Exogenous Fe2+ alleviated the toxicity of CuO nanoparticles on Pseudomonas tolaasii Y-11 under different nitrogen sources
- Published
- Accepted
- Received
- Academic Editor
- Monika Mortimer
- Subject Areas
- Microbiology, Natural Resource Management, Environmental Contamination and Remediation
- Keywords
- Ferrous ion, CuO-NPs, Nitrogen removal, Detoxify, Functional microorganisms
- Copyright
- © 2020 Yang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Exogenous Fe2+ alleviated the toxicity of CuO nanoparticles on Pseudomonas tolaasii Y-11 under different nitrogen sources. PeerJ 8:e10351 https://doi.org/10.7717/peerj.10351
Abstract
Extensive use of CuO nanoparticles (CuO-NPs ) inevitably leads to their accumulation in wastewater and toxicity to microorganisms that effectively treat nitrogen pollution. Due to the effects of different mediums, the sources of CuO-NPs-induced toxicity to microorganisms and methods to mitigating the toxicity are still unclear. In this study, CuO-NPs were found to impact the nitrate reduction of Pseudomonas tolaasii Y-11 mainly through the action of NPs themselves while inhibiting the ammonium transformation of strain Y-11 through releasing Cu2+. As the content of CuO-NPs increased from 0 to 20 mg/L, the removal efficiency of NO3− and NH4+ decreased from 42.29% and 29.83% to 2.05% and 2.33%, respectively. Exogenous Fe2+ significantly promoted the aggregation of CuO-NPs, reduced the possibility of contact with bacteria, and slowed down the damage of CuO-NPs to strain Y-11. When 0.01 mol/L Fe2+ was added to 0, 1, 5, 10 and 20 mg/L CuO-NPs treatment, the removal efficiencies of NO3- were 69.77%, 88.93%, 80.51%, 36.17% and 2.47%, respectively; the removal efficiencies of NH4+ were 55.95%, 96.71%, 38.11%, 20.71% and 7.43%, respectively. This study provides a method for mitigating the toxicity of CuO-NPs on functional microorganisms.
Introduction
The excessive use of nitrogen fertilizer coupled with the indiscriminate discharge of domestic sewage and industrial wastewater has caused increasingly severe nitrogen pollution (Liu et al., 2020; Paredes et al., 2020). Nitrogen pollution causes serious harm to the ecological environment, such as eutrophication of water, and causes health problems such as infant methemoglobinemia (Liu et al., 2020; Paredes et al., 2020; Zhang et al., 2020a; Zhang et al., 2016). Microbial nitrogen removal is an economical and effective method controlling nitrogen pollution in water. Biological nitrification and denitrification are therefore widely used for pollution control (He et al., 2020). However, the functional activities of those microorganisms are highly susceptible to external influences, such as nanoparticles (NPs) (Wang et al., 2017a; Wang et al., 2017b; Zhang et al., 2018a).
In recent years, with the rapid development of nanotechnology, NPs have been widely used in various consumer and industrial products (Nowack et al., 2015; Zhang et al., 2018a). For example, CuO-NPs are widely used in gas sensors, antibacterial textiles, batteries, catalysts, and metal coatings (Chen et al., 2019; Wang et al., 2017a). The increasingly widespread application of CuO-NPs inevitably leads to the release of CuO-NPs into industrial and municipal wastewater and accumulation in activated sludge (Hou et al., 2015; Jośko et al., 2019; Wang et al., 2017a; Zhang et al., 2018a; Zhang et al., 2017b). It was speculated that the concentration of CuO-NPs was about 1 mg/L in the environment and 50 mg/L in semiconductor wastewater (Zhang et al., 2020b; Zhang et al., 2018a). The introduction of NPs into the sewage treatment system will seriously threaten the activity of sewage treatment microorganisms, thus causing damage to the sewage treatment system. CuO-NPs are toxic to microorganisms in activated sludge. They have been found to destroy the integrity of the microbial cell membrane in activated sludge, reduce the diversity and activity of the bacterial community, and significantly reduce the effectiveness of nitrogen and phosphorus removal (Wang et al., 2017a; Wang et al., 2017b; Zhang et al., 2018a). Hou et al. (2016) found that the denitrification process of the biofilm reactor was inhibited by 50 mg/L CuO-NPs, reducing the nitrogen removal efficiency and significantly reducing the activity of nitrite reductase (NIR) and nitrate reductase (NAR). Huang et al. (2020) found that the propagation and ammonium removal of Pseudomonas putida strain Y-9 were inhibited significantly by 1 mg/L CuO-NPs. Wang et al. (2017b) found that 1 mg/L CuO-NPs had a significant inhibitory effect on ammonia monooxygenase (AMO) and nitrite oxidoreductase (NOR) activity.
There is no unified answer as to whether the toxicity of NPs is caused by the NPs themselves or the released metal ions (Huang et al., 2020; Wang et al., 2016). It is generally believed that metal oxide NPs can dissolve in aqueous media, causing heavy metal ions to be released into the media (Luo et al., 2018; Wang et al., 2016). Therefore, improving the stability of NPs in aqueous solutions can mitigate their toxicity to microorganisms. Studies have shown that the addition of organic matter can alleviate the damage caused by NPs to bacteria. Zhao et al. (2013) showed that the addition of fulvic acid could reduce the damage to the bacterial membrane caused by CuO-NPs. However, studies have also shown that when the organic matter content in the wastewater was high, the chemical oxygen demand (COD) content in the water body was increased, which was not conducive to the removal of nitrogen and the growth of bacteria (Pan et al., 2020). Therefore, other methods of mitigating the impact of NPs on functional bacteria need to be sought.
Some scholars improved the matrix of NPs by doping with iron to reduce the cytotoxicity of NPs. For example, Adeleye et al. (2018) and Naatz et al. (2017) reduced the toxicity to marine phytoplankton and zebrafish by doping Fe in CuO-NPs. The solubility and toxicity of ZnO NPs were significantly reduced by doping Fe in ZnO NPs (Fairbairn et al., 2011; George et al., 2010; Xia et al., 2011). However, the method of changing the CuO-NPs matrix to relieve cytotoxicity is not suitable for sewage treatment plants. Fe2+ is an essential element for microbial growth (Feng et al., 2020). The use of iron in sewage treatment to enhance nitrogen removal has been extensively studied (Feng et al., 2020; Liu et al., 2020; Liu & Ni, 2015; Qiao et al., 2013). However, there has been no research on the direct addition of Fe2+ in wastewater treatment to reduce the toxicity of NPs to functional bacteria.
In view of the fact that there are few studies on the differences in CuO-NP cytotoxicity given different nitrogen sources, this study used Pseudomonas tolaasii Y-11 which exhibits excellent denitrification and nitrification activity, as the bacterial source (He et al., 2016). A series of experiments were conducted to: (1) determine the effect of CuO-NPs on the proliferation and nitrogen removal of strain Y-11; (2) reveal the effect of CuO-NPs on ammonium and nitrate removal performance of strain Y-11; and (3) evaluate the detoxification ability of Fe2+ on CuO-NPs. We hoped to provide a method for mitigating the toxicity of CuO-NPs on functional microorganisms.
Materials & Methods
Cell cultivation and culture media
The aerobic denitrification and heterotrophic nitrification strain Pseudomonas tolaasii Y-11 (KP410741) used in this study was isolated from a winter paddy field by He & Li (2015).
The basal medium (BM) (Huang et al., 2020) was used to determine the nitrogen removal performance of strain Y-11 (1 L containing 0.31 g NaNO3 (BM1) or 0.25 g (NH4)2SO4 (BM2), 2.56 g CH3COONa, 1.5 g KH2PO4, 0.42 g Na2HPO4, and 0.1 g MgSO4 ⋅ 7H2O). BM1 and BM2were the BM for two different nitrogen sources. We added 0.05 g/L FeSO4 ⋅ 7H2O to the BM to explore the mitigation effect of Fe2+ on CuO-NP toxicity. Before exposure to CuO-NPs, strain Y-11 was cultivated in Luria-Bertani (LB) medium (1 L containing NaCl 10 g, tryptone 10 g, and yeast extract 5 g) at 15 °C and 150 rpm/min for 36 h.
The initial pH of all the media was adjusted to 7.3 with 0.1 M NaOH or 0.1 M HCl. Each 250 ml conical flask contained 100 ml of medium. The medium was sterilized at 121 °C, 0.11 MPa for 20 min and cooled naturally to room temperature.
Preparation of CuO-NPs suspension
The CuO-NPs (40 nm, 99.5% purity) used in this study were purchased from Zewu Company (Chongqing, China) as a dry powder. The suspension of the CuO-NPs (2000 mg/L) was prepared by adding 100 mg of the CuO-NPs to 50 mL ultrapure deionized water. Subsequently, the CuO-NPs suspension was sonicated (600 W and 40 kHz) for 20 min to increase their dispersion (Huang et al., 2020). The morphology of CuO-NPs was observed by a scanning electron microscope (SEM, Phenom World, Holland). The hydrodynamic diameter of ZnO-NPs in the suspensions were measured by Dynamic Light Scattering (DLS, Brookhaven, USA). A SEM image and hydrodynamic diameter of the CuO-NPs (2,000 mg/L) used for this study are available in the Figs. S1 and S2. Different concentrations of CuO-NPs in simulated sewage were obtained by diluting the suspension with basal medium.
P. tolaasii Y-11 exposure to CuO-NPs with or without Fe
To investigate the difference in toxicity of CuO-NPs to strain Y-11 with different nitrogen sources, the strain was exposed to 1, 5, 10, and 20 mg/L CuO-NPs in the basal medium. Basal medium without CuO-NPs was used as a control. Strain Y-11 was inoculated into BM, and shaken for 48 h at 15 °C and 150 rpm. The inoculation volume of the above experiments was 1% (v/v), and the initial optical density (OD600) was about 0.1. The above experiments were repeated three times. After 48 h, the OD600 of the medium was measured. The medium was centrifuged (8000 rpm, 5 min) to determine the amount of NO3−, NH4+, and metal ions (Cu2+). Three parallel measurements were preformed per sample.
P. tolaasii Y-11 exposure to Cu2+ with or without Fe2+
To investigate whether the toxicity of CuO-NPs was caused by dissolved Cu2+, the concentrations of Cu2+ in BM1 and BM2 were set at 0.01, 0.1, 0.5, and 1 mg/L and 0.01, 0.05, 0.1, and 0.15 mg/L, respectively. The above experiments were repeated in triplicate. The culture conditions and measurement indexes were the same as above.
Analytical methods
The OD600 was measured at an optical density of 600 nm. NO3− and NH4+ were detected in the supernatant after centrifugation (8000 rpm, 5 min) according to the method of He et al. (2019). Specifically, the concentrations of NO3− and NH4+ were determined by the hydrochloric acid photometric method and the indophenol blue method, respectively. The concentration of Cu2+ in the supernatant was analyzed via ICP-OES (ICP-OES 5110, Agilent, USA).
The removal efficiency of NO3− or NH4+ was calculated as follows: (1) where R is the NO3− or NH4+ removal efficiency (%), and T0 and T1 represent the initial and final NO3− or NH4+ concentrations in the system, respectively.
Statistical analyses
The significance of the results was tested using the one-way analysis of variance (ANOVA) in SPSS Statistics 20. Three parallel measurements were performed per sample and the results were expressed as mean ± standard deviation.
Results and Discussion
Effects of CuO-NPs on proliferation and nitrogen removal of strain Y-11 with and without Fe2+ addition
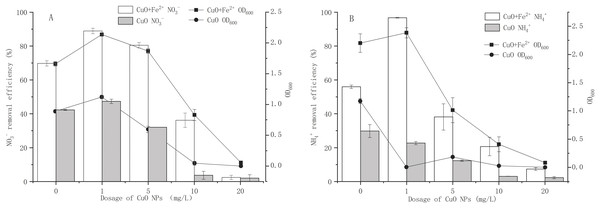
The effect of CuO-NPs on the proliferation and nitrogen removal performance of strain Y-11 with or without added Fe2+ is shown in Fig. 1. Interestingly, 1 mg/L CuO-NPs promoted cell proliferation and NO3− removal (Fig. 1A). In the Fe 2+-free treatment, as the CuO-NP content increased, cell proliferation was inhibited. At 20 mg/L CuO-NPs, the proliferation of bacteria stopped completely. Meanwhile, the NO3− removal efficiency also decreased from 42.29% to 2.05%. Hou et al. (2015) found that 50 mg/L CuO-NPs inhibited the denitrification process and significantly reduced the nitrogen removal rate. The addition of Fe2+ significantly promoted the growth of bacteria and the removal of NO3−. At 0, 1, 5, and 10 mg/L CuO-NPs, the OD600 reached 1.33, 2.14, 1.87, and 0.83, which were significantly higher values than those of Fe2+-free treatment. At 20 mg/L CuO-NPs, the bacteria grew slowly. In the Fe2+-containing treatment, when CuO-NP content was 0, 1, 5, 10, and 20 mg/L, the removal efficiencies of NO3− were 69.77%, 88.93%, 80.51%, 36.17%, and 2.47%, respectively. The addition of Fe2+ increased the activity of the bacteria and enhanced the resistance to CuO-NPs. Song et al. (2016) found that exogenous Fe2+ could improve the microbial activity of water samples and promote the removal of NO3−. Therefore, adding Fe2+ is a feasible method by which to increase bacterial tolerance to CuO-NPs.
Figure 1: Effects of CuO NPs on growth and nitrogen removal of strain Y-11 with and without Fe2+ addition.
(A) The effect of CuO NPs on the growth and NO removal of strain Y-11 in NO medium; (B) the effect of CuO NPs on the growth and NH removal of strain Y-11 in NH medium.In the Fe2+-free treatment, as the CuO-NPs content increased from 0 to 20 mg/L, OD600 decreased from 1.17 to 0, and the NH4+ removal efficiency decreased from 29.83% to 2.33% (Fig. 1B). Even 1 mg/L CuO-NPs were inhibitory to bacteria. This was consistent with the research results of Huang et al. (2020). Zhang et al. (2017a) showed through their research that 1 mg/L CuO-NPs slightly affected the removal of ammonia, and 3–10 mg/L promoted the removal of ammonia. This was different from the results of the present study. By comparing the cell proliferation under Fe2+-free treatment, it could be concluded that the cytotoxicity of CuO-NPs in NH4+ wastewater was stronger than that of NO3− wastewater. The cytotoxicity levels of CuO-NPs in BM1 and BM2 were different, and nitrification-related enzymes were more sensitive to CuO-NPs than denitrification-related enzymes. Ye et al. (2020) found that the denitrification process was more sensitive to the toxicity of ZnO NPs than the nitrification process. This finding was consistent with the results of this study. In the Fe2+-containing treatment, when the CuO-NP contents were 0, 1, 5, 10, and 20 mg/L, OD600 was 2.20, 2.39, 1.01, 0.41 and 0.08, respectively, and the NH4+ removal efficiencies were 55.95%, 96.71%, 38.11%, 20.71%, and 7.43%, respectively. It is worth noting that in the Fe2+-containing treatment, 1 mg/L CuO-NPs significantly promoted cell proliferation and NH4+ removal (compared to the control treatment). Fe plays an important role in the growth and metabolism of microorganisms and is a component of ferritin (Feng et al., 2020). Zhang et al. (2018b) found that Fe2+ (less than 5 mg/L) could significantly promote the removal of nitrogen in the ammonia oxidation system. Exogenous Fe2+ enhanced the resistance of strain Y-11 to CuO-NPs and promoted the removal of NH4+.
Effect of Fe2+ addition on Cu2+ dissolution from CuO-NPs
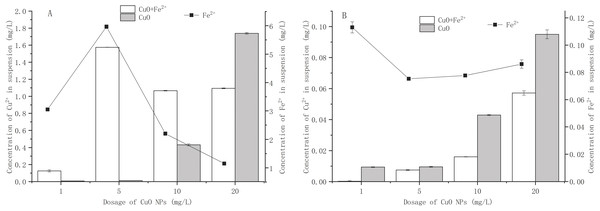
Due to the large specific surface area of CuO-NPs, Cu2+ would be released in the aqueous environment (Luo et al., 2018). Figure 2 shows the effect of Fe2+ addition on the release of Cu2+ from CuO-NPs. In the Fe2+-free treatment, the amounts of Cu2+ released by 1, 5, 10, and 20 mg/L CuO-NPs in BM1 were 0.008, 0.013, 0.432, and 1.739 mg/L, respectively (Fig. 2A). In the Fe2+- containing treatment, as the CuO-NPs content increased from 1 mg/L to 20 mg/L, the maximum release amount of Cu2+ of 1.575 mg/L occurred at 5 mg/L CuO-NPs. At 1 mg/L, 10 mg/L, and 20 mg/L CuO-NPs, the Cu2+ concentrations were 0.126 mg/L, 1.07 mg/L, and 1.09 mg/L, respectively. As the dose of NPs increased, the dissolved metal ions were adsorbed by the NPs, resulting in a decrease in the amount of metal ions released (Wang et al., 2016). Interestingly, although the addition of Fe2+ promoted the release of Cu2+ from CuO-NPs (except for 20 mg/L CuO-NPs), bacterial activity and the removal of NO3− were enhanced (Fig. 1A). Kang, Mauter & Elimelech (2009) found that higher concentrations of divalent cations (such as Mg and Ca) in wastewater may cause nano-metal oxide particles to accumulate more than in river water, thereby affecting their ecotoxicity. Adeleye et al. (2018) found that doping Fe in CuO-NPs significantly promoted the release of Cu2+, but this did not increase the toxicity of CuO-NPs to the marine phytoplankton. However, Naatz et al. (2017) found that the doping of Fe significantly reduced the dissolution of Cu2+ and reduced the toxicity of CuO-NPs to zebrafish embryos. Therefore, in BM1, the toxicity of CuO-NPs to strain Y-11 was caused by the NPs themselves. Exogenous Fe2+ enhanced the tolerance of strain Y-11 to CuO-NPs.
Figure 2: Effect of Fe2+ addition on Cu2+ dissolution from CuO NPs.
(A) The effect of Fe2+ addition on Cu2+ dissolution from CuO NPs in NO medium; (B) the effect of Fe2+ addition on Cu2+ dissolution from CuO NPs in NH medium.CuO-NPs released very little Cu2+ in BM2 (Fig. 2B). As the CuO-NPs content increased from 1 mg/L to 20 mg/L, the released Cu2+ content gradually increased. In the Fe2+-free treatment, the maximum release amount of Cu2+ reached 0.095 mg/L when the CuO-NPs content was 20 mg/L. This was much lower than the release amount of Cu2+ in BM1 at the same concentration (1.739 mg/L). The dissolution of NPs is affected by their surface area (Liu et al., 2018). Under the action of electrostatic attraction, NH4+ ions were adsorbed on the surface of CuO-NPs, which reduced the surface area of the CuO-NPs. In the Fe2+-containing treatment, less Cu2+ was released. The Cu2+ release amounts were 0.0003, 0.0075, 0.016, and 0.057 mg/L at 1, 5, 10, and 20 mg/L of CuO-NPs, respectively. Fe2+ played a role in inhibiting the dissolution of Cu2+ from CuO-NPs. Previous studies had shown that the composition of the solution (such as a high concentration of divalent cations) affected the dissolution of NPs (Chen et al., 2017; Kang, Mauter & Elimelech, 2009; Kunhikrishnan et al., 2015). Mao et al. (2020) found that the addition of metal cations promoted the aggregation of NPs. Exogenous Fe2+ increased the content of divalent cations in the medium and inhibited the dissolution of CuO-NPs. Although the solubility of CuO-NPs in BM2 was lower, CuO-NPs had a higher inhibitory effect on bacterial proliferation and NH4+ removal (Fig. 1B). Exogenous Fe2+ inhibited the dissolution of Cu2+, and the toxicity of CuO-NPs to strain Y-11 was reduced. Therefore, in BM2, the toxicity of CuO-NPs on the strain Y-11 was mainly caused by the release of metal ions.
Effects of Cu2+ on growth and nitrogen removal of strain Y-11 with and without Fe2+ addition
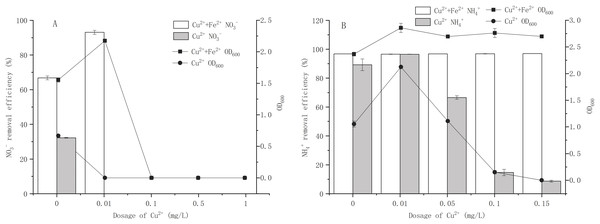
Figure 3: Effects of Cu2+ on growth and nitrogen removal of strain Y-11 with and without Fe2+ addition.
(A) The effect of Cu2+ on the growth and NO removal of strain Y-11 in NO medium; (B) the effect of Cu2+ on the growth and NH removal of strain Y-11 in NH medium.Metal ions are released from NPs in aqueous environment. According to the concentration of Cu2+ released from CuO-NPs in different nitrogen sources, the effects of Cu 2+ on cell proliferation and nitrogen removal of strain Y-11 were discussed (Fig. 3). In BM1, the release of Cu2+ exceeded 1 mg/L (Fig. 2A). Therefore, the concentrations of Cu2+ were set at 0, 0.01, 0.1, 0.5, and 1mg/L (Fig. 3A) for testing. Interestingly, when the concentration of Cu2+ exceeded 0.01 mg/L, severe toxicity was produced and cell proliferation stopped. At 0.01 mg/L Cu2+, the addition of Fe2+ improved the activity of bacteria, and the removal efficiency of NO3− reached 93.1% (66.8% for the control treatment). Low-dose CuO-NPs (1 mg/L) released less than 0.1 mg/L Cu2+ (Fig. 2A), which may be the main reason for the low dose of CuO-NPs (<1 mg/L) induced the synthesis of related enzymes (such as Cu-containing nitrite reductase) and material transfer to increase the activity of the bacteria (Huang et al., 2020; Wang & Chen, 2016; Zhang et al., 2017a). The addition of Fe2+ promoted the release of Cu2+. When Fe2+ was added, the toxicity of CuO-NPs did not increase but promoted cell proliferation and NO3− removal (Figs. 1A and 2A). However, the addition of Fe2+ in the treatment of Cu2+ did not achieve detoxification (Fig. 3A). Cu2+ had strong toxicity in cells. Therefore, we concluded that in BM1 the toxicity of CuO-NPs on strain Y-11 was mainly caused by the NPs themselves. The addition of exogenous Fe2+ caused the Fe2+ to be adsorbed onto the CuO-NPs and inhibited the direct contact between the CuO-NPs and cells, thereby reducing the damage caused by CuO-NPs to strain Y-11. Although exogenous Fe2+ promoted the release of Cu2+, Cu2+ may undergo a hydrolysis process to generate hydroxides and reduce the poisonous effects of Cu2+ (Wang et al., 2016).
In BM2, the maximum release amount of Cu2+ from CuO-NPs was less than 0.1 mg/L. We set the Cu2+ concentration gradient in BM2 to 0, 0.01, 0.05, 0.1, and 0.15 mg/L (Fig. 3B). In Fe2+-free wastewater, 0.01 mg/L Cu2+ significantly increased cell proliferation to 2.12 (1.05 for the Cu2+-free treatment). As the concentration of Cu2+ increased, cell proliferation was inhibited. The NH4+ removal efficiency decreased from 96.52% to 8.72% as Cu2+ concentration increased. The release of Cu2+ in BM2 was less, but the CuO-NPs showed a higher inhibition effect (Figs. 1B and 2B). With the addition of Fe2+, cell proliferation was maintained at about 2.5, and the NH4+ removal efficiency was higher than 96%. The addition of Fe2+ inhibited the release of Cu2+ from CuO-NPs and reduced its toxic effect on cells (Figs. 1B and 2B). In BM2, the aggregation of CuO-NPs due to the electrostatic effect effectively inhibited the distribution of CuO-NPs in the wastewater, and the free Cu2+ made a major contribution to the cytotoxicity. The addition of Fe2+ effectively reduced the dissolution of Cu2+ from CuO-NPs and reduced the toxic effects of Cu2+ on cells.
Effect of exogenous Fe2+ on the aggregation behavior of CuO-NPs
The hydrodynamic diameter could reflect the aggregation state of CuO-NPs in water medium. The hydrodynamic diameter of CuO-NPs under different nitrogen sources was shown in Table 1. The hydrodynamic diameter increased gradually with the increase of the concentration of CuO-NPs, indicating that CuO-NPs had aggregated. This may be due to the increased collision frequency (Mwaanga, Carraway & Van den Hurk, 2014). The hydrodynamic diameter of CuO-NPs in BM2 is larger than that of in BM1, which further revealed that the amount of Cu2+ released in BM2 is less than that of in BM1. Furthermore, it has been reported that the aggregated NPs can reduce toxicity (Hou et al., 2016). Exogenous Fe2+ further promoted the aggregation of CuO-NPs, and the hydrodynamic diameter increases to 2–3 times of the original. The aggregation of CuO-NPs effectively reduced the possibility of contact with bacteria, and reduced the damage of CuO-NPs themselves to cells.
| Particle size (nm) | CuO-NPs concentration (mg/L) | ||||
|---|---|---|---|---|---|
| 1 | 5 | 10 | 20 | ||
| NO3− wastewater | Fe2+-free | – | 659.95 | 743.73 | 1116.52 |
| Fe2+-containing | 2,522.24 | 2,254.52 | 2,027.38 | 1,827.41 | |
| NH4+ wastewater | Fe2+-free | 720.57 | 735.00 | 833.36 | 1,100.54 |
| Fe2+-containing | 1,556.45 | 1,201.48 | 1,528.13 | 1,331.07 | |
Notes:
Conclusions
The cytotoxicity of CuO-NPs in NO3− medium and NH4+ medium was caused by the NPs themselves and the released Cu2+, respectively. CuO-NPs showed higher cytotoxicity in NH4+ medium than in NO3− medium. Exogenous Fe2+ significantly promoted the aggregation of CuO-NPs, reduced the possibility of contact with bacteria, and slowed down the damage of CuO-NPs to strain Y-11. The results of this study provide a new method to alleviate the toxic effects of CuO-NPs on nitrogen-removing microorganisms.
Supplemental Information
Effects of CuO NPs on growth and nitrogen removal of strain Y-11 with and without Fe2+ addition
A is nitrate removal; B is ammonium removal. Error bars represent the standard deviation for three replicates.
Effect of Fe2+ addition on Cu2+ dissolution from CuO NPs
A is a nitrate source; B is an ammonium source. Error bars represent the standard deviation for three replicates.
Effects of Cu2+ on growth and nitrogen removal of strain Y-11 with and without Fe2+ addition
A is a nitrate source; B is an ammonium source. Error bars represent the standard deviation for three replicates.