Cloning and functional characterization of porcine AACS revealing the regulative roles for fat deposition in pigs
- Published
- Accepted
- Received
- Academic Editor
- Cong-Jun Li
- Subject Areas
- Agricultural Science, Genetics, Genomics, Molecular Biology, Zoology
- Keywords
- AACS, Subcutaneous fat, Fat deposition, Gene clone, Porcine
- Copyright
- © 2023 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Cloning and functional characterization of porcine AACS revealing the regulative roles for fat deposition in pigs. PeerJ 11:e16406 https://doi.org/10.7717/peerj.16406
Abstract
Fat deposition is a quantitative trait controlled by multiple genes in pigs. Using transcriptome sequencing, we previously reported that AACS is differentially expressed in the subcutaneous fat tissue of Dingyuan pigs with divergent backfat thickness. Therefore, with the aim of further characterizing this gene and its protein, we cloned the entire 3286-bp mRNA sequence of the porcine AACS, and the encoded AACS protein is a hydrophilic protein without a signal peptide or transmembrane sequence. Our findings suggested that among various tissues and pig breeds, AACS was highly expressed in subcutaneous fat. We have identified three completely linked SNP loci in the AACS gene: A-1759C, C-1683T, and A-1664G. The double luciferase activity test in the 5′ flanking region indicated that the flanking region of AACS contained several active regulatory elements. The three linked SNPs that were identified in one of the critical active elements, and might serve as important molecular markers regulating backfat thickness. Finally, we observed that AACS overexpression inhibited the proliferation and differentiation of subcutaneous preadipocytes. Collectively, our results suggest that AACS inhibits subcutaneous fat deposition in pigs. This study provides a new molecular marker for understanding the mechanism of porcine fat deposition.
Introduction
The main adipose tissue depot in pigs is the subcutaneous adipose tissue (SAT), accounting for over 70 % of the total body fat in pigs (Allen, 1976). In the actual production process, the excessive accumulation of subcutaneous fat increases production costs and reduces product quality, thus affecting economic benefits. Subcutaneous fat reduction is a critical aspect of pig breeding (Hocquette et al., 2010; Louveau et al., 2016). Interpreting the genetic determinants of fat deposition and identifying molecular markers that influence fat deposition are the theoretical basis for conducting molecular breeding; therefore, candidate genes that control subcutaneous fat deposition must be investigated.
Acetoacetyl-CoA synthase, also known as acetylacetic acid CoA (AACS), is a cytoplasmic ketone body (acetylacetic acid)-specific ligase found in various adipogenic tissues (Ito et al., 1986) that specifically converts acetylacetic acid to acetoacetyl-CoA for the synthesis of cholesterol and fatty acids (Endemann et al., 1982) . AACS mRNA is especially abundant in white adipose tissue, and its expression increases during adipocyte differentiation (Yamasaki et al., 2007). The expression pattern of AACS during preadipocyte differentiation is highly similar to that of acetyl-CoA carboxylase 1 (ACC-1), a key enzyme in fatty acid synthesis, suggesting that the function of AACS is related to fatty acid synthesis (Yamasaki et al., 2007; Yamasaki et al., 2005; Yamasaki et al., 2009). The C/EBP- α binding site was found in the AACS promoter region, suggesting that (C/EBP- α) is crucial for AACS expression in adipocyte differentiation (Hasegawa et al., 2008). AACS plays a vital role in the regulation of fat deposition.
We previously identified AACS as the key differentially expressed gene in the subcutaneous fat tissue of Dingyuan (DY) pigs with divergent backfat thicknesses using transcriptomic profiles (Zhang et al., 2022b). Therefore, we surmised that AACS is a crucial candidate gene for back subcutaneous fat thickness in pigs. Accordingly, we cloned porcine AACS to evaluate its role in regulating fat deposition and identified its functional molecular markers that might regulate its transcriptional expression. We believe that our results would help identify major genes or markers that may benefit the pig-breeding industry.
Materials & Methods
Ethics statements
Animal rearing and handling were performed in accordance with the Guide for the Care and Use of Laboratory Animals of China. All experimental protocols were approved by the Committee on the Ethics of Animal Experiments of the China Agricultural University (permit number: SKLAB-2012-04-07).
Experimental materials
Ear tissue samples were collected from Tibetan pigs (TP, n = 34), Landrace (LL, n = 34), Yorkshire (YY, n = 40), and Berding (a population that was bred from hybrid breed of Berkshire pig and Dingyuan pig, BD, n = 104) pigs for DNA extraction. The TP, LL, and YY pigs were raised at the Tibet Agriculture and Animal Husbandry College, while BD and DY pigs were raised at the Ankang Agriculture and Animal Husbandry Company, Dingyuan County, Anhui Province. BD individuals had phenotypic data of backfat thickness and age up to 70 kg. RNA was extracted from the subcutaneous fat tissue and longissimus dorsi muscle of six-month-old TP (n = 6), YY (n = 6), and DY (n = 6). Based on our observation and previous studies, the TP is a high backfat thickness breed, while the LL and YY pigs are low backfat thickness breeds (Zhang et al., 2022a; Wang et al., 2014). In our previous study, the DY pigs with high backfat thickness (HBF, n = 6) and low backfat thickness (LBF, n = 6) groups were selected from a DY population at similar age old and body weight (Zhang et al., 2022b). Six-day-old piglets were used to isolate primary preadipocytes. The animals are raised under the same conditions and have free access to food and water. The feed provided to the animals has a metabolizable energy content of 13.5 MJ/kg and a crude protein content of 16.0%. The pig ear samples were obtained as a general breeding monitoring procedure, after which the pigs were continued to be fed at their original breeding base. The animals that were used to collect the back adipose tissue for RNA extraction and cell isolation were slaughtered in accordance with the standard “Operating procedures of livestock and poultry slaughtering—Pig” (GB/T 17236-2019, China). Briefly, the pigs were fasted for 12 h with free access to fresh water. After that, the pigs were shocked at 90 V and 50 Hz for 10 s to stun them and then exsanguinate.
DNA, RNA, and cDNA preparation
Genomic DNA was isolated from the ear tissue using a standard phenol/chloroform extraction method. Total RNA was extracted from tissue samples with TRIZOL® Reagent (Invitrogen, Carlsbad, CA, USA). Use a NanoDrop 2000 Biophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and electrophoresis to verify its quality and integrity. Following the manufacturer’s instructions, we used the FastQuant Reverse Transcription Kit (TIANGEN, Beijing, China) to reverse transcribe 2 µg of RNA sample in a 20 µL reaction volume into cDNA.
Cloning and sequence analysis of AACS
The 5′- and 3′-end sequences of the cDNA encoding the AACS were obtained from the adipose tissue of TP using the Smarter™ RACE cDNA Amplification kit 5′/3′ according to the manufacturer’s instructions, and the remaining sequences were amplified using PCR; the specific primer sequences are listed in Table S1. Gel purification of PCR products, ligation to vectors, splicing of full-length mRNA sequences of porcine AACS genes, and prediction of physicochemical properties of AACS protein (including amino acid hydrophobicity, theoretical isoelectric point, and instability point index) were performed according to the methods described previously (Zhang et al., 2022a). Online software Signal P 3.0 (http://www.cbs.dtu.dk/services/Signal/P-3.0/) and TMHMM (HTTP://www.cbs.dtu.dk/services/TMHMM) were used to predict the signal peptide region and transmembrane structure of the protein, respectively. The BLast tool (Basic Local Alignment Search Tool, https://blast.ncbi.nlm.nih.gov/Blast.cgi) from NCBl was used to perform a comparison of amino acid and nucleotide sequence similarity.
Measurement of gene expression
A semi-quantitative reverse transcription and polymerase chain reaction (SqRT-PCR) was used to detect AACS expression in several pig tissues, and the assay was performed as previously described (Wang et al., 2014). Total RNA (2 µg) was reverse transcribed to cDNA using the FastQuant RT Kit (TIANGEN, Beijing, China) following the manufacturer’s specifications. Then, quantitative real-time PCR (qPCR) was performed using SYBR Green qPCR SuperMix (TIANGEN, Beijing, China). The amplification cycle system was as follows: 95 °C for 15 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. The relative gene expression levels were calculated using the 2−ΔΔCt method. The specific primer sequences are presented in Table S2, and the housekeeping gene, β-actin, served as the positive control.
SNP screening, genotyping, and correlation analysis
To detect potential functional regulatory sites in the 5′ lateral region, three pairs of primers for SNP screening of the AACS were designed using Primer Premier software (version 5.0; Premier Biosoft, Palo Alto, CA, USA) and are listed in Table S3. The amplicon sequences covered 2002-bp regions in the 5′-flanking (numbered starting from the start codon; the first base upstream of ATG was designated as −1, followed by sequential numbering until −2,164). First, we performed pooled sequencing using 8 individuals from each breed (TP, YY, and LL) to identify the existendce of SNPs. For sites with SNP, we amplified the DNA from each individual (TP, n = 34; LL, n = 34; YY, n = 40) separately for gene genotyping analysis. A single bright band of the final product was considered as qualified and sent to SinoGenoMax for sequencing. Chromas Pro (Technelysium Pty) and DNAMAN (version 8.0) were used to analyze sequence variation. Chi-square (χ2) test of SPSS (version 21.0) was used to analyze genotype distributions and differences.
Dual-luciferase reporter assays
To detect the AACS gene promoter region activity, the −2044–+116 fragment of this promoter region was amplified and then cloned into a pGL3-Basic vector using homologous recombination. The primers used for amplification are listed in Table S4. The target plasmid (900 ng) was co-transfected with the internal control plasmid pRL-TK (100 ng), while pRL-TK was co-transfected with empty pGL3-Basic as the external control. There were 4 replicates in each group. At 48 h post-transfection, the cells were lysed in 100 µL of lysis buffer and then assayed for promoter activity using a dual-luciferase reporter assay system (Promega, Madison, WI, USA) on the PerkinElmer 2030 Multilabel Reader (PerkinElmer, Waltham, MA, USA). The Renilla luciferase signal was normalized to the firefly luciferase signal.
Isolation and culture of subcutaneous preadipocytes
Subcutaneous adipocytes were stripped from subcutaneous deposits in the neck and back of six-day-old piglets. The adipose tissue was cut into small pieces and digested with 1 mg/mL collagenase type I (Invitrogen, Carlsbad, CA, USA) at 37 °C for 60 min in a reciprocating shaker bath, followed by filtration. Preadipocytes were cultured in a growth medium (GM) containing Dulbecco’s modified Eagle medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (PS, Gibco). All cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C.
Induced differentiation of porcine preadipocytes
When the primary cells reached 100% confluence, adipogenesis was induced with a differentiation medium for 2 days, which consisted of DMEM supplemented with 10% FBS, 0.5 mM isobutylmethylxanthine, 20 nM insulin, and 0.5 mM dexamethasone. Subsequently, cells were cultured in a maintenance medium consisting of 5 µg/mL insulin in GM.
Plasmid construction, lentivirus packaging, and lentivirus infection
To amplify the CDS region of the porcine AACS, the corresponding fragment was inserted into the EcoRI/BamHI site of the pLenti-CMV-EGFP-3FLAG-PGK-Puro vector (Obio Technology, Shanghai, China). Using Lipofectamine 2000-mediated transfection, 293T cells were co-transfected with the pLenti-CMV-EGFP-3FLAG-PGK-Puro vector, vesicular stomatitis virus G protein plasmid, or packaging plasmid. After 48 h, the cells generated mature lentivirus-containing supernatant. The preadipocytes were infected with the lentivirus and then screened with a concentration of 5 µg/mL of puromycin to obtain a stable AACS-overexpressed preadipocytes line.
Oil Red O staining and dye extraction analysis
After removing the growth medium, cells were fixed with 4% paraformaldehyde for 30 min at room temperature. The cells were washed with PBS and stained with Oil Red O working solution (Sigma, St. Louis, MO, USA) for 30 min. The cells were again washed with PBS and observed under a microscope. Finally, Oil Red O dye was extracted from the stained cells with isopropanol for 20 min, and the lipid droplet content was evaluated by spectrophotometrically measuring the absorbance at 490 nm. There were 3 replicates in each group.
CCK8 and EdU proliferation assays
The cells were inoculated in 96-well plates, and 10 µL of CCK8 (Beyotime Biotechnology, Shanghai, China) solution was added to each well before the assay. There were 9 replicates in each group. After incubation for 1 h, the absorbance was measured at 450 nm using a microplate reader (Biotek, Winooski, VT, USA).
The EdU assay was performed using an EdU assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Cell nuclei and EdU-positive cells were stained blue and red, respectively (n = 3).
Statistical analysis
Statistically significant differences were determined via the t-test using SPSS software (version 21.0; IBM, Chicago, IL, USA). Graphs were prepared using GraphPad Prism 7 software (GraphPad Software, San Diego, CA, USA). The results are expressed as mean ± standard deviation (SD), with statistical significance set at p < 0.05 and highly significant values at p < 0.01.
Results
Cloning and sequence analysis of AACS
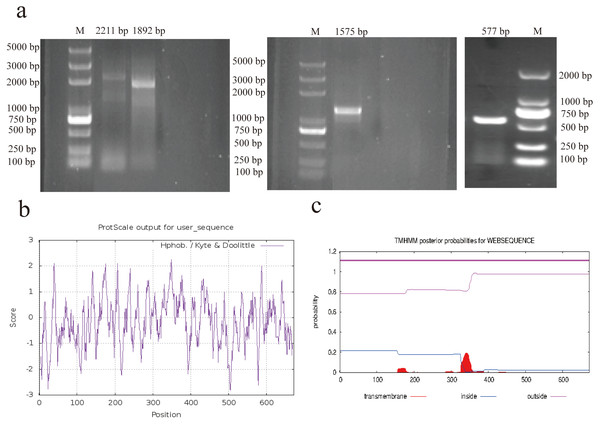
The complete mRNA sequence of AACS was successfully cloned using 5′ rapid amplification of cDNA ends (RACE), 3′ RACE, and PCR (Fig. 1A). The sequence with a 5′ end length of 1892 bp was obtained using nested PCR, with the first amplified product being 2211 bp. The sequence with a 3′ end length of 577 bp was obtained using 3′ RACE, and the intermediate sequence was obtained using PCR amplification with 1575 bp. After sequencing and assembling, the full-length 3286-bp AACS sequence (Accession Number, OP807955), including the coding sequence of 2019 bp, 5′ untranslated region of 161 bp, and 3′ untranslated region of 1106 bp, encoding a total of 672 amino acids, was obtained. Sequence analysis with ExPASy revealed that the isoelectric point of the AACS protein was 5.79, with a relative molecular mass of 75 kDa and an instability index of 32.82; therefore, it was presumed to be a stable protein. Hydrophobicity prediction analysis revealed strong hydrophilic regions in many parts of the protein. The hydrophobic regions were evenly distributed with an average value of −0.170, indicating that AACS is hydrophilic (Fig. 1B). Furthermore, no signal peptide sequence or transmembrane domain was present in this gene (Fig. 1C). The results indicated nucleotide similarities of 89.71, 89.14, 88.44, 88.15, and 86.68% and amino acid sequence similarities of 93.9, 93.45, 91.82, 91.67, and 90.62% for cattle, sheep, donkeys, horses, and humans, respectively, suggesting that the AACS is relatively conserved among these species.
Figure 1: RACE amplification and sequence analysis.
(A) Amplification of 5′ RACE (left), PCR (middle), and 3′ RACE (right). (B) Prediction of hydrophilicity of the AACS protein. (C) Transmembrane domain prediction of the AACS protein. M = Trans2K/Trans2K Plus DNA Marker(Transgene, Beijing, China).Expression profile of porcine AACS in tissues
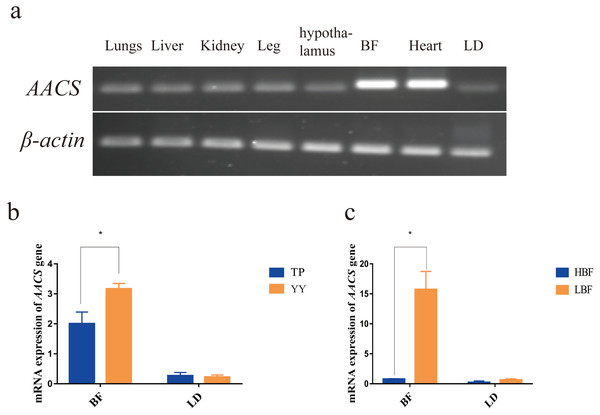
The AACS expression in the lung, liver, kidney, leg, hypothalamus, back fat (BF), heart, and longest dorsal (LD) muscle tissues of pigs were evaluated. The results revealed that AACS was expressed in all eight tissues, with high expression in the heart and BF tissues (Fig. 2A). To investigate AACS function in fat deposition, AACS expression was examined in the BF and LD tissues. In BF tissue, the mRNA level of AACS in TP was significantly lower than that in YY, and no significant differences were observed between these in LD tissue (Fig. 2B). Similar results were obtained in DY pigs with divergent backfat thicknesses. In BF tissue, AACS expression was significantly lower in the HBF group than in the LBF group, with no significant difference in LD tissue (Fig. 2C). These results suggest that AACS might play a negative regulatory role in BF deposition in pigs.
Figure 2: Expression analysis of AACS in tissues.
(A) AACS expression in different tissues of TP pigs using SqRT-PCR. (B) The mRNA expression of AACS in TP and YY pigs. (C) The mRNA expression of AACS in DY pigs. BF, back fat; LD, longest dorsal; TP, Tibetan pig; YY, Yorkshire pig; HBF, high back fat thickness; LBF, low back fat thickness. Each bar represents the means ± SD. * P < 0.05.SNP identification of 5′ flanking region in AACS
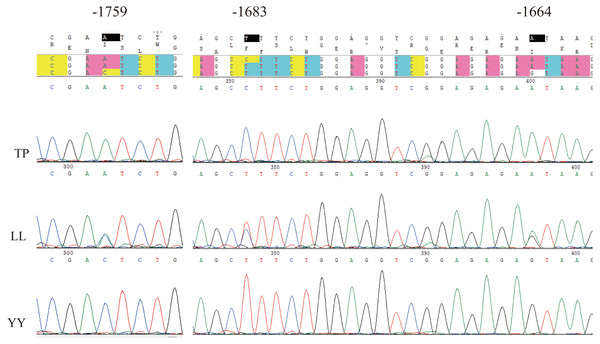
Using Sanger sequencing, three complete linkage mutation loci in the 5′ flanking region, namely A-1759C, C-1683T, and A-1664G, were identified (Fig. 3). The three SNPs were completely linked and formed two haplotypes, ACA and CTG. Thus, the frequencies of allele and genotypes of A-1759C could represent the polymorphisms at these three sites. For site A-1759C, three genotypes (AA, AC, and CC) were observed in the LL and YY groups, and two genotypes (AA and AC) in the TP group, and the genotype frequency distribution was significantly different between the two lean-type pigs (LL and YY) and the TP group (Table 1). This linkage site is likely a crucial SNP marker associated with fat deposition.
Figure 3: Sequencing map of three linked sites.
TP, Tibetan pig; YY, Yorkshire; LL, Landrace.| Breed | Genotype frequency (number/frequency) | Allele frequency | ||||
|---|---|---|---|---|---|---|
| AA | AC | CC | HWE χ2 value (P-value) | A | C | |
| TP | 32/0.9412 | 2/0.0588 | 0/0.0000 | 0.031 (P= 0.860) | 0.9706 | 0.0294 |
| LL | 2/0.0588 | 12/0.3529 | 20/0.5882 | 0.013 (P= 0.911) | 0.2353 | 0.7647 |
| YY | 6/0.15 | 17/0.425 | 17/0.425 | 0.137 (P= 0.934) | 0.3625 | 0.6375 |
To confirm this hypothesis, we genotyped A-1759C in 104 BD pigs and analyzed its association with backfat thickness and age up to 70 kg. The results helped identify 34, 58, and 12 pigs with the AA, AC, and CC genotypes, respectively. Association analysis showed that the backfat thickness of the AA and AC genotypes was significantly higher than that of the CC genotype (AA vs CC, p = 0.0041; AC vs CC p = 0.0019). No significant differences were observed between the three genotypes for age up to 70 kg (Table 2).
| Genotype | AA (n = 34) | AC (n = 58) | CC (n = 12) |
|---|---|---|---|
| Thickness of back fat | 14.16 ± 3.17a | 14.24 ± 3.28a | 10.95 ± 2.11c |
| Day old at 70 kg | 216.36 ± 15 | 213.24 ± 16.11 | 214.14 ± 12.32 |
Notes:
Transcription activity of AACS promoter region
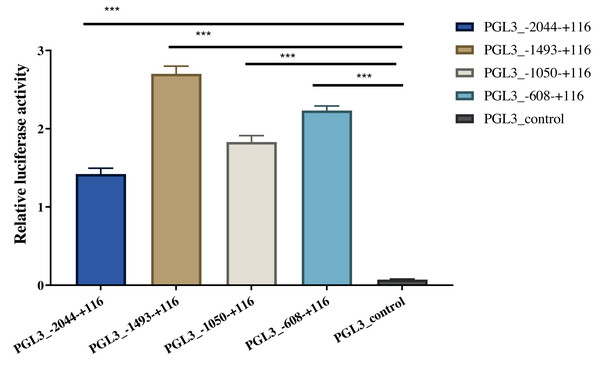
Four length fragments of the 5′ flanking region (−2044/+116, −1493/+116, −1050/ +116, and −608/+116) were amplified, and the promoter activity in these four regions was detected using the dual-luciferase reporter assay. The results indicated that the fluorescence activity of these four sections was significantly higher than that of the control group (PGL3-control), indicating that these four sections contained critical regulatory elements. Among these, the −608/+116 region may have vital regulatory elements that enhance promoter activity, and the −1493/−2044 region may have critical active elements that inhibit promoter activity (Fig. 4). The identified A-1759C, C-1683T, and A-1664G linkage sites were all located in the −1493/−2044 region; thus, their polymorphisms might affect the expression of AACS and fat deposition in pigs.
Figure 4: Dual-luciferase analysis for promoter activity.
The control group was co-transfected with PGL3-basic (900 ng) and PRL-TK (100 ng). The target plasmid (PGL3_−2044–+116, PGL3_ −1493–+116, PGL3_ −1050–+116, PGL3_ −608–+116; 900 ng/each) was co-transfected with the internal control plasmid pRL-TK (100 ng). There were 4 replicates in each group. Each bar rep-resents the means ± SD. ***p < 0.001.AACS inhibits the proliferation of subcutaneous preadipocytes
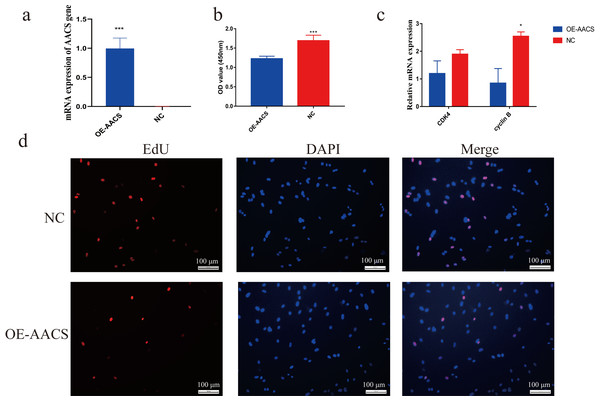
Porcine subcutaneous preadipocytes were infected with a lentivirus to overexpress AACS, and unsuccessfully infected cells were removed using puromycin. The results confirmed the successful overexpression of AACS using lentiviral transfection (Fig. 5A). The CCK8 assay demonstrated that the number of living cells decreased significantly in the AACS overexpression group compared with that in the control group (Fig. 5B). Similar results were obtained in the EDU assay. AACS overexpression resulted in reduced EdU positivity compared to the control group (Fig. 5D). We detected the mRNA expression levels of CDK4 (cyclin-dependent kinase 4) and cyclin B, which are considered proliferation-marker genes, and found that the expression levels of both genes decreased after AACS overexpression, with cyclin B showed significant differences (Fig. 5C).
Figure 5: Overexpression of AACS inhibits the proliferation of porcine subcutaneous preadipocytes.
(A) Detection of overexpression efficiency after lentivirus infection. (B) Overexpression of AACS inhibited the proliferation of porcine subcutaneous preadipocytes using CCK8 assay (n = 9). (C) The mRNA expression levels of proliferation-related genes (n = 3). (D) The proliferation of porcine subcutaneous preadipocytes after overexpression of AACS for 24 h was detected using EdU staining (n = 3). Each bar represents the means ± SD. *P < 0.05, ***P < 0.001.AACS inhibits adipogenic differentiation of subcutaneous preadipocytes
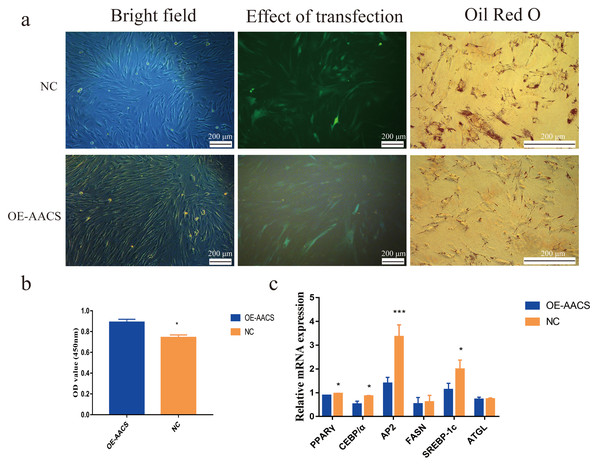
To validate the AACS-mediated regulation of the differentiation of subcutaneous preadipocytes, Oil Red O staining was performed on day 6 of preadipocyte differentiation to detect the number of lipid droplets generated (Fig. 6A). The results revealed that AACS overexpression in porcine preadipocytes significantly reduced the lipid droplet production (Fig. 6B), and the expression of the marker genes of adipogenesis, PPAR γ, CEBP α, AP2, and SREBP-1C, were significantly lower in the overexpression group than in the control group (Fig. 6C). These results indicate that AACS plays an inhibitory role in the differentiation of subcutaneous preadipocytes in pigs.
Figure 6: Overexpression of AACS inhibited the differentiation of porcine subcutaneous preadipocytes.
(A) Image of porcine adipocytes stained with oil red O after six days of differentiation. (B) Oil-red O staining was used to determine the content of lipid droplets in porcine subcutaneous adipocytes (n = 3). (C) The mRNA expression of marker genes associated with adipogenesis in porcine subcutaneous adipocytes (n = 3). Each bar represents the means ± SD. *P < 0.05, ***P < 0.001.Discussion
This study is the first to clone AACS mRNA sequence and report that AACS plays an inhibitory role in the differentiation of porcine subcutaneous preadipocytes. These findings confirm the crucial role of AACS in porcine subcutaneous adipogenesis and proliferation. Our findings also elucidate the potential effect of AACS on the improvement of subcutaneous fat deposition and provide theoretical implications for its role in the improved breeding of pigs.
AACS directly activates ketone bodies in the cytosol to synthesize cholesterol and fatty acids (Endemann et al., 1982). We observed AACS expression in the adipose tissue of DY pigs with divergent backfat thicknesses, suggesting that AACS plays a role in the subcutaneous fat deposition in pigs. Porcine AACS genes were cloned and compared using the NCBI for Biotechnology Information website. The high similarity of amino acid and nucleotide sequences across species indicates that the mRNA sequence of the cloned AACS gene is relatively accurate and that the gene is relatively conserved among species.
Highly conserved genes often share common regulatory mechanisms (Chikina & Troyanskaya, 2011; Gerstein et al., 2014; Stuart et al., 2003). This study, along with others, demonstrated that AACS genes are highly expressed in adipose tissue (Yamasaki et al., 2007) and play a negative regulatory role in the deposition of subcutaneous fat in pigs and that upregulation of AACS inhibits the differentiation of subcutaneous preadipocytes. In contrast, in 3T3-L1 cells, the downregulation of AACS inhibited cell differentiation (Hasegawa et al., 2012). Similar results have been found in mice, where the AACS gene expression level in white adipose tissue was lower in Zucker fatty rats than in lean rats. However, in high-fat, diet-induced obese rats, the expression levels of AACS were increased (Yamasaki et al., 2007). In the epididymal adipose tissue, AACS protein expression decreases in mice fed a short-term high-fat diet (Plubell et al., 2017). The expression trend of AACS in different species indicates that this is not only affected by obesity and species type but also by the location of fat deposition, where the mechanism of fat deposition differs at different locations (Luo et al., 2022; Mendizabal et al., 1997; Zhou et al., 2010). The formation of adipose tissue mainly involves an increase in the number of adipocytes (proliferation) and their hypertrophy (differentiation) (Choe et al., 2016; Ghaben & Scherer, 2019). High expression of AACS inhibits the proliferation and differentiation of porcine subcutaneous preadipocytes, resulting in a decrease in flat droplet generation.
Active promoter elements with vital functions are present in the 5′ flanking region of the gene (Kimura et al., 2001; Li et al., 2017). We detected the promoter activity of this region and observed that each flanker region gained strong promoter activity by truncating the amplified fragment, among which the −1,493/−2,044 region had important promoter-inhibiting active elements. Notably, an important linkage mutation site (A-1759C) was identified in this region, validated in a population of 104 pigs, and demonstrated to be significantly associated with backfat thickness, which may be a vital molecular marker for pig breeding.
Conclusions
We cloned porcine AACS mRNA sequences for the first time and confirmed that AACS is a negative regulator of subcutaneous preadipocyte differentiation in pigs. This study provides evidence that elevated AACS genes lead to the downregulation of PPAR γ, CEBP/α, and AP2 genes which are key regulators of fat formation. Additionally, we explored luciferase activity in the 5′ flanking region of the AACS gene and obtained molecular markers that could be used for genetic improvement in pigs. Although we present preliminary findings, we confirmed that AACS is a vital regulator of porcine subcutaneous fat deposition and can have practical implications in improving pig breeding.
Supplemental Information
Supplementary methods
Reaction systems and reaction procedures for reverse transcription cDNA.