most MdIAAs might interact with ARFs by forming stable homo- and hetero-dimers.
The Arabidopsis and rice Aux/IAA gene families have been well characterized. Useful information will be provided about the biological functions of the apple through comparative studies on phylogenetic relationships. We concomitantly monitored the expression patterns of 17 MdIAA genes from different apple tissues. Plants floral initiation is an important stage in their life cycle. In Arabidopsis, the gain-of-function mutant IAA7/AXR2 confers delayed flowering under short-day light (Mai, Wang & Yang, 2011). Interestingly, MdIAA21, a homologous gene of AtIAA7, was highly expressed in flowers (Fig. 4), which suggested that MdIAA7 might play an important role in the apple flowering process. However, AtIAA14 proved to have an important role in the development of both lateral and adventitious root development (Lopez-Bucio et al., 2015). The orthologous gene of AtIAA14 in apple, MdIAA21, was preferentially expressed in the flowers, which indicated that the apple IAA family genes may vary in the evolution for their own growth and development. Furthermore, a phylogenetic tree was constructed to reveal the evolutionary relationships of IAA genes between apple and rice. Interestingly, in rice, the OsIAA11 gene was reported to play an essential role in the development of the root system (Zhu et al., 2012; Ni et al., 2014). According to the phylogenetic tree analysis, the homologous gene of OsIAA11 in the apple was MdIAA21, which was preferentially expressed in flowers. This suggested that the MdIAA genes might not perform the same biological functions as the IAA genes in other already reported species.
As an important primary auxin-responsive gene family, auxin treatment rapidly induces the expression of the IAA family. According to the results of the IAA treatment, 17 apple IAA genes were selected and their expression patterns were studied. The expression of MdIAA genes was either higher or lower than the control after different amounts of exposure to IAA treatment. We concluded that the treatment time of IAA played a crucial role in the up-regulation or down-regulation of auxin responsive genes.
Extensive studies have shown that the changes in auxin concentration, redistribution, and signal transmission are affected by various integrated environmental factors (Shibasaki et al., 2009). Mounting evidence shows that the auxin-response genes, including Aux/IAA, GH3, and SAUR are related to stress and defense responses in Arabidopsis, maize, rice, and Sorghum bicolor (Ghanashyam & Jain, 2009; Jain & Khurana, 2009; Zhang et al., 2009; Wang et al., 2010a). In our research, MdIAAs showed various expression patterns under different stress conditions. The expression levels of some MdIAAs increased after drought, cold, salt, and ABA exposure. However, the expression levels of some MdIAAs decreased after treatments. Similar results have been shown in other species. In the tomato, the up-regulated expression of most SlIAAs under drought and salt stress treatments was observed, while the expression levels of SlIAA3, SlIAA11, and SlIAA14 decreased after stress treatments (Wu et al., 2012). Here, drought-inducibility elements (MBS), LTR motifs, and defense and stress responsiveness elements (TC-rich repeats) were shown to be upstream of the promoter regions in some MdIAAs. These cis-elements related to abiotic stress and auxin signaling regulating pathways might result in different expression patterns of MdIAA genes. However, the results from qRT-PCR analysis were inconsistent with the analysis of the promoter region on MdIAA genes. For instance, although some LTR motifs were found in MdIAA24 and MdIAA33, no significant alterations in their expression levels were observed under the cold conditions and conversely, in the promoter regions of MdIAA10 and MdIAA20, no cis-elements related to salt stress were found. However, qRT-PCR analysis showed that the two genes in question were significantly responsive to stress. This indicated that the expression of those MdIAAs in stress response might be regulated by some unidentified cis-regulated elements in the apple.
In the present study, as compared with the wild type, the OE of MdIAA9 in tobacco seedlings increased their root lengths and fresh weights. Furthermore, this OE also induced a series of changes in tobacco seedlings related to abiotic stress responses, including REL, total chlorophyll concentration, free proline, and MDA content. The lower levels of REL and MDA and increased levels of proline and total chlorophyll in the overexpressed lines indicated that MdIAA9 has a significant role in enhancing resistance to osmotic stress. The present study indicated that OE of MdIAA9 in tobacco seedlings led to an enhancement of drought resistance.
Conclusions
A total of 34 MdIAA genes were identified and characterized in this study. A comprehensive MdIAA gene family genome-wide analysis was presented, which included the phylogeny, chromosome locations, gene structures, and conserved motifs. Our research shows that most members of the MdIAA gene family have different patterns of spatio-temporal transcript accumulation. Exogenous auxin can induce MdIAAs at the transcriptional level, most of which may be induced by exposure to drought stress, salt, cold, and ABA in the apple. Our research also shows that the OE of MdIAA9 in tobacco seedlings leads to an enhanced drought resistance. The comprehensive information from the results enhances our recognition of how MdIAA genes function at different points in the life cycle and under various abiotic stresses. Notably, the present study implies that the stress-responsive genes may be appropriate candidates to create stress-tolerant apple rootstocks and cultivars using transgenic technology.