Genome-wide identification and expression analysis of auxin response factors in peanut (Arachis hypogaea L.)
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Agricultural Science, Bioinformatics, Genomics, Molecular Biology, Plant Science
- Keywords
- Pod development, Auxin response factors, Auxin, Evolutionary analysis, Hormonal response
- Copyright
- © 2021 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Genome-wide identification and expression analysis of auxin response factors in peanut (Arachis hypogaea L.) PeerJ 9:e12319 https://doi.org/10.7717/peerj.12319
Abstract
Auxin response factors (ARFs) are transcription factors that regulate the expression of auxin response genes, and have important functions in plant growth and development. In this study, available genome data for peanut (Arachis hypogaea L.) were used to identify AhARF genes. In total, 61 AhARFs and 23 AtARFs were divided into six groups (I–VI). Molecular structural analysis revealed that the protein members of AhARF contain at least two domains, the B3 domain and the Auxin-resp domain, and that some have a C-terminal dimerisation domain. Screening of the transcriptome data of 22 tissues of A. hypogaea cv. Tifrunner in a public database showed high expression levels of AhARF2 and AhARF6. AhARF6 was expressed more highly in the stem and branch than in the root and leaf of the wild species Arachis monticola (A. mon) and cultivated species H103. After treatment with exogenous auxin (NAA), the expression of AhARF6 was inhibited, and this inhibition was greater in A. mon than in H103. The transcriptome map revealed that the expression of AhARF6 was higher in the larger pods of H8107 and ZP06 than in the medium pods of H103 and small pods of A. mon. Moreover, AhARF6-5 was proven to be localised in the nucleus, consistent with the location of AtARF6. These results suggest that AhARF6 may play an important role in pod development in peanut.
Introduction
Auxins play a very important role in the regulation of plant growth and development processes (De Smet & Jurgens, 2007; Jain & Khurana, 2009; Zhao, 2010), such as vascular elongation, embryogenesis, lateral root initiation, flower and fruit development, and apical dominance. Auxins can regulate the activity of auxin response factors (ARFs) (Guilfoyle & Hagen, 2007; Okushima et al., 2005; Yang et al., 2013), which are transcription factors that regulate the expression of auxin response genes. To our knowledge, ARFs were first reported in Arabidopsis thaliana (Ulmasov et al., 1997), and the ARF gene family was subsequently identified in Arabidopsis. A typical ARF consists of a highly conserved N-terminal B3-like DNA-binding domain (B3 domain, PF02362, InterPro entry IPR003340) and a C-terminal dimerisation domain. The middle region (Auxin-resp domain, PF06507, InterPro entry IPR010525), which is located between the B3 domain and C-terminal dimerisation domain, has been proposed to function as an activation or repression domain (Tiwari, Hagen & Guilfoyle, 2003; Ulmasov, Hagen & Guilfoyle, 1999). A previous study indicated that ARFs also have a C-terminal convergent functional domain Aux_IAA (Hagen & Guilfoyle, 2002). ARFs can specifically combine with the auxin response element (AuxRE) “TGTCTC” in the promoter region of auxin response genes to regulate them.
ARFs have been identified in many species, including Arabidopsis (Okushima et al., 2005), tomato (Solanum lycopersicum) (Wu et al., 2011), rice (Oryza sativa) (Wang et al., 2007), corn (maize) (Xing et al., 2011), soybean (Glycine max) (Chien et al., 2013), populus (Populus trichocarpa) (Kalluri et al., 2007), rape (Brassica napus) (Li et al., 2019), and litchi (Litchi chinensis Sonn.) (Zhang et al., 2019a). In Arabidopsis, ARFs play a vital role in plant growth and development processes, especially root development, leaf aging, flower formation, and fruit development (Goetz et al., 2007; De et al., 2008; Wu et al., 2020). In potato (Solanum tuberosum L.) crown bud, the expression level of StARF6 was higher around the apical meristem than in other tissues, suggesting that StARF6 is related to tissue growth and vascular bundle development (Wu, Tian & Reed, 2006). AtARF6 and AtARF8 are homologous genes in Arabidopsis that have been proven to have similar functions (Nagpal et al., 2005). Both AtARF6 and AtARF8 are involved in regulating plant growth and flower maturation. Double or single mutants of arf6 and/or arf8 have similar phenotypes, such as delayed flower maturation, the appearance of small petals, short stamens, and delayed anther release (Nagpal et al., 2005). Previous studies have shown that ARF6 and ARF8 regulate the jasmonic acid (JA) biosynthetic pathway by inhibiting KNOX gene expression. ARF6 and ARF8 promote JA synthesis, which induces the expression of the MYB21 and MYB24 required for the growth of stamens and pistils during flowering (Finet et al., 2010; Nagpal et al., 2005; Reeves et al., 2012; Tabata et al., 2010). Genes belonging to the ARF6 subfamily are targeted by microRNA167. Overexpression of microRNA167 in transgenic tomato plants inhibited the expression of SlARF6 and SlARF8, leading to organ development defects in tomato flowers (Liu et al., 2014; Wu et al., 2011). An optical signal can inhibit the DNA-binding ability of ARF through direct interactions among the photoreceptors CRY1, phyB, and ARF (Mao et al., 2020), and then inhibit auxin signal transduction and induce hypocotyl elongation in Arabidopsis. SlARF7 is expressed highly in unpollinated mature ovaries. Moreover, studies have shown that the transcript level of SlARF7 increases during tomato flower development, is maintained at a constant high level in mature flowers, and decreases within 48 h after pollination, suggesting that SlARF7 regulates fruit setting and development (De et al., 2008).
Peanut (Arachis hypogaea L.) is a major oil crop grown worldwide. Seeds of peanut have a high oil content and abundant protein. Peanut is an allotetraploid with 40 chromosomes (AABB = AA × BB, 2n = 4x = 40). The genome sequences of the peanut tetraploid cultivated species A. hypogaea cv. Tifrunner (Bertioli et al., 2019), A. hypogaea var. Shitouqi (Zhuang et al., 2019), and the two diploid wild species A. duranensis and A. ipaensis (Bertioli et al., 2016; Chen et al., 2016; Lu et al., 2018; Yin et al., 2020) have been completed. The genome sequence of the wild allotetraploid species A. monticola (A. mon) has also been completed (Yin et al., 2018). A. mon plays an important role in the domestication of peanut, from the wild diploid to cultivated tetraploid species (Yin et al., 2020). Extensive data provide a strong foundation for study of the specific gene families of peanut. In this study, the genome version of A. hypogaea var. Tifrunner was used to identify ARF gene family members. Comprehensive information about the ARF family in peanut, including chromosome locations, phylogenetic relationships, expression patterns, and gene structures, is provided here. The results may facilitate functional studies of the AhARF gene family in peanut.
Materials & Methods
Identification of peanut ARF gene family
A. hypogaea Tifrunner version 1.0 was used here. First, the amino acid sequences of AtARFs were downloaded from the Arabidopsis Information Resource (TAIR9, https://www.arabidopsis.org/). Then, homologs of peanut were searched in PeanutBase (https://peanutbase.org/) using BlastP (P = 0. 001) according to the amino acid sequences of AtARFs. Second, BlastP searches of the ARF domain “PF06507” were performed on the PeanutBase website to find ARF genes. The results of the two searches were integrated and redundant genes were discarded. Subsequently, through a domain analysis performed using a hidden Markov model (HMM) (http://pfam.xfam.org/), we selected sequences with P < 0.001, and then deleted incomplete and redundant amino acid sequences. We identified 62 ARFs in A. hypogaea Tifrunner, 28 in A. ipaensis, 27 in A. duranensis, and 33 in A. mon. Each ARF contained a B3 domain (PF02362) and Auxin-resp domain (PF06507). Among these, there was one gene without a chromosomal location in the family of Tifrunner, which was deleted. Finally, 61 AhARF gene family members were identified in Tifrunner, and the AhARF genes were named AhARF1-1 to AhARF19-5 according to their annotation and chromosome location (Additional File 2: Table S1). For example, AhARF6-5 was annotated as auxin response factor 6 with fifth rank according to position on all of the chromosomes of the AhARF6 subfamily.
Sequence analyses and the construction of evolutionary tree
The whole proteins sequences of 61 AhARFs and 23 AtARFs (Additional file 2: Table S5) were performed using ClustalW in the software of MEGA-X (https://www.megasoftware.net/). Then, the above results were used to construct an unrooted evolutionary tree by Maximum Likelihood method (1,000 bootstrap replications).
Chromosomal distribution and tandem duplication analysis of AhARF genes
The aforementioned AhARFs were used to screen ARFs in the A. ipaensis K30076 1.0, A. duranensis V14167 1.0, and A. mon genome databases (Yin et al., 2018). The location of each AhARF gene was identified according to the physical location information of the peanut genome annotated by A. hypogaea Tifrunner 1.0. According to the amino acid sequence of AhARF from Tifrunner, the similarity for repeated sequence analysis using the BLAST method was set to 90%. Finally, tandem duplication analysis was performed and chromosomal location diagrams were generated using the program Circos-0.69-6 (http://circos.ca) (Krzywinski et al., 2009).
Expression patterns of AhARF genes in different tissues of Tifrunner
The transcription levels of AhARF genes in different tissues of Tifrunner were obtained from a public database (https://peanutbase.org/). At 10 days post-emergence, 22 types of tissues were sampled (Clevenger et al., 2016). The samples included seedling leaf, main stem leaf, lateral stem leaf, vegetative shoot tip, reproductive shoot tip, root, nodules, perianth, gynoecium, androecium, aerial gynophore tip, subterranean gynophore tip, Pattee 1 pod, Pattee 1 stalk, Pattee 3 pod, Pattee 5 pericarp, Pattee 5 seed, Pattee 6 pericarp, Pattee 6 seed, Pattee 7 seed, Pattee 8 seed, and Pattee 10 seed (Additional File 2: Table S2). Fragments per kilobase million (FPKM) data were used here. TBtools software (Chen et al., 2020) was applied to visualise these publicly available data. A heatmap was constructed with ClustalW and TreeView using the average data.
Proteins structure analysis of ARF6 sub-family
Total 16 amino acid sequences of ARF6, including peanut (six ARF6 sub-family amino acid sequences), corn (one ARF6), rice (two ARF6), tomato (two ARF6), Arabidopsis (one ARF6), soybean (one ARF6), Medicago (two ARF6), Lupinus micranthus Guss (one ARF6) were obtained from NCBI. DNAMAN was used to align the ARF6 amino acid sequences of peanut and other species, and HMMER was used for domain prediction. The AhARF6 sequence motifs were identified using the MEME program (http://meme-suite. org/tools/meme), and AhARF6 gene structures were deduced using the Gene Structure Display Server (GSDS2, http://gsds.cbi.pku.edu.cn/). TBtools software was used to combine the result of evolutionary tree, domain and conserved motif of ARF6. AhARF6 proteins properties were analyzed, including the number of amino acids, molecular weight, theoretical pI, instability index, aliphatic index, grand average of hydropathicity (GRAVY), and subcellular localization prediction by using ProtParam (https://web.expasy.org/protparam/).
Transcriptomics analysis of four peanut varieties with different pod/seed sizes
Four peanut varieties with different pod/seed sizes were used here. Among these, ZP06 and H8107 are large-pod varieties, H103 is a medium-pod variety, and A. mon is a small-pod variety. These varieties were planted in the same area (Henan, China). The size of the planted area was 0.34 ha. Pod samples for further experiments were collected at different specific developmental stages (15, 35, 55, and 75 days after flowering). Pods were manually separated into shells and seeds. The shells and seeds for each developmental stage of each variety were mixed, immediately frozen in liquid nitrogen, and stored at −80 °C for RNA extraction and subsequent transcriptomics analysis. Three replications were performed. FPKM values of AhARF6 subfamily members were collected to evaluate the expression levels of the different varieties. A heatmap of AhARF6 was generated using TBtools.
Construction of GFP vector and subcellular localization of AhARF6-5 gene
To investigate the subcellular localization of the AhARF6-5 protein, we transiently expressed green fluorescent protein (GFP) with AhARF6-5 protein in tobacco (Nicotiana benthamiana) leaf epidermal cells. The CDS of AhARF6-5 was cloned using Phanta Max Super-Fidelity DNA Polymerase (P505; Vazyme, Nanjing, China) and divided into three segments. Three pairs of homologous recombination primers (Additional file 2: Table S3) were designed using Vazyme’s official website (https://crm.vazyme.com/cetool/simple.html). The amplification products were cloned using the ClonExpress Ultra One Step Cloning Kit (C115, Vazyme, Nanjing, China) and ligated into the PFGC5941-35S-GFP (35S-GFP) vector. The recombined plasmids were then transformed into Agrobacterium tumefaciens strain EHA105; transient expression and infiltration were performed using previously published protocols (Sparkes et al., 2006; Zhang et al., 2019b). Leaves transformed with the 35S-GFP vector alone were used as the controls. Fluorescence and bright-light images of transiently infected tobacco leaves were obtained 48–72 h after infiltration by using a laser scanning confocal microscope (LSM710; Axio Observer Z1, Zeiss, Jena, Germany).
Plant materials and hormone treatments
H8107, ZP06 and H103 are tetraploid cultivated species, and A.mon is tetraploid wild species. Mutation identification of each member of AhARF6 sub-family was done in four varieties. The cultivar H8107, ZP06 and H103 were developed in our laboratory. Here, H103 and A.mon were selected to be materials corresponding to cultivated species and wild species of peanut. For hormone treatments, six seedlings of peanut were grown in 1/2 Hoagland solution in a light incubator under a 16 h photoperiod (32 °C) and 8 h dark (25 °C) period. When A.mon and H103 were grown up to fifth leaf stage, 100 µM NAA was subjected to their leaves. Around 100 mg of the roots, leaves, stems, and branches were sampled at 2, 4, 8, and 12 h after NAA treatment. Control was collected without NAA treatment at the same stage, immediately frozen in liquid nitrogen, and then stored at −80 °C until RNA extraction. Three replications were done.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
RNA was extracted from the roots, stems, leaves, and branches by using TransZol Plant (TaKaRa, Dalian, China). The RNA concentration and quality were evaluated with a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA) and standard agarose gel electrophoresis (1%, w/v). The RNA was used for the reverse transcription of cDNA with EasyScript One-Step gDNA Removal and CDNA Synthesis SuperMix (TaKaRa, Dalian, China). The primers were designed using the Real-time PCR (TaqMan) Primer and Probes Design Tool website (https://www.genscript.com/tools/real-time-pcr-taqman-primer-design-tool). The total PCR volume was 20 µL, and it contained 0.4 µL of the forward primer, 0.4 µL of the reverse primer, two µL of cDNA, 10 µL of TransStart Top Green qPCR SuperMix (TaKaRa), and 7.2 µL of nuclease-free H2O. TB Green Premix ExTaq II (Tli RNaseH Plus) Mix (TaKaRa, Dalian, China) was used for qRT-PCR on the CFX96 Touch real-time PCR system (Bio-Rad, Hercules, CA, USA). The PCR protocol was as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Each reaction biology experiments were repeated three times, and the 2−ΔΔCT (Livak & Schmittgen, 2001) method was used to calculate the relative gene expression levels of AhARF6 genes in peanut. The primers for qRT-PCR are listed in Additional file 2: Table S4. AhActin (EG030635) was amplified as endogenous controls for qRT-PCR (Reddy et al., 2013). The specific primers of AhARF6 sub-family members used to detect transcripts are listed in (Additional file 2: Table S4).
Results
Identification of peanut ARF gene family
A total of 61 AhARF genes were identified and named AhARF1-1 to AhARF19-5 (Additional File 2: Table S1) according to their annotation and chromosome location in PeanutBase (https://peanutbase.org/). All AhARFs contained B3 and Auxin-resp domains, and some of them had the Aux_IAA domain.
Phylogenetic analysis of ARF genes
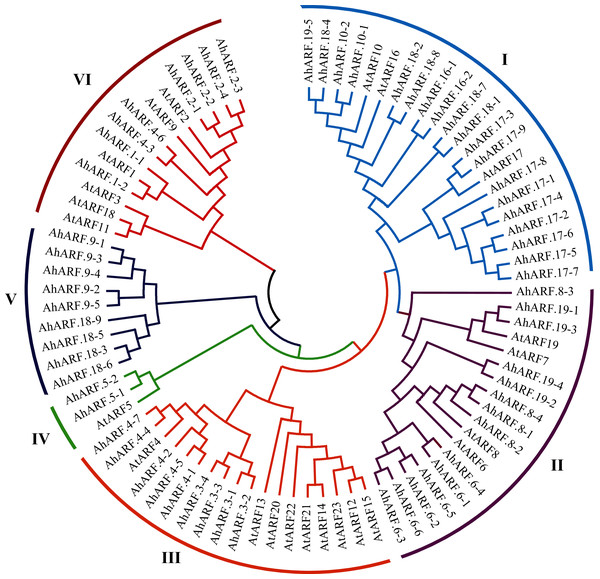
Phylogenetic analysis of the ARF proteins from peanut and Arabidopsis was conducted to study the evolutionary relationships (Additional File 2: Table S5). The results demonstrated that AhARF proteins can be divided into six subgroups according to the clades and classifications for Arabidopsis (Fig. 1). Group I contains the highest number of ARF proteins (n = 22: 19 AhARF proteins from peanut and three from Arabidopsis). Group II has 18 ARF proteins (14 AhARF proteins from peanut and 4 AtARF proteins from Arabidopsis). Group VI has 14 ARF proteins (eight AhARF proteins from peanut and six from Arabidopsis). These results indicate that most peanut AhARF proteins have high homology with Arabidopsis AtARF proteins. Class A ARFs (Cédric et al., 2013), including ARF5, ARF6, ARF7, ARF8, and ARF19, are transcriptional activators. Among these, AhARF6/AtARF6, AhARF8/AtARF8, and AhARF19/AtARF19 belong to Group II, and AhARF5/AtARF5 belong to Group IV. There is no AhARF7 in peanut. The results show that many peanut ARF genes have high similarity with ARF genes of Arabidopsis.
Figure 1: Phylogenetic analysis of auxin response factor (ARF) proteins from peanut and Arabidopsis.
The phylogenetic tree was constructed using the maximum likelihood method in MEGA-X. The numbers represent the confidence of the branches.Chromosomal distribution and tandem duplication analysis of AhARF genes
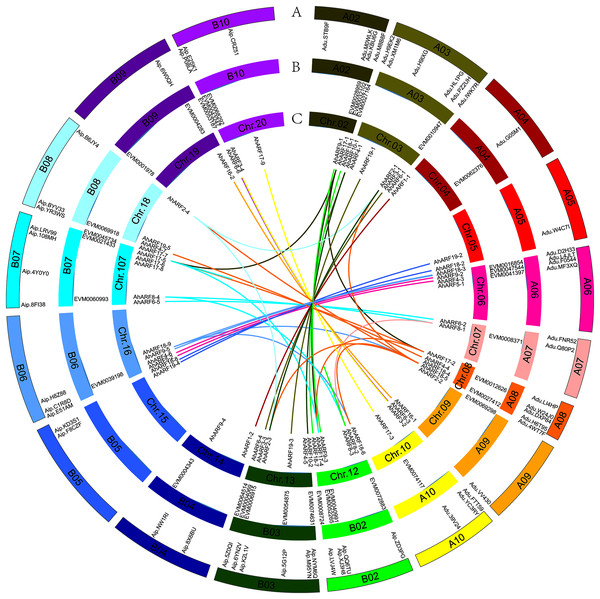
Based on their annotated genomic locations, the 61 AhARF genes were found to be widely distributed among the peanut chromosomes (except for chromosomes 01 and 11) (Fig. 2). The number of genes on homologous chromosomes was not exactly the same. Chromosome 3 contained the most AhARF genes (n = 7). Chromosomes 12, 13, and 17 had six genes. Chromosome 8 had five AhARF genes. Chromosomes 6 and 16 had four genes. Chromosomes 10, 18, and 20 possessed three genes. Chromosomes 5, 14, and 15 had two genes, and chromosomes 4, 9, and 19 had only one.
Figure 2: Distribution and synteny analysis of AhARF gene family on peanut chromosomes.
Distribution and synteny analysis of AhARF gene family on peanut chromosomes. The approximate chromosomal locations of the AhARF genes are indicated on the periphery. The colored lines linking genes from different chromosomes denote segmental duplication events. A. A. duranensis (diploid wild species) and A. ipaensis (diploid wild species). B. A. mon (tetraploid wild species). C. A. hypogaea (tetraploid cultivated species). Two genes on the same curve are a couple of segmentally duplicated genes at A. hypogaea.Duplication (fragment and tandem duplication) is important in the analysis of the evolution of gene families. Twenty-six segmental duplication events have occurred according to the tetraploid A. hypogaea peanut amino acid sequence (Fig. 2): AhARF6-2 to AhARF6-5, AhARF6-3 to AhARF6-6, AhARF6-1 to AhARF6-4, and so on (Fig. 2). No tandem duplication was observed. The results suggest that only segmental duplication took part in the evolution of the peanut AhARF gene family. The number of ARF genes was identified; there were 28 in A. ipaensis, 27 in A. duranensis, 33 in A. mon, and 61 in A. hypogaea. There is no evolutionary rule regarding the different numbers of ARF genes in diploid wild species, tetraploid wild peanut, or tetraploid cultivated peanut, possibly because the genome of A. mon has not been completely assembled.
Expression pattern analysis of AhARF genes in different tissues
To understand the functions of AhARF genes in peanut, we investigated the transcription levels of AhARF genes in different tissues by using publicly available transcriptome datasets (Clevenger et al., 2016). Hierarchical clustering analysis was performed, and heatmaps were generated to display the expression patterns of the AhARF genes (Additional File 1: Fig. S1) by using TBtools. AhARF6 genes were expressed in all tissues of peanut. The expression levels of AhARF6 were highest in stamen, stem, and branch, followed by fruit needle and pod; the expression levels were relatively low in the other parts. However, AhARF6-2 and AhARF6-5 were expressed at lower levels than the other four AhARF6 subfamily genes in all tissues. These results suggest that AhARF6 genes may play a role in flower organ development, tissue organ growth, and pod maturation. The AhARF2 subfamily was expressed highly in all tissues, suggesting key roles in tissue development. The expression levels of other ARF genes, especially AhARF3, AhARF4, AhARF10, AhARF17, and AhARF18, were very low or even absent. Different AhARF genes may play different roles in growth and tissue development in peanut. Understanding the expression patterns of AhARF genes in different tissues can provide a foundation for identifying the functional genes of peanut.
AhARF6 protein properties and sequence analyses of ARF6 in peanut and other plants
Analysis of the protein properties of AhARF6 (Additional File 2: Table S7) showed that the length and molecular weight of AhARF6 proteins are relatively similar, ranging from 810–923 amino acids and 89.83–101.67 kDa, respectively. The theoretical isoelectric point varied from 4.13 to 11.38. All AhARF6 proteins were considered unstable, because the instability index was higher than 40 (between 61.49 and 69.08). The aliphatic index of the AhARF6 proteins was predicted to range from 72.70 to 76.57. Because of a relatively low average hydrophilic value (<0), all AhARF6 proteins were predicted to be hydrophilic. Subcellular localisation analysis showed that all AhARF6 proteins are localised in the nucleus, indicating that AhARF6 may play a role.
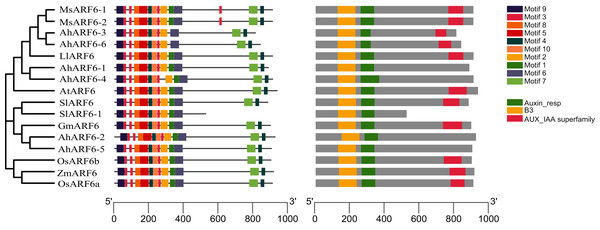
To investigate the structural features of ARF6 proteins, 16 ARF6 amino acid sequences of Arabidopsis, maize, tomato, rice, soybean, medicago, and Lupinus micranthus Guss. were obtained from the National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov/). The ARF6 evolution analysis (Fig. 3A) showed that AhARF6 had the highest similarity with MsARF6, LmARF6, and GmARF6, indicating that peanut is most closely related to medicago, Lupinus micranthus Guss., and soybean. AhARF6 had the lowest homology with maize and rice. Ten conserved motifs (Fig. 3B) (motifs 1–10) were identified. Most of the motifs are located within the N-terminal region; very few (motifs 4 and 7) are within the C-terminal region. Eleven of the ARF6 protein sequences (Fig. 3C) contain the B3, Auxin-resp, and Aux_IAA domains, and five protein do not have the Aux_IAA domain. In this phylogenetic tree, closely related ARF6 genes showed a conserved intron–exon structure and similar motifs in terms of alignment, which suggests that ARF6 members with similar structures clustered along the same branch may have similar biological functions.
Figure 3: ARF6 protein sequence analyses of peanut, corn, rice, Arabidopsis, soybean, tomato, medicago and Lupinus micranthus Guss.
(A) Phylogenetic tree was constructed using MEGA-X with the NJ method. Corn, ZmARF6. Rice, OsARF6. Peanut, AhARF6. Arabidopsis thaliana, AtARF6. Soybean, GmARF6. Tomato, SlARF6. Medicago, MsARF6. Lupinus micranthus Guss, LmARF6. (B) Motif distribution of ARF6 proteins. Different motifs are indicated by different colors. The sequence information for each motif is provided in Additional file 2: Table S8. (C) Structural analysis of ARF6 proteins.Analysis of pod expression of the AhARF6 subfamily in four varieties and subcellular localisation of AhARF6-5 gene
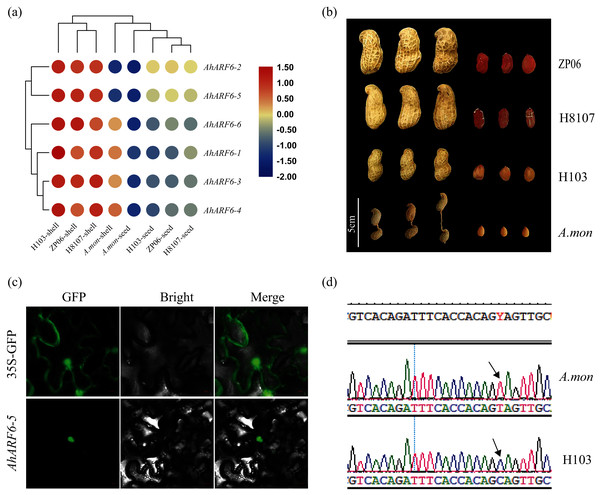
To understand the function of the AhARF6 gene subfamily in peanut, we measured its expression levels in four pod varieties (Additional File 2: Table S9). The FPKM data were retrieved from the transcriptome data of the four varieties: H8107, ZP06, H103, and A. mon. A heatmap was constructed from the results (Fig. 4A). All AhARF6 subfamily genes were expressed in the pods, but the expression levels varied. The expression of AhARF6 was significantly higher in pod shell than seed. All six AhARF6 genes were more highly expressed in the shell of H103 than in the shell of the other three varieties. Additionally, the expression in shell and seed was lower in A. mon than in the other samples. In seed, AhARF6 showed higher expression in large-pod cultivars (ZP06 and H8107) than in the medium-pod cultivar (H103), and it showed the lowest expression in the super-small pod A. mon (Figs. 4A, 4B). In summary, AhARF6 expression appears to be associated with the trait of pod size. Quantitative trait locus (QTL) or other types of analysis are needed to better understand the regulation of fruit size by AhARF6.
Figure 4: Heatmap for pod expression in four varieties and subcellular localization of AhARF6-5 proteins.
Heatmap for pod expression in four varieties and subcellular localization of AhARF6-5 proteins. (A) Peanut pod and seed phenotype of ZP06, H8107, H103 and A.mon. (B) Expression analysis of seeds of ZP06, H8107, H103 and A. mon by transcriptome analysis. Bar showed log2 (FPKM). (C) Mutation identification of AhARF6-5 gene in A. mon and H103. (D) Subcellular localization of AhARF6-5 proteins in tobacco leaves.Subcellular localisation analysis of AhARF6-5 was performed. Confocal laser microscopy revealed green fluorescent protein (GFP) in the nucleus (Fig. 4C), demonstrating that the AhARF6-5 protein is localised in the nucleus. Mutation identification of each member of the AhARF6 subfamily was done for all four varieties. In cultivated species H103 and tetraploid wild species A. mon, AhARF6-5 has a C > T mutation in the coding region (Fig. 4D), which results in early termination of translation in A. mon. Auxin transcription factors were expressed in the nucleus and regulate downstream genes (Tiwari, Hagen & Guilfoyle, 2003).
Expression of AhARF6 genes in different tissues
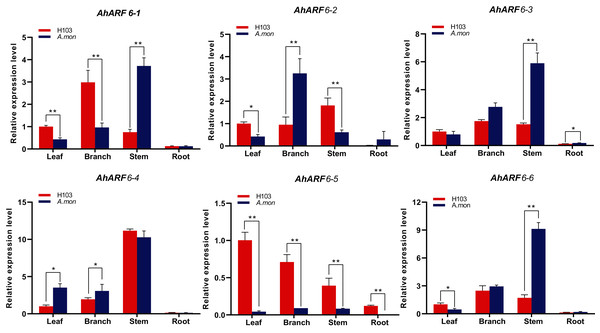
We investigated the expression levels of AhARF6 genes in four tissues of A. mon and H103: leaves, branches, stems, and roots at the fifth leaf stage. The different materials were applied for quantitative reverse transcription polymerase chain reaction (qRT-PCR). The results showed that AhARF6 subfamily genes were expressed in the leaves, branches, stems, and roots of H103 and A. mon (Fig. 5), indicating that they are constitutively expressed genes. Further analysis revealed that most of the AhARF6 genes were expressed most highly in stems, followed by branches, and that the expression levels were lowest in roots (Fig. 5). However, the expression of AhARF6-5 was higher in leaves than in the other tissues. The expression levels of AhARF6 genes differed between A. mon and H103. In stem, AhARF6-1, AhARF6-2, AhARF6-3, AhARF6-4, and AhARF6-6 were expressed at levels 1.06–4.95 times higher in A. mon than in H103. These results indicate that ARF6 genes are mainly expressed in branches and stems, and that the expression pattern of AhARF6-5 is different from the other five AhARF6 genes. In summary, AhARF6 may play key roles in the processes of peanut pod/seed growth and development.
Figure 5: Expression analysis of AhARF6 sub-family genes in different tissues.
qRT-PCR analysis of AhARF6 transcript levels in leaves, roots, stems, and branches was performed. Values are means ± SD (n = 3). Significant difference between H103 and A. mon at P < 0.05 and P < 0.01 (two sample t-test) is denoted by * and **, respectively.Early responses of AhARF6 to exogenous NAA treatments
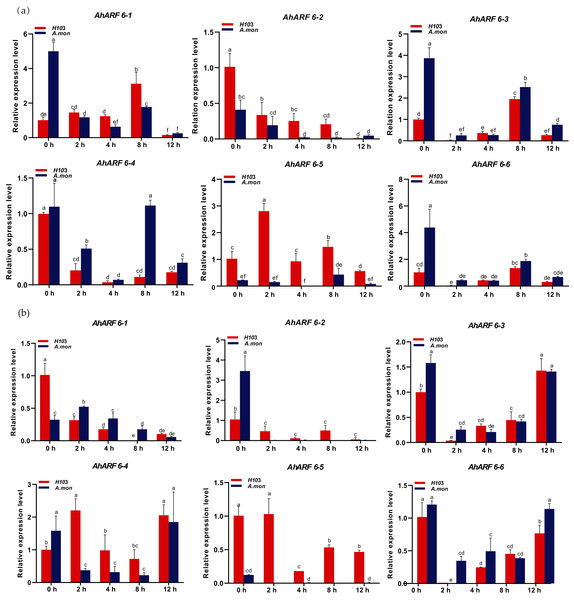
To investigate the early responses of AhARF6 to exogenous auxin (NAA) application, 100 µM NAA was sprayed, and fifth-stage leaves of H103 and A. mon were analysed. The expression levels of AhARF6-1, AhARF6-2, AhARF6-3, AhARF6-4, and AhARF6-6 in the main stem in H103 and A. mon initially decreased and then increased gradually over 12 h with NAA treatment (Fig. 6A). However, the expression level of AhARF6-5 in stem initially increased and then decreased in H103, in contrast to the case of A. mon, which is different from the early responses of the other five AhARF6 genes to NAA. The six AhARF6 genes were downregulated to various degrees in branch at 4 h after exogenous NAA treatment, but the four AhARF6 genes (AhARF6-3, AhARF6-4, AhARF6-5, AhARF6-6) showed increased expression. AhARF6-5 exhibited much higher branch expression levels in H103 than in A. mon within 12 h of exogenous NAA treatment (Fig. 6B). Exogenous NAA inhibits the expression of AhARF6 genes, and the expression of AhARF6 was decreased in the stems and branches of H103 and A. mon after treatment (Additional File 2: Table S10). AhARF6 genes also showed downregulation in leaves and roots (Additional File 1: Figs. S2, S3). The results show that the responses to NAA treatment varied among different tissues.
Figure 6: Expression analysis of AhARF6 sub-family genes in response to NAA treatment in peanut stem and branch.
qRT-PCR analysis of AhARF6 transcript levels was performed at the fifth leaf stage of plants treated with NAA for 0, 2, 4, 8 h. (A) Expression in the stem. (B) Expression in the branch. Values are means ± SD (n = 3). Different lowercase letters denote significant differences between the any two stages (P < 0.05, one-way ANOVA and Tukey’s test for multiple comparisons).Discussion
ARFs are transcription factors specific to plants, and play important regulatory roles in the growth and development of plants. Zhuang et al. (2019) identified 114, 28, and 28 ARF proteins by BLAST in Shitouqi, A. duranensis, and A. ipaensis using the protein sequence from the A. hypogaea ARF gene, respectively; these were grouped into nine clusters according to their maximum likelihood tree. A recent Chinese report identified 62 AhARF gene family members, and named them AhARF1–AhARF62 according to their chromosome location in Tifrunner (Tang et al., 2020). In this study, 61 ARF genes (a gene not located on the chromosome was deleted) were identified in Tifrunner and divided into 13 functional subfamilies. Moreover, 27, 28, and 33 members of AhARF were found in A. duranensis, A. ipaensis, and A. mon, respectively. This study observed 26 segmental duplication events in 61 ARF proteins based on the criterion of 90% similarity, which is less than the 33 segmental duplication events in 62 ARF proteins identified in a previous report (Tang et al., 2020). The number of ARF genes is higher in peanut than in most other plants, such as Arabidopsis (23 members) (Okushima et al., 2005), tomato (21 members) (Wu et al., 2011), rice (25 members) (Wang et al., 2007), maize (31 members) (Xing et al., 2011), soybean (51 members) (Chien et al., 2013), populus (39 members) (Kalluri et al., 2007), barley (17 members), peach (17 members) (Diao et al., 2020), strawberry (12 members) (Wang et al., 2019), and millet (24 members) (Zhao et al., 2016), and is similar to the number in allopolyploid B. napus (67 members) (Wen et al., 2019). The reason for this may be that peanut is an allotetraploid plant, with 40 chromosomes and two sets of independent chromosomes. Thus, the genome of peanut (2.38 Gb) is many times larger than those of Arabidopsis and rice (0.125 and 0.466 Gb, respectively). An evolution analysis of the 61 AhARFs of peanut and 23 AtARFs of Arabidopsis showed that they can be divided into six groups, and that eight AtARFs are homologous to AhARFs. The amino acid sequence analysis of OsARFs in rice (Wang et al., 2007) and AtARFs in Arabidopsis showed that they can be divided into five classes, similar to ZmARFs and AtARFs (Xing et al., 2011). The results showed that ARF clustering results differ among species.
A recent report on AhARF focused on the expression patterns of AhARF10 (AhARF3-1 here), AhARF20 (AhARF8-1 here), AhARF23 (AhARF4-4 here), and AhARF46 (AhARF5-2 here) during seed germination and found that played a positive role in the process (Tang et al., 2020). Previous studies on other crops showed that ARF6 is mainly expressed in the flowers of plants, and is involved in the maturation of flower organs and development of seeds and/or fruits (Kumar, Tyagi & Sharma, 2011; Nagpal et al., 2005). For example, SlARF8 plays a negative role in controlling fruit size, and the inhibition of SlARF8 expression enhances tomato fruit development (Goetz et al., 2007). Transgenic plants with decreased SlARF7 transcription levels formed seedless (parthenocarpic) fruits (De et al., 2008). Previous studies have shown that ARF8 regulates fruit formation in Arabidopsis thaliana and tomato (Goetz et al., 2007). Expression analysis of CmARF9, CmARF16-like, CmARF19-like, CmARF19, CmARF1, CmARF2, CmARF3, and CmARF5 showed that they may be associated with fruit growth in melon during early development (Wu et al., 2020). In Tifrunner, all AhARF6 genes were expressed most highly in stamens, stems, and branches, followed by fruit needles and pods (Clevenger et al., 2016). Among these, four AhARF6 genes (AhARF6-1, AhARF6-3, AhARF6-4, and AhARF6-6) were expressed at higher levels than AhARF6-2 and AhARF6-5 in 22 tissues of peanut. Here, the transcriptome data of H8107, ZP06, H103, and A. mon showed that all six AhARF6 subfamily genes were expressed in pods. Importantly, the large-seed varieties (ZP06 and H8107) showed higher expression of AhARF6 than the medium-seed variety (H103), and the small-seed A. mon showed the lowest expression. These results suggest that AhARF6 may be related to pod/seed growth and development, although this requires further verification by QTL and other methods.
In Arabidopsis, AtARF6 is mainly expressed in flowers; together with AtARF8, it regulates the maturation of flower organs and development of the seed coat (Nagpal et al., 2005; Wu et al., 2011). In tomato, SlARF6 is expressed in all tissues. SlARF6A is expressed more highly in flowers and fruits, and is involved in flower organ development and fruit ripening (Kumar, Tyagi & Sharma, 2011; Maas et al., 2014). In peach, PpARF6 is expressed in roots, stems, leaves, and flowers, and is involved in fruit ripening (Diao et al., 2020; Li, Ran & Sun, 2016). FvARF6 is highly expressed in old leaves and might participate in the late growth of strawberry (Wang et al., 2019). We analysed the expression levels of peanut AhARF6 genes in roots, stems, leaves, and branches and found that they were all expressed in these four tissues, indicating that AhARF6 genes are constitutively expressed. The expression levels of AhARF6-1, AhARF6-2, AhARF6-3, AhARF6-4, and AhARF6-6 were higher in A. mon than H103. ARF6 is expressed in different tissues in different plants, indicating that ARF6 may have different functions during the growth and development of different tissues. As well as regulating auxin gene expression, ARFs also participate in the regulatory pathways of various hormones, such as gibberellins (O’Neill et al., 2010; Weston, Reid & Ross, 2009), ethylene, abscisic acid, brassinolide (Vert et al., 2008), and salicylic acid. The early expression of AhARF6 genes following exogenous NAA treatments confirmed that AhARF6 genes are highly sensitive to auxins. The expression of AhARF6 genes either decreased or increased after 2 h of treatment with NAA. Most AhARF6 genes showed a negative response to NAA treatment of the stems and branches. The detailed mechanism of the response should be explored in the near future. An analysis of the AhARF family proteins revealed that all AhARF proteins occur in the nucleus. We also found that 16 ARF6 proteins from different crops have the B3 conserved domain and auxin-resp domain, while some contain the Aux_IAA domain (Tiwari, Hagen & Guilfoyle, 2003; Ulmasov, Hagen & Guilfoyle, 1999). The structure of AhARF6 subgroup proteins is more similar to those of MsARF6, LmARF6, and GmARF6. In this study, AhARF6-5 had a C > T mutation in the coding region of A. mon and H103 (Fig. 4D), which results in the early termination of translation in A. mon. According to its expression in the pod of different species, AhARF6-5 may be involved in the control of pod size.
Basically, ARFs, as transcription factors localised in the nucleus, regulate downstream genes. The division of ARF proteins in cells and spatiotemporal expression of ARF genes in plants provides an important basis for research on the function of ARFs in the processes of plant growth and development. In peach, PpARF4, PpARF6, PpARF10A, and PpARF12 fusion proteins were observed in the nucleus (Diao et al., 2020). The BnARF protein of Brassica napus and AtARF protein of Arabidopsis are located in the nucleus (Truskina et al., 2021; Wen et al., 2019). Here, subcellular localisation analysis by the transient agroinfiltration method with a GFP fusion construct showed that AhARF6-5 is located in the nuclear area. The aforementioned results indicate that ARFs are typical nuclear-localised transcription factors. As an important part of the auxin signaling pathway, ARFs combine with AuxRE in the auxin response gene promoter to activate or inhibit auxin response gene expression and regulate auxin synthesis (Tiwari, Hagen & Guilfoyle, 2003). AhARF6-5 is a Class A ARF, and AhARF6-5 is a transcription activator of auxin gene expression. Auxin regulates cell division and elongation, and also determines the size of plant fruit (Perrot-Rechenmann, 2010; Woodward & Bartel, 2005). All of the data from cell culture studies indicate that auxin is a permissive signal of cell division that enables initiation of the cell cycle. AhARF6 may control peanut pod size by accommodating the expression of downstream auxin genes.
Conclusions
In this study, 61 AhARF genes were identified in the peanut genome and subsequently divided into six groups with 23 AtARFs. The AhARF6 subfamily consists of constitutively expressed genes expressed in leaves, stems, branches, and roots. The expression of AhARF6 in stems and branches of H103 and A. mon was decreased after NAA treatment. Exogenous NAA inhibits the expression of AhARF6 genes. The expression levels of AhARF6 in shell and seed were lower in A. mon than H103, and our findings showed that AhARF6 genes may play a role in pod/seed development.
Supplemental Information
Expression patterns of AhARF genes in different tissues.
The dates are from an expression atlas (Clevenger et al., 2016), Bar showed log2 (FPKM) colored blue to red. Information on 22 tissues has been provided in Additional file 2: Table S2. Fragments per Kilobase per Million mapped reads (FPKM) values of the AhARF genes are listed in Additional file 2: Table S6.
Expression analysis of AhARF6 sub-family genes in response to NAA treatment in peanut leaf.
qRT-PCR analysis of AhARF6 transcript levels was performed at the fifth leaf stage of plants treated with NAA for 0, 2, 4, 8, and 12 h. Values are means ± SD (n = 3). Different lowercase letters denote significant differences between the any two stages (P < 0.05, one-way ANOVA and Tukey’s test for multiple comparisons).
Expression analysis of AhARF6 sub-family genes in response to NAA treatment in peanut root.
qRT-PCR analysis of AhARF6 transcript levels was performed at the fifth leaf stage of plants treated with NAA for 0, 2, 4, 8, and 12 h. Values are means ± SD (n = 3). Different lowercase letters denote significant differences between the any two stages (P < 0.05, one-way ANOVA and Tukey’s test for multiple comparisons).