Agerinia marandati sp. nov., a new early Eocene primate from the Iberian Peninsula, sheds new light on the evolution of the genus Agerinia
- Published
- Accepted
- Received
- Academic Editor
- William Jungers
- Subject Areas
- Anthropology, Biodiversity, Paleontology, Taxonomy, Zoology
- Keywords
- Adapiformes, Notharctidae, Paleogene, Spain
- Copyright
- © 2017 Femenias-Gual et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Agerinia marandati sp. nov., a new early Eocene primate from the Iberian Peninsula, sheds new light on the evolution of the genus Agerinia. PeerJ 5:e3239 https://doi.org/10.7717/peerj.3239
Abstract
Background
The Eocene was the warmest epoch of the Cenozoic and recorded the appearance of several orders of modern mammals, including the first occurrence of Euprimates. During the Eocene, Euprimates were mainly represented by two groups, adapiforms and omomyiforms, which reached great abundance and diversity in the Northern Hemisphere. Despite this relative abundance, the record of early Eocene primates from the European continent is still scarce and poorly known, preventing the observation of clear morphological trends in the evolution of the group and the establishment of phylogenetic relationships among different lineages. However, knowledge about the early Eocene primates from the Iberian Peninsula has been recently increased through the description of new material of the genus Agerinia from several fossil sites from Northeastern Spain.
Methods
Here we present the first detailed study of the euprimate material from the locality of Masia de l’Hereuet (early Eocene, NE Spain). The described remains consist of one fragment of mandible and 15 isolated teeth. This work provides detailed descriptions, accurate measurements, high-resolution figures and thorough comparisons with other species of Agerinia as well with other Eurasian notharctids. Furthermore, the position of the different species of Agerinia has been tested with two phylogenetic analyses.
Results
The new material from Masia de l’Hereuet shows several traits that were previously unknown for the genus Agerinia, such as the morphology of the upper and lower fourth deciduous premolars and the P2, and the unfused mandible. Moreover, this material clearly differs from the other described species of Agerinia, A. roselli and A. smithorum, thus allowing the erection of the new species Agerinia marandati. The phylogenetic analyses place the three species of Agerinia in a single clade, in which A. smithorum is the most primitive species of this genus.
Discussion
The morphology of the upper molars reinforces the distinction of Agerinia from other notharctids like Periconodon. The analysis of the three described species of the genus, A. smithorum, A. marandati and A. roselli, reveals a progressive change in several morphological traits such as the number of roots and the position of the P1 and P2, the molarization of the P4, the reduction of the paraconid on the lower molars and the displacement of the mental foramina. These gradual modifications allow for the interpretation that these three species, described from the early Eocene of the Iberian Peninsula, are part of a single evolutionary lineage. The stratigraphical position of Masia de l’Hereuet and Casa Retjo-1 (type locality of A. smithorum) and the phylogenetic analyses developed in this work support this hypothesis.
Introduction
One of the most important steps in the early radiation of the primate clade was the appearance and diversification of Euprimates, also known as true primates or primates of “modern aspect” (Bloch et al., 2007; Silcox et al., 2015). Within Euprimates, two main groups were differentiated in the early Eocene, Omomyiformes and Adapiformes, which may be related to the main clades of living primates (haplorhines and strepsirrhines, respectively) following the more accepted theory (e.g., Seiffert et al., 2009; Ni et al., 2013; Godinot, 2015). However, several researchers consider Adapiformes as the stem group of the clade Haplorhini (e.g., Gingerich et al., 2010; Gingerich, 2012).
The first records of these groups in Europe correspond to the omomyiforms Teilhardina, Melaneremia and Nannopithex and the adapiforms Donrussellia, Cantius, Protoadapis and Agerinia (Gebo, 2002; Godinot, 2015). Despite this relative diversity, the early Eocene primate record is still scarce and poorly known, preventing the establishment of clear phylogenetic relationships among known taxa. As the most common elements found in the fossil record are teeth, changes observed in dental morphology are the primary basis for distinguishing evolutionary lineages.
Recent works dealing with European Eocene primates have focused on the description of new material (Hooker, 2007; Hooker, 2012; Hooker & Harrison, 2008; Marigó, Minwer-Barakat & Moyà-Solà, 2011; Marigó, Minwer-Barakat & Moyà-Solà, 2013; Gebo, Smith & Dagosto, 2012; Gebo et al., 2015; Minwer-Barakat, Marigó & Moyà-Solà, 2012; Minwer-Barakat et al., 2013; Femenias-Gual et al., 2015), the revision of previous taxonomic assignations (Minwer-Barakat, Marigó & Moyà-Solà, 2013; Minwer-Barakat, Marigó & Moyà-Solà, 2016; Marigó et al., 2014) and the establishment of relationships between different taxa (Smith, Rose & Gingerich, 2006; Marigó, Minwer-Barakat & Moyà-Solà, 2010; Marigó, Minwer-Barakat & Moyà-Solà, 2013; Minwer-Barakat et al., 2017), with some exceptions focused on the diet (Ramdarshan, Merceron & Marivaux, 2012), the locomotor behaviour (Marigó et al., 2016) and the endocranial anatomy (Ramdarshan & Orliac, 2016) of several species. However, only a few contributions have been published regarding European primates from the early Eocene, recently including the revision of Agerinia roselli from Les Saleres and the description of the new species Agerinia smithorum from Casa Retjo-1 (Femenias-Gual et al., 2016a and Femenias-Gual et al., 2016b, respectively). The former work allowed for the identification of several traits of A. roselli that were not described previously, such as the presence of two roots situated mesially with respect to the P3 or the presence of a tiny paraconid on the M1. On the other hand, A. smithorum is characterized by the presence of a two-rooted P2, a well-developed paraconid on the M1 and a tiny one on the M2, among other features. Based on these primitive traits, these authors proposed A. smithorum as a probable ancestor of A. roselli.
Here we present the first detailed study of new euprimate material found in the locality of Masia de l’Hereuet (early Eocene, NE Spain), where the presence of the plesiadapiform Arcius was already noted by Marigó et al. (2012). A preliminary study of this material was made by Femenias-Gual et al. (2014), who did not give a taxonomic determination. In the present work, after comparison with the material of A. roselli from Les Saleres and A. smithorum from Casa Retjo-1, all the euprimate teeth found from Masia de l’Hereuet can be confidently assigned to the genus Agerinia. Moreover, some morphological traits different from those of A. roselli and A. smithorum allow the erection of a new species. The material from Masia de l’Hereuet allows the first description of the deciduous upper and lower teeth of Agerinia; in addition, this sample includes several upper molars, which were still unknown for A. roselli and A. smithorum, and only known for some small samples of Agerinia sp. such as those from Casa Ramón and Condé-en-Brie (Peláez-Campomanes, 1995; Herbomel & Godinot, 2011).
Geological setting
The fossil site of Masia de l’Hereuet is located to the south of the path that connects the villages of Corçà and Agulló (Fig. 1), in the western sector of the Àger valley (Lleida province, NE Spain). Geologically, this locality is situated in the continental Eocene deposits of the Corçà formation in the Àger sub-basin, included in the south Pyrenean foreland basin (Puigdefàbregas et al., 1989). The continental deposits of the Corçà Formation overlie early Eocene transitional deltaic deposits of the Ametlla Formation (Mutti et al., 1985; Dreyer & Fält, 1993; Zamorano, 1993), and are mainly made up of different terrigenous deposits, including clays and sandstones interbedded with some conglomeratic levels. Fine grained deposits (mainly clays) are interpreted as floodplain deposits, while sandstones and conglomerates are related to complex multi-storey stacking of braided and meandering river channels (Crusafont-Pairó & Rosell-Sanuy, 1966; Solé, 1985; Checa, 1995; Poyatos-Moré et al., 2013).
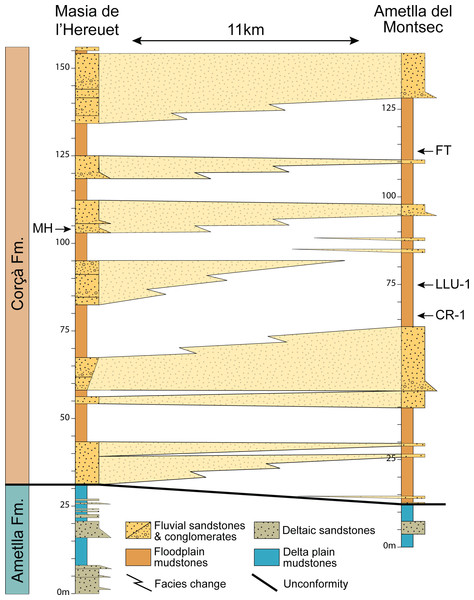
Figure 1: Map of the Àger sub-basin (Southern Pyrenean Basins, NE Spain).
Red stars represent the placement of the early Eocene fossil sites Masia de l’Hereuet (MH) and Casa Retjo-1 (CR-1). Modified from Femenias-Gual et al. (2016b).Several fossil-bearing levels have been identified within the deposits of the Corçà Formation in the Àger sub-basin, including the locality of Casa Retjo-1, type locality of the species Agerinia smithorum. Two representative sedimentary logs were measured in the sections of Masia de l’Hereuet and L’Ametlla del Montsec (where Casa Retjo-1 is located), separated by approximately 11 km (Fig. 1). Their stratigraphic correlation is shown in Fig. 2. In both sections, the lithology is mainly composed of several 5–20 m-thick fluvial sand-rich units alternating with 10’s m-thick, fine-grained packages of floodplain mudstones (Corçà Fm.), and overlying transitional deltaic deposits (Ametlla Fm.). The correlation between the two studied sections allows placing the Masia de l’Hereuet fossil site stratigraphically two fluvial units above Casa Retjo-1, indicating a relative younger age for the former locality.
Figure 2: Regional correlation between the sections of Masia the l’Hereuet and L’Ametlla del Montsec.
The scheme shows the thickness and lateral facies changes of the main sandstone units and the location of the fossil sites: Masia de l’Hereuet (MH), Font del Torricó (FT), Casa Llúcio-1 (LLU-1) and Casa Retjo-1 (CR-1).The mammalian fossil remains found in Masia de l’Hereuet allowed Antunes et al. (1997) to assign this site to the Grauvian (MP10, Reference Level of the mammalian biochronological scale for the European Palaeogene, Schmidt-Kittler, 1987; Aguilar, Legendre & Michaux, 1997). Later on, Badiola et al. (2009) considered that this fossil site was older than previously thought, and assigned Masia de l’Hereuet to the Neustrian (MP8+9) after a revision of the rodents, artiodactyls and perissodactyls from this locality. Recently, the lizards from Masia de l’Hereuet have also been described by Bolet (in press).
Material and Methods
Studied material
The material studied comes from the fossil site Masia de l’Hereuet and consists of a right mandible fragment (IPS-82807) that preserves the teeth from P2 to P4 and the root of the P1, and 15 isolated teeth identified as: one right and one left dP4 (IPS-82796; IPS-82797); one left P4(IPS-82806); two complete and one broken left M1 (IPS-82800; IPS-82801; IPS-82802); one complete left M2 and one fragment of a right M2 (IPS-82805; IPS-82798); two left M3 (IPS-82803, IPS-82804); one right dP4 (IPS-82814); one entire and one broken left M1 (IPS-82808; IPS-82809); one left M2 (IPS-82815); and one left M3 (IPS-82799). All this material is housed at the Institut Català de Paleontologia Miquel Crusafont, ICP (Sabadell, Spain).
Comparative sample
The material studied from Masia de l’Hereuet has been directly compared with the specimens of Agerinia roselli from Les Saleres (Spain) and Agerinia smithorum from Casa Retjo-1 (Spain), both stored at the ICP collections. In addition, the studied sample has been compared with Agerinia cf. roselli from Azillanet (France), belonging to the collections of the Université de Montpellier; and with Agerinia sp. from Condé-en-Brie (France), Donrussellia gallica, Pronycticebus gaudryi and Protoadapis curvicuspidens, which are stored at the MNHN. It has also been compared with high-quality casts of Periconodon huerzeleri, Donrussellia magna, Donrussellia provincialis, Europolemur klatti, Protadapis ignoratus, Cantius eppsi, Marcgodinotius indicus and Asiadapis cambayensis, also stored in the MNHN. Finally, comparisons with Agerinia sp. from Casa Ramón (Spain), cf. Agerinia from Rians (France), Periconodon sp. from Eckfeld Maar (Germany), Periconodon lemoinei, Periconodon jaegeri, Donrussellia lusitanica, Donrussellia russelli, Donrussellia louisi, Darwinius masillae, Europolemur koenigswaldi, Europolemur dunaifi, Europolemur kelleri, Protoadapis angustidens, Protoadapis brachyrhynchus, Protoadapis weigelti, Protoadapis muechelnensis and Cantius savagei, are based on published data.
Dental nomenclature, measurements and micrographs
The dental nomenclature used in the descriptions is that proposed by Szalay & Delson (1979). Measurements have been taken with an optic caliper “Nikon measuroscope 10” connected to a monitor “Nikon SC112”, using the criteria described by Marigó, Minwer-Barakat & Moyà-Solà (2010). Micrographs have been taken using the Environmental Scanning Electron Microscope (ESEM) at Universitat de Barcelona.
New zoological taxonomic name
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:729814E7-5509-48C1-9FF0-84D3E515D909. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Phylogenetic analyses
Two phylogenetic analyses were run using a version of a character-taxon matrix of living and extinct primates as well as euarchontan outgroups that was originally published by Seiffert et al. (2005). This matrix has been successively modified (Seiffert et al., 2009) and a recent version was used by Marigó et al. (2016). The matrix analysed here (Data S1) includes 391 characters and 109 taxa for the first analysis, or 112 taxa for the second analysis (see ‘Results of the Phylogenetic Analyses’). The three taxa added in the matrix for the second analysis are Donrussellia gallica, Periconodon huerzeleri and Darwinius masillae. Their codification was taken from older versions of the matrix used by Seiffert et al. (2010) and Marigó, Minwer-Barakat & Moyà-Solà (2011). In both analyses some multistate characters were treated as ordered, and those with polymorphisms scored as intermediate states were scaled to a half-step so that transitions between adjacent “fixed” states in morphoclines were equal to one full step. Both parsimony analyses were run in PAUP 4.0b10 (Swofford, 1998) for 5,000 replicates with random addition sequence and the tree-bisection-reconnection branch-swapping algorithm. Both analyses were constrained by a molecular scaffold using a constraint tree (Data S2), and treated premolar loss as reversible.
Systematic Paleontology
| Order PRIMATES Linnaeus, 1758 |
| Suborder STREPSIRRHINI Geoffroy Saint-Hilaire, 1812 |
| Infraorder ADAPIFORMES Hoffstetter, 1977 |
| Family NOTHARCTIDAE Trouessart, 1879 |
| Genus AGERINIA Crusafont-Pairó, 1973 |
AGERINIA MARANDATI sp. nov.
urn:lsid:zoobank.org:act:02E82A3B-58B0-4FB8-AE2C-64E1517A0F24
Derivation of name. This species is named after Bernard Marandat (Institut des Sciences de l’Évolution, Université de Montpellier, France), in recognition of his outstanding contribution to the knowledge of Paleogene mammals.
Holotype. Left isolated M1 (IPS-82801) from Masia de l’Hereuet, stored in the Institut Català de Paleontologia Miquel Crusafont (ICP), Sabadell, Spain.
Hypodigm. Right mandible fragment preserving the root of P1 and the teeth from P2 to P4 (IPS-82807); one right and one left dP4 (IPS-82796; IPS-82797); one left P4 (IPS-82806); two complete and one broken left M1 (IPS-82800; IPS-82801; IPS-82802); one complete left M2 and one fragment of a right M2 (IPS-82805; IPS-82798); two left M3 (IPS-82803, IPS-82804); one right dP4 (IPS-82814); one entire and one broken left M1 (IPS-82808; IPS-82809); one left M2 (IPS-82815); and one left M3 (IPS-82799), all from Masia de l’Hereuet.
Occurrence. Masia de l’Hereuet, Àger sub-basin (Southern Pyrenean Basins, Lleida province, NE Spain); early Eocene (Neustrian, MP8+9, Mammal Paleogene Reference Level).
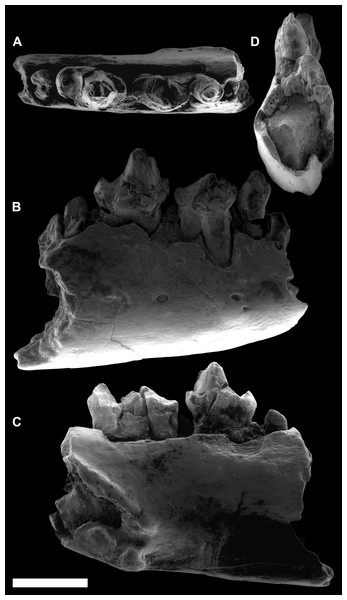
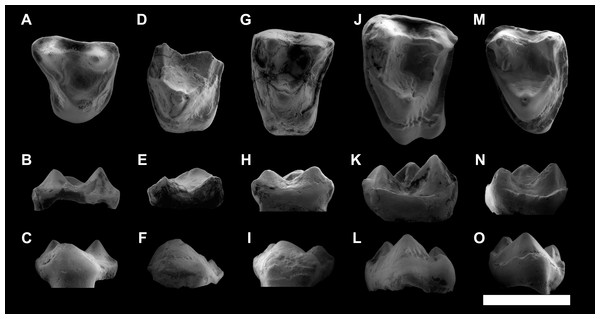
Figure 3: ESEM images of Agerinia marandati sp. nov. from Masia de l’Hereuet.
Right mandible (IPS-82807) with alveoli of the canine and P1, premolars from P2 to P4, and mesial root of the M1 in occlusal (A), buccal (B), lingual (C) and mesial (D) views. Scale bar represents 3 mm.Figure 4: ESEM images of isolated lower teeth of Agerinia marandati sp. nov. from Masia de l’Hereuet.
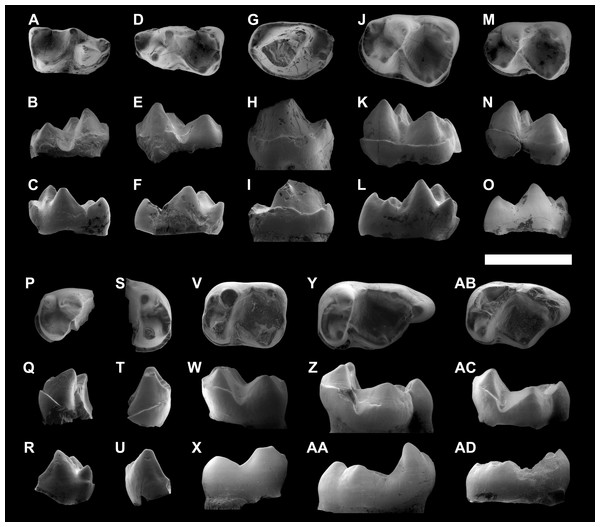
IPS-82796, right dP4 in occlusal (A), buccal (B) and lingual (C) views. IPS-82797, left dP4 in occlusal (D), buccal (E) and lingual (F) views. IPS-82806, left P4 in occlusal (G), buccal (H) and lingual (I) views. IPS-82801 (holotype), left M1 in occlusal (J), buccal (K) and lingual (L) views. IPS-82800, left M1 in occlusal (M), buccal (N) and lingual (O) views. IPS-82802, fragment of left M1 in occlusal (P), buccal (Q) and lingual (R) views. IPS-82798, fragment of right M2 in occlusal (S), buccal (T) and lingual (U) views. IPS-82805, left M2 in occlusal (V), buccal (W) and lingual (X) views. IPS-82803, left M3 in occlusal (Y), buccal (Z) and lingual (AA) views. IPS-82804, left M3 in occlusal (AB), buccal (AC) and lingual (AD) views. Scale bar represents 3 mm.Figure 5: ESEM images of isolated upper teeth of Agerinia marandati sp. nov. from Masia de l’Hereuet.
IPS-82814, right dP4 in occlusal (A), buccal (B) and lingual (C) views. IPS-82809, fragment of left M1 in occlusal (D), buccal (E) and lingual (F) views. IPS-82808 left M1 in occlusal (G), buccal (H) and lingual (I) views. IPS-82815, left M2 in occlusal (J), buccal (K) and lingual (L) views. IPS-82799, left M3 in occlusal (M), buccal (N) and lingual (O) views. Scale bar represents 3 mm.Diagnosis. Medium-sized notharctid. P1 and P2 single-rooted. P4 with well-developed protoconid and metaconid, distinct paraconid, hypoconid and cristid obliqua. M1 with a voluminous paraconid, open trigonid basin, trigonid clearly narrower than the talonid, protocristid oblique to the lingual and buccal borders. M2 and M3 without paraconid, closed trigonid basin, trigonid nearly equal in width to the talonid, and a protocristid subperpendicular to the lingual and buccal borders. Upper molars with paraconule more developed than the metaconule, and without pericone. M1 and M2 with distinct hypocone and well-developed preparaconule crista and hypoparacrista, joining paracone and paraconule.
Differential diagnosis. Agerinia marandati differs from A. roselli in having a less molarized P4 (lacking an entoconid), a relatively large M1 paraconid, an open M1 trigonid basin and a protocristid that is more oblique to the lingual and buccal borders on the M1. Agerinia marandati differs from A. smithorum in having a single-rooted P2, a more molarized P4 (showing distinct paraconid and hypoconid) and in lacking a paraconid on the M2. Agerinia marandati differs from Pronycticebus gaudryi in the less bulbous cuspids, the single-rooted P1, the absence of paraconid on the M2 and M3, and the less developed hypocone, parastyle and metastyle on the upper molars. It further differs from Europolemur in the smaller size and in the much more developed paraconid on the M1. It differs from Protoadapis in the much less robust cusps, in having P3 and P4 similar in height and in the well-developed paraconid on the M1. Agerinia marandati differs from Cantius eppsi and Cantius savagei in its smaller size and less inflated cusps. It further differs from Cantius eppsi in the lack of paraconid on the M2 and M3, in the better-developed hypocone, hypoparacrista and hypometacrista and the less-developed paraconule, metaconule and lingual cingulum on the upper teeth. Furthermore, it differs from Cantius savagei in the narrower M1 and in the slightly longer talonid basin. Besides, Agerinia marandati differs from Marcgodinotius indicus in its smaller size, in the single-rooted P2, the differentiated paraconid and metaconid on the P4, the lack of paraconid on the M2 and M3, and in the less concave mesial and distal borders in the upper molars. A. marandati differs from Asiadapis cambayensis in the presence of a P1, the better-developed paraconid on the M1, and in the more developed hypocone, paraconule and metaconule on the upper molars.
Measurements. See Table 1.
| Catalogue number | Tooth | Length | Width |
|---|---|---|---|
| IPS-82796 | dP4 | 2.98 | 1.61 |
| IPS-82797 | dP4 | 3.18 | 1.81 |
| IPS-82807 | P2 | ≥1.58 | ≥1.22 |
| P3 | ≥2.68 | ≥1.61 | |
| P4 | ≥3.16 | ≥1.99 | |
| IPS-82806 | P4 | 3.21 | 2.25 |
| IPS-82800 | M1 | 3.11 | 2.14 |
| IPS-82801 | M1 | 3.70 | 2.60 |
| IPS-82802 | M1 | – | – |
| IPS-82805 | M2 | 3.05 | 2.50 |
| IPS-82798 | M2 | – | – |
| IPS-82803 | M3 | 4.18 | 2.51 |
| IPS-82804 | M3 | 3.55 | 2.36 |
| IPS-82814 | dP4 | 3.13 | 3.19 |
| IPS-82808 | M1 | 3.00 | 3.86 |
| IPS-82809 | M1 | – | – |
| IPS-82815 | M2 | 3.72 | 4.68 |
| IPS-82799 | M3 | 2.94 | 3.83 |
Description
Mandible. This specimen preserves the distal part of the canine alveolus, which is placed just mesially with respect to the P1, with no diastema between these two teeth. The P1 root is preserved, which corresponds to a small premolar, and is aligned with the rest of the teeth on the longitudinal axis of the mandible. The mandible preserves all the teeth from P2 to P4 and the mesial root of the M1; however, they are all damaged. Furthermore, on the lingual side of the mandible, below the P1 and P2, a slightly protruding stripe can be observed descending from the mesial to the distal part, probably indicating an unfused mandible. On the buccal side of the mandible there are three mental foramina of similar size and a tiny one mesially situated just on the bottom of the central one. The mesial-most foramen is broken and placed between the alveoli of the canine and P1. The central foramina are situated between the root of the P2 and the mesial root of the P3 and the distal-most foramen is placed underneath the mesial root of the P4.
dP4. The trigonid is elongated, much longer than wide; it is slightly shorter and clearly narrower than the talonid. There is a broad space separating the paraconid and metaconid. The trigonid is open lingually. The paraconid is well differentiated, clearly smaller than protoconid and metaconid, and placed in the mesiolingual extreme of the tooth. The paracristid runs buccally from the paraconid and curves distally reaching the protoconid. The protoconid is located mesially with respect to the metaconid. Both cuspids are similar in height and connected by a protocristid, oblique to the buccal and lingual borders. There is no premetacristid. The cristid obliqua reaches the apex of the metaconid. The talonid basin is deep. The hypoconid is placed mesially with respect to the entoconid. The postcristid is thickened at its central part, but it does not bear a distinct hypoconulid. There is a well-marked notch that separates the preentocristid and the postmetacristid. The buccal cingulid is slightly marked and restricted to the mesiobuccal corner of the tooth, from the buccal base of paraconid to the distal base of protoconid. There are two roots.
P2. The P2 is slightly longer than broad, with an oval outline. It is larger than P1 (based on the size of the roots), and clearly smaller than P3 and P4. The morphology is rather simple, with a single distinguishable cuspid (protoconid) and two sharp cristids directed mesially and distally from the apex to the base of the crown. At the distal end of the tooth, there is a small but distinguishable bulge centrally located. There is a weak cingulid on the mesial part of the tooth, and a more developed cingulid occupying the distolingual and the distobuccal borders. There is only one root.
P3. There is only one P3 known, twice long as it is broad. The crown is strongly damaged, preventing the observation of the dental morphology. However some traits can be described, particularly the presence of a protuberance on the distal end of the crown, centrally located, that does not constitute a clear cuspid. The buccal and lingual cingulids are faintly marked and restricted to the mesial and distal ends of the tooth. On the distolingual corner, the lingual cingulid encloses an incipient and shallow talonid basin. There are two roots.
P4. The P4 is larger than P3. The outline is oval, somewhat more mesiodistally elongated in specimen IPS-82807 than in IPS-82806. There is a distinct paraconid in mesiolingual position. The paracristid descends from the protoconid apex, curving lingually at its end and reaching the paraconid. The metaconid, clearly distinguishable, is attached to the distolingual side of the protoconid in specimen IPS-82806 (the poor preservation of specimen IPS-82807 prevents the observation of this trait). There is a distinct cristid obliqua that runs from the joint between protoconid and metaconid to the hypoconid, which is centrally located at the distal side of the tooth (this trait is also only observed in specimen IPS-82806). The hypoconid is as high as the paraconid. The buccal and lingual cingulids, high and well marked, surround the entire base of the tooth in specimen IPS-82806; in IPS-82807 these cingulids seem less marked, but it can be due to the bad preservation of this specimen. The lingual cingulid encloses a shallow talonid basin, restricted to the distolingual corner. There are two roots.
M1. The trigonid is shorter and much narrower than the talonid. The trigonid basin is as long as it is wide and it is open between the paraconid and the metaconid. The paraconid is well differentiated but clearly smaller than the other trigonid cuspids; it is placed on the mesiolingual corner of the tooth, closer to the metaconid than to the protoconid. The paracristid runs mesially from the protoconid and, at the mesiobuccal corner of the tooth, curves buccally reaching the paraconid. The protoconid is located in a more mesial position than the metaconid. Both cuspids are connected by a protocristid, clearly oblique to the lingual and buccal borders, that shows a well-marked V-shaped valley. In the specimens IPS-82800 and IPS-82801, there is no premetacristid, whereas IPS-82802 shows a cristid directed mesially from the metaconid, which does not reach the paraconid. In this latter specimen, the metaconid apex is slightly curved distally. The cristid obliqua reaches the trigonid wall at the level of the buccal base of the metaconid. The talonid basin is clearly deeper than the trigonid basin. The hypoconid is placed mesially with respect to the entoconid. The postcristid connects the hypoconid and entoconid, which shows several enamel swellings that do not constitute a distinct hypoconulid. The preentocristid connects to the postmetacristid closing the talonid lingually. IPS-82801 shows a small but well differentiated bulge on the middle of the preentocristid. The buccal cingulid runs from the mesiobuccal base of the paraconid to the distolingual base of the hypoconid. This cingulid is very strong on the mesial border and below the protoconid, and weaker at the level of the talonid, being interrupted below the hypoconid in IPS-82800.
M2. The trigonid is wider than long; it is much shorter than the talonid, but similar in width, and therefore the outline of the tooth is rectangular. There is no paraconid. The paracristid runs from the protoconid, surrounding the mesial border and continuing into a premetacristid that reaches the metaconid, closing the trigonid basin lingually. The protoconid is located in a slightly more mesial position than the metaconid, so the protocristid is almost perpendicular to the buccal and lingual borders of the tooth. The cristid obliqua reaches the trigonid wall close to the lingual base of protoconid. The talonid basin is closed, moderately shallow and as wide as it is long. The hypoconid is slightly more voluminous than the entoconid and placed in a slightly more mesial position. The postcristid joins hypoconid and entoconid; at its distobuccal part, it thickens forming a tiny hypoconulid. The preentocristid and postmetacristid are connected, closing the talonid basin lingually. The buccal cingulid is weak and restricted to the base of the protoconid.
M3. The trigonid is much shorter than the talonid and similar in width. There is no paraconid. The paracristid continues into a premetacristid reaching the metaconid, so the trigonid basin is closed. The protoconid is placed in a slightly more mesial position than the metaconid. The protocristid is nearly perpendicular to the buccal and lingual margins of the tooth. The cristid obliqua is straight and reaches the trigonid wall in a more buccal position than in the M2. The talonid basin is closed, moderately deep and longer than wide. The hypoconid is slightly more voluminous and placed more mesially than the entoconid. Preentocristid and postmetacristid are connected, closing the talonid buccally. The hypoconulid lobe is prominent, but longer and better differentiated from the talonid basin in IPS-82803 than in IPS-82804. The postentocristid shows a slightly marked valley between the hypoconulid and the entoconid in specimen IPS-82803. The buccal cingulid is visible on the base of the protoconid. On IPS-82803 a very weak cingulid is hardly observed on the buccal base of the hypoconid.
dP4. The specimen is eroded, lacking the enamel. The outline is subtriangular, with the buccal side longer than the lingual side and it has three main cusps: paracone, metacone and protocone. There is no hypocone. The trigon basin is quite deep. The preparacrista is slightly marked and runs from the apex of the paracone, curving buccally, reaching the mesiobuccal corner, which is broken. The postparacrista runs distally from the paracone and connects to the premetacrista, which reaches the metacone. The postmetacrista is straight and connects the metacone to the distobuccal corner of the tooth. The protocone is slightly lower than paracone and metacone. The preprotocrista connects the protocone to a small paraconule. The preparaconule crista runs around the mesiobuccal half of the tooth, from the paraconule to the mesiobuccal corner. The metaconule is more developed than the paraconule. Three cristae run from the metaconule: a well-marked postprotocrista that connects to the protocone, a short premetaconule crista, which is directed to the metacone but does not reach its apex, and a longer postmetaconule crista (also known as lateral posterior transverse crista) that borders the distal margin of the tooth and reaches the postmetacrista at the distobuccal corner of the tooth. There is a faint anterocingulum that runs from the mesiolingual base of the protocone to the mesial half of the tooth, reaching the preparaconule crista. The postcingulum is restricted to the distolingual corner of the tooth, reaching the postmetaconule crista. There are three roots.
M1. The outline is subtriangular, being much wider than long and with the buccal side somewhat longer than the lingual one. The trigon basin is deep. The paracone is somewhat larger than the metacone; the protocone is slightly lower than the buccal cusps, and the hypocone is clearly smaller and lower than the rest. The preparacrista is weak. From the apex of the paracone, a straight postparacrista descends distally and connects with the premetacrista that reaches the metacone apex. The postmetacrista is straight and runs from the apex of metacone to the distobuccal corner of the tooth. The paraconule is much more developed than the metaconule; it has two buccal cristae: a preparaconule crista, reaching the end of the preparacrista at the mesiobuccal corner of the tooth, and a hypoparacrista that reaches the apex of the paracone. These cristae enclose a well-developed basin mesiolingual to the paracone. The preprotocrista is well marked and connects the paraconule and the protocone. The metaconule is connected to the metacone by a hypometacrista and to the protocone by a postprotocrista. There is a small but distinct hypocone at the distolingual corner of the tooth; it is weakly connected to the base of the protocone by a very short and low postprotocingulum. There is a weak buccal cingulum restricted to the central part of the buccal border, between paracone and metacone. The anterocingulum is marked and connects to the preparaconule crista; it is longer in IPS-82809 than IPS-82808, surrounding the mesiolingual corner in the former. The posthypocone crista continues in a long and well-marked postcingulum that occupies the entire distal border, being interrupted at the distobuccal corner, without connecting to the postmetacrista. The specimen IPS-82808, shows a slightly wrinkled enamel surface at the lingual base of the protocone.
M2. It is larger than the M1, subtriangular, with the lingual side slightly shorter than the buccal side. The trigon basin is very deep. The paracone is slightly larger than the metacone and similar in height to the protocone. The preparacrista is well marked and straight; it connects the paracone to a small parastyle. The postparacrista and premetacrista are sharp and descend strongly, so their connection is placed in a very low position. The postmetacrista descends from the apex of the metacone and curves slightly towards the buccal side, reaching the distobuccal corner of the tooth. It thickens at its distal end, forming an incipient metastyle. Paraconule and metaconule are similar in size. From the paraconule, a preparaconule crista borders the mesial part of the tooth, reaching the parastyle. There is a deep basin mesiolingual with respect to the paracone, enclosed by the preparaconule and hypoparacrista. From the protocone, the preprotocrista and the postprotocrista connect to the paraconule and the metaconule, respectively. The hypometacrista is well marked and joins the metaconule with the metacone. There is a well-developed hypocone, placed in a marginal position at the distolingual corner of the tooth and protruding strongly on the outline of the molar. It is connected to the distal base of protocone by a short postprotocingulum. There is no pericone. The buccal cingulum is well developed and runs from the parastyle to the distobuccal corner of the tooth, without meeting the metastyle. The anterocingulum is also strong; it starts at the mesiolingual corner of the tooth and connects to the preparaconule crista. The anterocingulum continues into a lingual cingulum, which is crenulated at the base of the protocone. The posthypocone crista continues in a postcingulum that extends to the distobuccal corner of the tooth, without connecting to the metastyle. This cingulum encloses a very small talon basin. This specimen shows slight enamel wrinkling, especially on the trigon basin and on the distolingual base of protocone.
M3. The outline is triangular. The paracone is notably larger than the metacone and similar in height to the protocone. The trigon basin is deep. There is no hypocone. The preparacrista is curved lingually and connects the paracone to a hardly distinct parastyle. The postparacrista and premetacrista are well marked. The postmetacrista is very weak and curved buccally; it reaches the distobuccal corner of the tooth, without forming a metastyle. The conules are much less developed than in the M1 and M2: the paraconule is a mere thickening of the preprotocrista, and the metaconule is absent. The preparaconule crista is sharp and long, reaching the parastyle. The hypoparacrista is weaker than the preparaconule crista; it descends lingually from the paracone but does not reach the paraconule. The postprotocrista connects the protocone and the metacone. The buccal cingulum starts at the mesiobuccal base of the paracone, without reaching the parastyle. This cingulum is strong at the base of paracone, but weak and discontinuous at the base of metacone. The anterocingulum starts at the base of the paraconule, surrounds the mesiolingual border of the tooth and continues in a strong postcingulum that occupies the whole distal border. This cingulum shows some bulges at its lingual base that do not constitute distinct cuspids. The enamel is slightly wrinkled on the distolingual side.
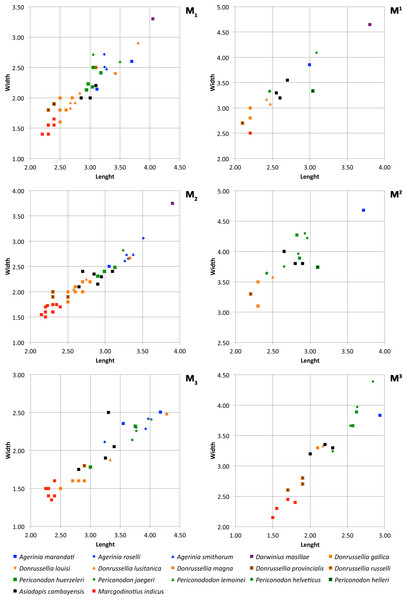
Figure 6: Size graphs (length × width, in mm) of the molars of the different species of Agerinia, Darwinius, Donrussellia, Periconodon, Asiadapis and Marcgodinotius.
Measurements of Agerinia marandati are those presented in this work. Measurements of Periconodon helveticus have been taken directly on high-resolution casts. Data of Agerinia roselli and Agerinia marandati after Femenias-Gual et al. (2016a) and Femenias-Gual et al. (2016b), respectively; data of Darwinius masillae after Franzen et al. (2009); data of Donrussellia gallica after Russell, Louis & Savage (1967); data of Donrussellia louisi, Donrussellia russelli, Periconodon lemoinei and Periconodon huerzeleri after Gingerich (1977); data of Donrussellia lusitanica after Estravís (2000); data of Donrussellia magna after Godinot et al. (1987); data of Donrussellia provincialis after Godinot (1981); data of Periconodon jaegeri after Godinot (1988); data of Periconodon helleri after Thalmann (1994); data of Asiadapis cambayensis and Marcgodinotius indicus after Rose et al. (2007) and Rose et al. (2009).
Comparisons
Comparisons with other samples attributed to Agerinia. The new species Agerinia marandati is quite similar in size and in some morphological traits to Agerinia roselli from Les Saleres (Fig. 6; Crusafont-Pairó, 1967; Szalay, 1971; Femenias-Gual et al., 2016a) such as the single-rooted P2, the distinct paraconid on the P4 or the lack of paraconid on the M2. However, A. marandati clearly differs in other several traits. The root of the P1 of A. marandati is centrally located on the mesiodistal axis of the mandible, while in A. roselli it is clearly shifted buccally (here, we consider that the most mesial root of A. roselli described by Femenias-Gual et al. (2016a) corresponds to the P1, see Discussion). The metaconid and hypoconid of the P4 are slightly better differentiated in A. roselli than in A. marandati. In addition, the P4 of A. marandati lacks the distinct entoconid that is present in A. roselli. Besides, the M1 paraconid is clearly larger in A. marandati, while it is very small in A. roselli. Finally, the distal and intermediate mental foramina of A. roselli are clearly more mesially located than in A. marandati.
Regarding Agerinia smithorum from Casa Retjo-1 (Femenias-Gual et al., 2016b), it is also very similar in size to A. marandati (Fig. 6). Furthermore, both species share several traits such as the central position of the P1 on the mesiodistal axis of the mandible, the lack of entoconid on the P4, the well-developed paraconid on the M1, or the similar disposition of the distal and intermediate mental foramina, this latter being only a little more mesially located in A. marandati than in A. smithorum. Nevertheless, there are several differences between these species. The P1 alveolus is more compressed mesiodistally in A. marandati than in A. smithorum, thus suggesting a more reduced premolar (the P1 crown is not preserved in these species). The number of roots of P2 is different, being double-rooted in A. smithorum and single-rooted in A. marandati. Additionally, the latter species differs from A. smithorum in the molarization of the P4. A. marandati shows a well developed metaconid and distinct paraconid and hypoconid, while in A. smithorum the metaconid is smaller and the paraconid and hypoconid are absent. Furthermore, A. marandati lacks the paraconid on the M2, while A. smithorum shows a tiny one. Finally, the mesial-most mental foramen is clearly more mesially located in A. marandati than in A. smithorum.
Godinot (1983) described two partial mandibles of Agerinia cf. roselli from Azillanet (MP10, France), which are similar in size or slightly larger than A. marandati. Furthermore, the M1 from Azillanet clearly differs from that of A. marandati in lacking the paraconid.
Peláez-Campomanes (1995) documented the presence of Agerinia sp. in the fossil site Casa Ramón (MP11, N Spain), which is clearly smaller than A. marandati. Moreover, they differ in several traits. In the M1 from Casa Ramón, the paracristid forms an acute angle in the mesiobuccal corner; this angle is obtuse in A. marandati. The M2 of Agerinia sp. is proportionally narrower than that of A. marandati, and the paracristid of the latter is higher than in the specimen from Casa Ramón. Besides, the protocristid of A. marandati is more perpendicular to the buccal and lingual borders of the tooth than in Agerinia sp. from Casa Ramón. The M1−2 of Casa Ramón lacks the hypoparacrista that is well marked in the M1 and M2 of A. marandati. Finally, the hypocone is connected to the distal base of protocone by a short postprotocingulum in the M1 and M2 of A. marandati but it is isolated in Agerinia sp.
Herbomel & Godinot (2011) described some specimens from Condé-en-Brie (MP8+9, France), preliminarily assigned to Agerinia sp. This form is, in general terms, somewhat larger than A. marandati and shows slightly more bulbous cusps. The paraconid of the M1 is larger in A. marandati than in Agerinia sp. from Condé-en-Brie, where it varies from small to moderate. The M1 and M2 of A. marandati show a hypoparacrista that connects the paracone with the paraconule, while in Agerinia sp. there is only a short hypoparacrista that does not reach the paraconule. Furthermore, the hypocone of Agerinia sp. varies in size from large to small or even absent in many M1 and M2, whereas it is well marked in the M1 and M2 of A. marandati. The pericone is absent in the upper molars of A. marandati, while in some M2 from Condé-en-Brie the anterior cingulum thickens forming a real pericone. The M3 of Agerinia sp. frequently display one or two lingual cusps; on the contrary, in the M3 of A. marandati there are some bulges at the level of the connection of the anterocingulum and the postcingulum, which do not constitute distinct cusps.
There is a single M2 from Rians (MP7, France) described by Godinot (1983), which was determined as cf. Agerinia. This specimen is clearly larger than the M2 of A. marandati and shows some morphological differences. For instance, the difference in width between the trigonid and the talonid is much more marked in the M2 of cf. Agerinia than in that of A. marandati, which shows a more squared outline. Furthermore, cf. Agerinia shows a well-developed paraconid that is absent in the M2 of A. marandati. Finally, the M2 from Rians shows an expansion of the distolingual corner and has the entoconid more distally placed with respect to the hypoconid than in A. marandati.
Comparisons with other Eurasian Notharctidae. The new species A. marandati has been compared to Periconodon and Darwinius, which together with Agerinia, form a close taxonomic group following Godinot (2015). Moreover, it has been compared with Donrussellia, the only other Euprimate genus found in the Iberian Peninsula in the early Eocene, as well as with other Eocene Eurasian notharctids, such as the genera Pronycticebus, Europolemur, Protoadapis, Cantius, Marcgodinotius and Asiadapis.
Although the taxonomy of Periconodon is uncertain, five species and a specimen without specific attribution constitute this genus (following Godinot, 1988; Godinot, 2015): P. helveticus from Ergerkingen (MP13; Rütimeyer, 1891), P. lemoinei from Grauves (MP10, Gingerich, 1977), P. huerzeleri from Bouxwiller (MP13, Gingerich, 1977), P. helleri from Geiseltal (MP13-14, Schwartz, Tattersall & Haubold, 1983), P. jaegeri from Bouxwiller (MP13, Godinot, 1988) and Periconodon sp. from Eckfeld Maar (MP13, Franzen, 2004). Regarding size, the teeth of Agerinia marandati are larger than those of P. huerzeleri and P. helveticus, similar in size to those of P. jaegeri and P. lemoinei, and shorter and broader than those of Periconodon sp. from Eckfeld Maar (Fig. 6; there are no available published measurements of P. helleri). In any case, A. marandati shows strong morphological differences with the genus Periconodon, including the lack of pericone on the upper molars, the better differentiated metaconid on the P4, the presence of a distinct paraconid on the M1 and of an entoconid on the M3 (Franzen, 2004; Godinot, 2015).
Regarding Darwinius masillae from Messel (MP11, Franzen et al., 2009), it is clearly larger than A. marandati (Fig. 6) and lacks the first lower premolar. The upper molars of D. masillae lack the metaconule that is present in A. marandati; the paraconule is barely marked in Darwinius. The hypoparacrista is faint and does not reach the paraconule. Moreover, the talon basin is clearly broader in the upper molars of Darwinius than in those of A. marandati. The anterocingulum and postcingulum of D. masillae are more developed than those of A. marandati. The M1 of Darwinius lacks a paraconid and shows a closed trigonid basin, whereas in A. marandati this tooth shows an open trigonid basin with a well-differentiated paraconid. Furthermore, the M1 of Darwinius shows a well-differentiated metastylid that is absent in A. marandati. Finally, the mandible of D. masillae shows only one mental foramen below the P2, whereas there are three foramina on A. marandati.
Furthermore, A. marandati clearly differs from the genus Donrussellia, which includes six species: D. gallica, D. russelli and D. louisi from Avenay, France (MP8+9, Russell, Louis & Savage, 1967; Gingerich, 1977), D. provincialis from Rians, France (MP7, Godinot, 1978), D. magna from Palette, France (MP7, Godinot et al., 1987) and D. lusitanica from Silveirinha, Portugal (MP7, Estravís, 2000). Concerning the size, A. marandati is clearly larger than Donrussellia provincialis, D. gallica and D. lusitanica and similar in size or slightly smaller than D. magna, D. russelli and D. louisi (Fig. 6). Regarding the morphology, Donrussellia clearly differs from A. marandati in the presence of a well-developed paraconid in all lower molars, whereas A. marandati only shows a well-marked cuspid in the M1. Furthermore, Donrussellia differs from A. marandati in having a double-rooted P2 while in the latter it is single rooted. In the M1 of Donrussellia the trigonid is as long as the talonid, while in A. marandati it is shorter than the talonid. The M1 and M2 of A. marandati clearly differ from those of Donrussellia in having a more developed hypoparacrista. Besides, the hypocone is distinct in the M1 and M2 of A. marandati, whereas this cusp is only present in some M2 of Donrussellia.
Additionally, A. marandati is smaller than Pronycticebus gaudryi from Mermerlein in France (MP20, Grandidier, 1904; Szalay, 1971). The cusps are generally more bulbous in P. gaudryi than in A. marandati. Regarding the lower teeth, they differ in the number of roots of the P2, being double-rooted in P. gaudryi and single-rooted in A. marandati. Besides, the former species shows a paraconid in all lower molars, whereas A. marandati only displays a paraconid in the M1. The upper teeth of A. marandati show less developed hypocone, parastyle and metastyle than those of P. gaudryi. Furthermore, the trigon basin of A. marandati is wider than in P. gaudryi, in which it is as long as it is wide. Moreover, the M3 of P. gaudryi displays a hypocone, and the hypoparacrista reaches the paraconule, whereas in the M3 of A. marandati the hypoparacrista does not join the paraconule and the hypocone is absent.
The genus Europolemur includes four species following Godinot (2015): E. koenigswaldi and E. kelleri from Messel (MP11, Franzen, 1987; Franzen, 2000), E. klatti from Geiseltal (MP13, Thalmann, 1994) and E. dunaifi from Bouxwiller (MP13, Tattersall & Schwartz, 1983; Godinot, 1988). All these species are much larger than A. marandati. The paraconid is much more developed in the M1 of A. marandati than in Europolemur, in which this cuspid can be small or absent. Furthermore, among other differences, E. koenigswaldi and E. klatti lack the P1 whereas A. marandati preserves it. Besides, E. klatti shows a double-rooted P2, while this premolar is single rooted in A. marandati. The size of the hypocone is variable in the upper molars of Europolemur, being less developed in E. kelleri than in A. marandati, and more developed in E. dunaifi than in A. marandati.
The genus Protoadapis comprises six species according to Godinot (2015): Protoadapis angustidens and Protoadapis brachyrhynchus from unknown levels of the Quercy phosphorites (Russell, Louis & Savage, 1967; Gingerich, 1975), Protoadapis curvicuspidens from different sites including Grauves (MP10, Russell, Louis & Savage, 1967) and Protoadapis weigelti, Protoadapis ignoratus and Protoadapis muechelnensis from Geiseltal (MP12, Thalmann, 1994). All these species are poorly known and only represented by lower teeth, except P. curvicuspidens. In any case, there are several important differences between Protoadapis and A. marandati. The genus Protoadapis is much larger and displays more robust cusps than Agerinia. In addition, the P3 is clearly higher than the P4 in Protoadapis, whereas in Agerinia these premolars are more similar in height. Moreover, the paraconid of the lower molars of Protoadapis is shown as a residual cuspule, whereas A. marandati displays a well-developed paraconid on the M1.
Several species are included in the genus Cantius, but only two are recorded from Europe: Cantius eppsi from Abbey Wood (MP8+9; Hooker, 2010) and Cantius savagei from Muntigny and Avenay (MP8+9; Gingerich, 1977). These two species show notable differences with A. marandati. Both C. eppsi and C. savagei are clearly larger and show more inflated cusps than Agerinia. Besides, Agerinia marandati differs from C. eppsi in having a paraconid only on the M1, whereas the latter generally shows a well-developed paraconid in all lower molars. Regarding the upper molars, A. marandati shows better-developed hypocone, hypoparacrista and hypometacrista than C. eppsi. In addition, A. marandati has slightly wrinkled enamel on the M2 an M3, whereas the teeth of C. eppsi have smooth enamel. Besides, C. eppsi shows a postprotocingulum, while in A. marandati it is absent. Furthermore, C. eppsi displays more developed paraconule, metaconule and lingual cingulum than A. marandati. The M1 of Cantius savagei is broader than that of A. marandati and displays a slightly shorter talonid basin.
The asiadapine Marcgodinotius indicus from Vastan and Tadkehwar mines (early Eocene, India; Bajpai et al., 2007; Rose et al., 2007; Rose et al., 2009; Smith et al., 2016) differs from A. marandati in several traits. Regarding the size, A. marandati is larger than M. indicus (Fig. 6). Marcgodinotius has a double-rooted P2, whereas in A. marandati this premolar is single rooted. Moreover, the P4 of A. marandati displays small but differentiated paraconid and metaconid, whereas the P4 of M. indicus usually lacks these cuspids. Some specimens of M. indicus show a low metaconid posterolingually placed in relation to the protoconid; on the contrary, in the P4 of A. marandati the metaconid is higher than in Marcgodinotius and lingually attached to the protoconid. The lower molars of M. indicus display a slighly longer trigonid than those of A. marandati. Furthermore, the M1 and M2 of M. indicus show a small, low and buccally shifted paraconid, while A. marandati only displays a well-developed paraconid on the M1. Furthermore, the outline of the talonid basin of A. marandati is clearly more rounded than in M. indicus. Regarding the upper teeth, the decidual P4 of A. marandati lacks the hypocone and the hypoparacrista that are well marked in M. indicus. The difference in length between the buccal and the lingual sides is less marked in the upper molars of A. marandati than in M. indicus. The outline of the upper molars is also different, showing concave mesial and distal borders in M. indicus. Besides, M. indicus differs from A. marandati in having a more marked buccal cingulum and styles in the M1. The M3 of M. indicus is much wider than that of A. marandati and also differs from the latter in the more developed parastyle and in the presence in some specimens of a small and low paraconule, premetaconule and postmetaconule cristae.
Concerning Asiadapis cambayensis from Vastan mine (early Eocene, India; Rose et al., 2007; Rose et al., 2009), it is smaller than A. marandati (Fig. 6). In addition, Asiadapis lacks the first lower premolar, whereas A. marandati has a small P1. The P4 of A. marandati has distinct paraconid and metaconid, whereas theses cuspids are absent in some specimens of A. cambayensis. The paraconid of the M1 is better developed in A. marandati. Furthermore, some M2 and M3 of A. cambayensis show a paraconid, which is absent in A. marandati. Besides, the talonid basin has a rounded outline in the lower molars of A. marandati, whereas in A. cambayensis the talonid basin is more elongated mesiodistally. Regarding the upper teeth, A. marandati shows more developed hypocone, paraconule and metaconule, especially in the M2. Furthermore, A. marandati displays slightly wrinkled enamel on the M2 and M3, which is smooth in A. cambayensis.
Results of the Phylogenetic Analyses
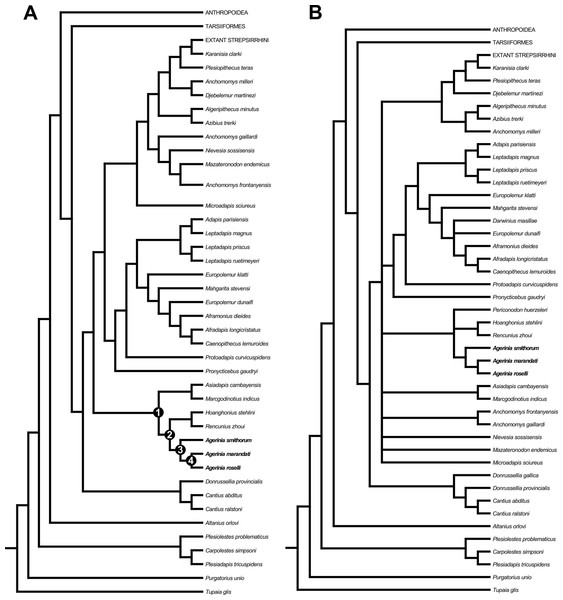
The two developed phylogenetic analyses agree in placing all the species of Agerinia together in the same clade. Besides, both analyses place A. smithorum as the most primitive of the three species of the genus (Fig. 7). However, both analyses present different results. On the one hand, the first analysis (Fig. 7A), performed taking into account 109 taxa, places the genus Agerinia as closely related to the sivaladapids Hoanghonius and Rencunius and, to a lesser extent, the asiadapines Asiadapis and Marcgodinotius. In this analysis, the clade formed by Agerinia, sivaladapids and asiadapines would not be nested within a monophyletic Adapiformes. These results have been obtained in previous analyses (see Marigó et al., 2016).
Figure 7: Strict consensus trees derived from parsimony analyses of the 391 character matrix.
(A) original data matrix using 109 taxa, strict consensus of 3 equally parsimonious trees (tree length (TL) = 4292.5, consistency index (CI) = 0.163, retention index (RI) = 0.571) recovered by 5,000 heuristic search replicates in PAUP 4.10b10. (B) data matrix with 112 taxa (addition of Donrussellia gallica, Periconodon huerzeleri and Darwinius masillae), strict consensus of 103 equally parsimonious trees (tree length (TL) = 4,345, consistency index (CI) = 0.162, retention index (RI) = 0.567) recovered by 5,000 heuristic search replicates in PAUP 4.10b10. Unambiguous synapomorphies supporting nodes 1, 2, 3 and 4 are provided in Data S3.Three unambiguous synapomorphies (see Data S3) support the placement of asiadapines as the sister group of the clade formed by Agerinia and sivaladapids on the strict consensus tree, and all three synapomorphies are related to dental features. Four unambiguous synapomorphies support the clade formed by Agerinia and sivaladapids, and all four are also related to dental features.
On the other hand, when performing the same analysis but taking into account three more taxa (Donrussellia gallica, Periconodon huerzeleri and Darwinius masillae), the placement of many taxa remains unresolved (Fig. 7B). For instance, the clade formed by Agerinia, sivaladapids and asiadapines in the previous analysis, in this second analysis is formed by Agerinia, sivaladapids and Periconodon, whereas asiadapines are in polytomy with anchomomyins (fully resolved in the previous analysis), as well as “adapiforms” and stem and crown strepsirrhines.
The addition of these three adapiform taxa, which present many characters coded as missing or unknown, results in unresolved conditions because only those groups that are found in all trees are included in the consensus tree (Rohlf, 1982). Thus, we conclude that, even if some of these taxa have traditionally been suggested as being closely related to Agerinia, their inclusion in phylogenetic analyses may not be the best option until these taxa are further studied or more material is recovered.
Discussion
The new material presented here represents the most complete sample of the genus Agerinia known to date and includes some dental elements previously undescribed, such as the upper molars, the upper and lower deciduous fourth premolars and the P2. Besides, the clear differences observed between this material and the previously described species A. roselli and A. smithorum have allowed erecting the new species Agerinia marandati.
The only mandibular fragment of A. marandati shows an oblique protruding stripe on the lingual surface of the mandible that suggests an unfused mandible. This feature is observed for the first time in the genus (because the mandibles of A. smithorum and A. roselli do not preserve this part). Several early Eocene notharctids, such as the genus Cantius or the asiadapines Marcgodinotius and Asiadapis, also display unfused mandibles (Rose et al., 2007; Rose et al., 2009; Godinot, 2015; Smith et al., 2016). Therefore, this feature observed in Agerinia could be interpreted as a primitive trait within Notharctidae.
It is also worth noting that A. marandati is the only formally described species of Agerinia preserving the upper molars. The lack of pericone in the specimens from Masia de l’Hereuet gives further support to the distinction between Agerinia and Periconodon, this latter genus being mainly characterized by a well-developed pericone.
Besides, the sample from Masia de l’Hereuet allows the evolution of several traits in the three known species of Agerinia to be observed. Agerinia marandati is similar in size to A. smithorum and A. roselli (Fig. 6), but displays some morphological differences that have allowed the description of a new species. Indeed, A. marandati shows a set of intermediate features between A. smithorum and A. roselli, suggesting that it represents a transitional step in the evolution of this lineage. Therefore the description of this new species reinforces the idea of the ancestor-descendant relationship between the other mentioned species, as proposed by Femenias-Gual et al. (2016b). These trends are supported by the stratigraphic position of the studied localities: as explained, the type locality of A. marandati, Masia de l’Hereuet, is situated in an upper stratigraphic position with respect to Casa Retjo-1, type-locality of A. smithorum.
| A. smithorum | A. marandati | A. roselli | |
|---|---|---|---|
| P1 root | |||
| Position in the mandible | Central | Central | Shifted buccally |
| Section of the root | Circular | Slightly mesiodistally compressed | Very mesiodistally compressed |
| P2 roots | Two | One | One |
| P4 molarization | |||
| Paraconid | Absent | Distinct | Distinct |
| Metaconid | Distinct but small | Distinct | Well-differentiated |
| Hypoconid | Absent | Distinct | Distinct |
| Entoconid | Absent | Absent | Distinct |
| M1 paraconid | Well-developed | Well-developed | Tiny |
| M2 paraconid | Tiny | Absent | Absent |
| Position of the mental foramina: | |||
| Distal foramen | Below the P4 mesial root | Below the P4 mesial root | Below the P3 distal root |
| Intermediate foramen | Below the P3 mesial root | Below the P3 mesial root | Below the P2 root |
| Mesial foramen | Below the P1 root | Between P1 and canine | Not observable |
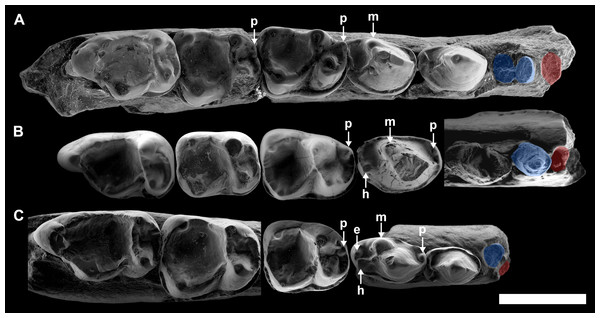
Table 2 summarizes the main morphological traits in the three described species of Agerinia. One of the most remarkable differences is the arrangement and the number of roots of the lower premolars. Although P1 is not preserved in any species of the genus, the size and the position of its root changes from A. smithorum to A. roselli (Fig. 8). In A. smithorum, the root of the P1 has a circular section and is located centrally in the mesiodistal axis of the mandible. In A. marandati, the root of the P1 is also centred, but more compressed mesiodistally than in A. smithorum. Finally, in A. roselli the root of the P1 is small and markedly displaced towards the buccal part of the mandible. The existence of two roots mesial to the P3 in A. roselli led Femenias-Gual et al. (2016a) to consider two different possibilities: the existence of single-rooted P1 and P2, or the presence of a double-rooted P2 extremely oblique to the mandible axis. This latter disposition of the roots of the P2 has been observed in some specimens of Marcgodinotius indicus (Rose et al., 2007; Rose et al., 2009; Smith et al., 2016). However, the mesial-most root of the mandible of A. roselli from Les Saleres is much more shifted buccally than in the case of Marcgodinotius so, if the two roots of A. roselli would correspond to a double-rooted P2, this premolar would be virtually transversal to the longitudinal axis of the mandible (while it is centrally placed and mesiodistally oriented in both A. smithorum and A. marandati). For this reason, Femenias-Gual et al. (2016a) considered these roots to correspond to single-rooted P1 and P2. This interpretation is consistent with the rest of features observed in the three known species of Agerinia. The material from Les Saleres displays more derived features than A. marandati (such as the more molarized P4 or more reduced paraconid in the M1), which agrees with a reduction and buccal displacement of the P1. On the contrary, it seems less plausible that a double-rooted P2 evolves from the single-rooted P2 of A. marandati (Fig. 8).
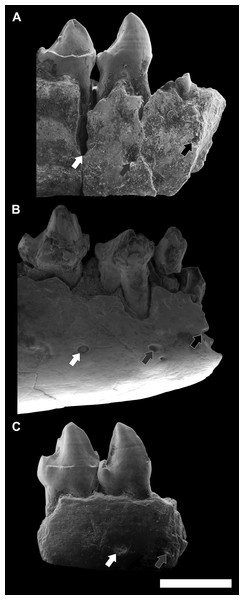
Figure 8: Comparison among the species of the genus Agerinia.
(A) A. smithorum from Casa Retjo-1: IPS-84291, holotype, right mandible with alveoli of the canine and P1, roots of the P2 and all teeth from P3 to M3. (B) A. marandati from Masia de l’Hereuet: IPS-82807, right mandible fragment with alveoli of the canine and P1, and teeth from P2 to P4 (the P4 of this specimen is not shown because of its bad preservation); IPS-82806, left P4 (reversed); IPS-82801, holotype, left M1 (reversed); IPS-82805, left M2 (reversed); IPS-82803, left M3 (reversed). (C) A. roselli from Les Saleres: IPS-2543, left mandible fragment with roots of the P1 and P2, and teeth from P3 to P4 (reversed); IPS-82793, right M1; IPS-1981, holotype, left mandible fragment with M2 and M3 (reversed). White arrows indicate the cuspids: p, paraconid; m, metaconid; h, hypoconid; e, entoconid. The root of the P1 is highlighted in red and those of the P2, in blue. All specimens are represented in occlusal view. Scale bar represents 3 mm.Despite the small sample size, the observed differences in the P1 and P2 of these three species seem to indicate a trend towards the reduction of the lower premolars from A. smithorum to A. roselli, involving the decrease in size of these teeth, the reduction of the number of roots of the P2 and the displacement of the P1. Such a change has been observed in other primate lineages as Teilhardina, in which the P1 is progressively reduced and displaced toward the buccal side from older to younger species (Smith, Rose & Gingerich, 2006), being even lost in the youngest species, T. americana.
A change in the morphology of the P4 can be also observed from A. smithorum to A. roselli, towards an increase in number and a better development of the cuspids (Fig. 8). Agerinia smithorum shows distinct protoconid and metaconid; Agerinia marandati shows, in addition, well-developed paraconid and hypoconid; finally, A. roselli also shows a entoconid as developed as the paraconid and hypoconid. Furthermore, the metaconid is progressively better differentiated from the protoconid from A. smithorum to A. roselli.
Regarding the lower molars, in A. smithorum the M1 has a well-developed paraconid and an open trigonid basin, and the M2 shows a tiny paraconid and a closed trigonid basin. Agerinia marandati has a similar morphology of the M1, with a large paraconid and an open trigonid basin, but lacks the paraconid on the M2. On the contrary, A. roselli only displays a tiny paraconid on the M1, in which the trigonid basin is closed. It is widely known that the lower molar paraconid tends to be reduced during the evolution of different primate lineages (Ankel-Simons, 2007; Godinot, 2015); for example in the genera Pseudoloris and Necrolemur the size of the paraconid decreases with time, changing from a distinct cuspid to a crest or even becoming completely absent (Minwer-Barakat, Marigó & Moyà-Solà, 2010; Minwer-Barakat, Marigó & Moyà-Solà, 2015; Minwer-Barakat et al., 2015). The same trend can be identified in the species A. smithorum, A. marandati and A. roselli (Fig. 8).
Figure 9: Comparison among the species of the genus Agerinia.
(A) A. smithorum from Casa Retjo-1: IPS-84291, holotype, right mandible with alveoli of the canine and P1, roots of the P2 and all teeth from P3 to M3 (this figure only shows teeth from P3 to P4). (B) A. marandati from Masia de l’Hereuet: IPS-82807, right mandible fragment with alveoli of the canine and P1, and teeth from P2 to P4. (C) A. roselli from Les Saleres: IPS-2543, left mandible fragment with roots of the P1 and P2, and teeth from P3 to P4 (reversed). White arrows indicate the position of the distal-most mental foramen; grey arrows indicate the position of the intermediate mental foramen; black arrows indicate the position of the mesial-most mental foramen. All specimens are represented in buccal view. Scale bar represents 3 mm.Finally, another remarkable trait that changes in the three species of Agerinia is the position of the mental foramina, which shift mesially from A. smithorum to A. roselli (Fig. 9). In A. smithorum and A. marandati, the distal-most mental foramen is placed under the mesial root of the P4, whereas it is placed at the level of the distal root of the P3 in A. roselli. The intermediate mental foramen is placed at the level of the mesial root of the P3 in A. smithorum and A. marandati (slightly more mesial in the latter), and at the level of the P2 root in A. roselli. Finally, the mesial-most mental foramen is located at the level of the P1 root in A. smithorum and between the P1 and the canine alveoli in A. smithorum (this foramen cannot be observed in A. roselli, since the mandible is broken at its mesial part).
All these considerations are supported by the phylogenetic analyses developed in this work. Both analyses place the three known species of Agerinia in the same clade, being A. smithorum the most primitive species. These results clearly reinforce the hypothesis of a single evolutive lineage, in which A. marandati represents a transitional step in the evolution between A. smithorum and A. roselli. Regarding the relationships of Agerinia with other primates, both analyses group this genus with the sivaladapids Rencunius and Hoanghonius. In the first analysis (Fig. 7A), Agerinia and sivaladapids are closely related to the asiadapines Marcgodinotius and Asiadapis. In our analyses, “Adapiformes” is not a monophyletic group, as has been previously hypothesized by several authors (Beard et al., 1988; Seiffert et al., 2009; Seiffert et al., 2010; Marigó, Minwer-Barakat & Moyà-Solà, 2011; Marigó, Minwer-Barakat & Moyà-Solà, 2013; Marigó et al., 2016 among others). These results support the idea that the phylogeny of “adapiforms” may be more complicated than previously thought, and highlights this controversy as still one of the most debated topics in paleoprimatology.
To sum up, there are several morphological traits that change progressively from A. smithorum to A. marandati and finally to A. roselli: the number of roots and the position of the first and second lower premolars, the degree of molarization of the P4, the development of the paraconid in the M1 and M2, and the position of the mental foramina. The observed trends suggest that these species constitute a single anagenetic lineage that evolved during the early Eocene in the Iberian Peninsula, in which A. marandati represents an intermediate stage of evolution between A. smithorum and A. roselli.
Conclusions
Here we present the most complete sample of the genus Agerinia described to date, coming from Masia de l’Hereuet (NE Spain). This material displays clear differences with the other species of Agerinia, allowing the erection of the new species Agerinia marandati, which is characterized by single rooted P1 and P2; P4 with distinct paraconid, protoconid, metaconid and hypoconid; paraconid well-developed in the M1 and absent in the M2 and M3; upper molars with the paraconule more developed than the metaconule and without pericone; M1 and M2 with a distinct hypocone and well-developed hypoparacrista and preparaconule crista. Moreover, this work describes for the first time the upper and lower fourth deciduous premolars of the genus, as well as some traits of the mandible. The material from Masia de l’Hereuet also includes several upper molars, which were not known for the other species of Agerinia. The description of the upper molars reinforces the distinction between the genera Agerinia and Periconodon, which has been a controversial issue in the past.
Agerinia marandati displays intermediate morphological characters between A. roselli and A. smithorum. A trend has been observed from A. smithorum to A. roselli for a set of features, including the reduction of the mesial lower premolars, the molarization of P4, the reduction of paraconid in the lower molars and the mesial displacement of the mental foramina. These trends allow for the interpretation that the three species of Agerinia known from the early Eocene of Europe, A. smithorum, A. marandati and A. roselli, are integrated in a single evolutionary lineage. Masia de l’Hereuet is situated stratigraphically above Casa Retjo-1 (type locality of A. smithorum), which indicates a younger age for A. marandati and is therefore consistent with the interpretation of these two species as ancestor and descendant. Finally, the phylogenetic analyses developed in this work support the hypothesis of a single clade including the three species of Agerinia, and indicate that Agerinia smithorum is the most primitive species of the genus.