Maternal sitagliptin treatment attenuates offspring glucose metabolism and intestinal proinflammatory cytokines IL-6 and TNF-α expression in male rats
- Published
- Accepted
- Received
- Academic Editor
- Daniela Foti
- Subject Areas
- Diabetes and Endocrinology, Drugs and Devices, Gastroenterology and Hepatology, Metabolic Sciences
- Keywords
- Sitagliptin, Fetal programming, Inflammatory, Maternal diet, Intestine
- Copyright
- © 2020 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Maternal sitagliptin treatment attenuates offspring glucose metabolism and intestinal proinflammatory cytokines IL-6 and TNF-α expression in male rats. PeerJ 8:e10310 https://doi.org/10.7717/peerj.10310
Abstract
Increasing evidence shows that maternal overnutrition may increase the risk of diabetes in offspring. We hypothesized that maternal sitagliptin intervention may improve glucose intolerance through gut targeting. Female Sprague-Dawley (SD) rats were fed a normal diet (ND) or a high-fat diet (HFD) for 4 weeks before mating. ND pregnant rats were divided into two subgroups: ND group (ND alone) and the ND-sitagliptin group (ND combined with 10 mg/kg/day sitagliptin treatment). HFD pregnant rats were randomized to one of two groups: HFD group (HFD alone) and the HFD-sitagliptin group (HFD combined with 10 mg/kg/day sitagliptin treatment) during pregnancy and lactation. Glucose metabolism was assessed in offspring at weaning. Intestinal gene expression levels were investigated. Maternal sitagliptin intervention moderated glucose intolerance and insulin resistance in male pups. Moreover, maternal sitagliptin treatment inhibited offspring disordered intestinal expression of proinflammatory markers, including interleukin-6 (Il6), ll1b, and tumor necrosis factor (Tnf), at weaning and reduced intestinal IL-6, TNF-α expression by immunohistochemical staining and serum IL-6, TNF-α levels. However, maternal sitagliptin intervention did not affect offspring serum anti-inflammatory cytokine IL-10 level. Our results are the first to show that maternal sitagliptin intervention moderated glucose metabolism in male offspring. It may be involved with moderating intestinal IL-6 and TNF-α expression in male rat offspring.
Introduction
Currently, it is estimated that four hundred and fifteen million adults have type 2 diabetes mellitus (T2DM) worldwide (Williams et al., 2020). T2DM is characterized by abnormalities in glucose metabolism, which leads to pancreatic cell insulin secretion disorder and peripheral insulin resistance (Virally et al., 2007). T2DM and its complications create a societal and commercial burden. Genetic and environmental factors are involved in the pathology of T2DM. Traditional environmental factors mainly include lifestyle factors, including excess energy intake and sedentary lifestyle. Recent epidemiological evidence (Eriksson et al., 2014; Jornayvaz et al., 2016) and animal studies (Boucher & Leung, 2015; Ohta et al., 2017) suggest that exposure to maternal overnutrition in utero increases the risk of metabolic diseases, such as hypertension, cardiovascular disease, and diabetes in offspring. Researchers regard in utero malnutrition status as an important environmental risk factor for developing T2DM (Bianco-Miotto et al., 2017). Maternal glucose dysmetabolism needs to be reversed for the wellbeing of offspring.
Dipeptidyl peptidase (DPP)-4 inhibitors can increase the activity level and duration of action of glucagon-like peptide (GLP)-1. Thus, they have become a new class of antidiabetic drugs in recent years. Once oral nutrients are given, GLP-1 is released from intestinal L cells (Reimann et al., 2008). GLP-1 has several important blood glucose-lowering effects. However, native GLP-1 is rapidly enzymatically hydrolyzed and inactivated by DPP-4 (Kieffer, McIntosh & Pederson, 1995). Since DPP-4 inhibitors prevent the degradation of endogenous GLP-1, they have now entered the clinic to treat patients with T2DM (Lovshin & Drucker, 2009). Sitagliptin is an orally active, fully reversible DPP-4 inhibitor that was approved by the US Food and Drug Administration (FDA) in 2006 (Bergman et al., 2007). As a highly selective DPP-4 inhibitor, sitagliptin prevents degradation of GLP-1 and improves glycaemia, reduces glycated haemoglobin level, stimulates insulin secretion and suppresses glucagon secretion (Gallwitz, 2007; Pratley & Salsali, 2007). In clinical trials, sitagliptin is an effective hypoglycemic agent at every stage of type 2 diabetes (Deacon & Holst, 2013). Recently, Professor Reimer et al. administered sitagliptin to high-fat diet (HFD)-induced obese rats before pregnancy. Their results showed that this intervention strategy did not have lasting effects on fasting blood glucose in offspring (Dennison, Eslinger & Reimer, 2017). Another group reported that sitagliptin attenuates severe acute pancreatitis-associated intestinal inflammation in vivo and in vitro (Zhou et al., 2019). In a double-blind, randomized and placebo controlled clinical trial, pregnant gestational diabetes mellitus (GDM) women in the 2nd trimester were treated with sitagliptin. After 16 weeks of treatment, improved fasting blood glucose, serum insulin, HOMA-IR and HOMA-β was observed in sitagliptin treatment group (Sun et al., 2017). Another trial evaluated the combination of sitagliptin and metformin in GDM patients. Compared with metformin treatment alone, sitagliptin and metformin combination treatment had improved glucose metabolism parameters (Elkind-Hirsch et al., 2018). Sitagliptin was well-tolerated in these two trials, and did not increase the incidence of gastrointestinal side effects. To date, there has been no report on the relationship between sitagliptin and the gut in offspring that underwent in utero overnutrition. Furthermore, the potential intestinal mechanism remains poorly defined.
The objective of the present study was to determine the effect of maternal early sitagliptin intervention on glucose metabolism in offspring. Specifically, we examined intestinal gene expression in the offspring of dams consuming a HFD with or without sitagliptin intervention during pregnancy and lactation.
Materials and Methods
Animal treatments and diets
Ethical approval for the study was granted by the Peking Union Medical Hospital Animal Ethics Committee (Project XHDW-2015-0051, 15 Feb 2015) and conformed to the NIH Animal Care guidelines (NIH publication No. 85-23, revised 1996). Rats were placed in a 12-h light/dark cycle and temperature (22 ± 1 °C) and humidity (65%–70%) controlled housing and were given food and water ad libitum. Eight-week-old female Sprague-Dawley rats (obtained from the Institute of Laboratory Animal Sciences of the Chinese Academy of Medical Sciences and Peking Union Medical College in Beijing, China, n = 32) were randomized into one of two groups for 4 weeks: normal diet (America Institute of Nutrition-93, AIN-93, kcal %: 10% fat, 20% protein, and 70% carbohydrate; 3.85 kcal/gm, ND, n = 16) or HFD (kcal %: 45% fat, 20% protein, and 35% carbohydrate; 4.73 kcal/gm, n = 16). Then, female rats were bred with male rats. After the identification of a copulation plug, dams were housed individually. To evaluate the effect of sitagliptin on mother rats with different diet and their offspring, we divided dams fed an ND into two subgroups: the ND only group and ND-sitagliptin group (n = 8 for each group). Meanwhile, dams fed a HFD were randomized to two subgroups: the HFD only group or the HFD-sitagliptin group (n = 8 for each group). The typical human daily dose of sitagliptin is 100 mg/60 kg body weight. Thus, according to the formula: drat =(37 × dhuman)/6 (Nair & Jacob, 2016), the corresponding dose of sitagliptin for rats is 10.28 mg/kg per day. Therefore, HFD-sitagliptin group rats were fed a HFD supplemented orally with 10 mg/kg/day sitagliptin treatment (Merck, West Point, PA, USA) as described in a previous study (Mega et al., 2011; Samaha, Said & Salem, 2019; Wojcicka et al., 2019). Dams treated with this protocol through pregnancy and lactation. The size of every litter was culled to 6 pups (3 male and 3 female rats) to ensure that there was no nutritional bias between litters. On the day of birth, one male offspring and one female offspring from each litter was randomly selected for experimental study (8 male and 8 female in each group). At weaning, offspring from all dams (8 male and 8 female for each group) were sacrificed following intraperitoneal injection of pentobarbital sodium (150 mg/kg). The intestines were immediately stored at −80 °C. Other surviving rats were kept feeding for other study. Fig. 1 shows the animal experimental protocol.
Figure 1: Animal experiment timeline.
Body weight, glucose tolerance, serum insulin and inflammatory cytokines assay
Dams were weighed on the day of confirmation of pregnancy and during the pregnancy. Pups were weighed on the day after birth and weaning. At weaning, pups underwent an oral glucose tolerance test (OGTT). Briefly, after overnight food deprivation, blood was sampled from the tip of the tail in rats before and 30, 60, and 120 min after oral glucose administration via gavage (2 g/kg). Blood glucose concentrations were determined by using a blood glucose meter (Contour TS glucometer, Bayer, Hamburg, Germany). The area under the glucose tolerance curve (AUC) of the OGTT was calculated as previously described (Zhang et al., 2018). At weaning, the pups were anesthetized after 10 h of fasting. Blood samples were collected from the intraorbital retrobulbar plexus. Serum insulin was measured using an ELISA kit (EZRMI-13K, Millipore, Billerica, MA, USA). Insulin sensitivity was assessed using HOMA-IR as previously described (Zhang et al., 2018). The levels of serum proinflammatory cytokines interleukin-6 (IL-6), TNF-α and anti-inflammatory cytokine IL-10 were measured using ELISA kits (RAB0311, Merck, Darmstadt, Germany, ab46070, Abcam, Cambridge, MA, USA and RAB0246 Merck, Darmstadt, Germany).
RNA isolation, microarray processing and analysis
Previous studies showed that the programming effects of maternal high fat diet occurred in a sexually dimorphic manner (Yokomizo et al., 2014; Zheng et al., 2014), possible due to the influence of confounding factors related to female hormone profile and estrous cycle (Kleinert et al., 2018). Thus, this study mainly focused on male offspring. Total RNA was isolated from the intestines of male pups in the HFD and HFD-sitagliptin groups by using TRIzol reagent (Life Technologies Inc., Carlsbad, CA, USA). Gene expression in the intestine was detected by an Affymetrix GeneChip Rat Gene 2.0 ST whole transcript-based array (Affymetrix Technologies, Santa Clara, CA). The differential gene criteria between the two groups was 1.50-fold or higher (p < 0.05). The data obtained have been deposited in the NCBI Gene Expression Omnibus (GEO) database (accession number GSE134070).
The Gene Ontology (GO) classification system and Kyoto Encyclopedia of Genes and Genomes (KEGG) were used to assign biological meaning to the group of different genes and pathway enrichment through Database for Annotation, Visualization, and integrated Discovery (DAVID) software. STRING software (Biobyte Solution, Heidelberg, Germany) was used to analyze the connections among differentially expressed genes.
Real-time PCR
To validate the gene array results, the expression of genes (Il1b, Il6, and tumor necrosis factor (Tnf)) was analyzed using real-time PCR. Total RNA from the four groups was reverse-transcribed by Superscript II (Life Technologies, Carlsbad, CA). The primers are shown in Table 1. Real-time PCR was performed with an ABI Prism 7500 Real-Time System (Applied Biosystems, Foster City, CA) using ABI SYBR Mix (Applied Biosystems, Foster City, CA). The mRNA levels of the target gene were corrected by glyceraldehyde 3-phosphate dehydrogenase (Gapdh) using the 2−ΔΔCt method.
| Gene symbol | GenBank ID | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|---|
| Il6 | NM_012589 | AGCGATGATGCACTGTCAGA | GGAACTCCAGAAGACCAGAGC | 127 |
| Il1b | NM_031512 | GACTTCACCATGGAACCCGT | GGAGACTGCCCATTCTCGAC | 104 |
| Tnf | NM_012675 | GAACTCAGCGAGGACACCAA | GCCAGTGTATGAGAGGGACG | 124 |
Notes:
- Il6
-
interleukin 6
- Il1b
-
interleukin 1b
- Tnf
-
tumor necrosis factor
Immunohistochemistry for IL-6 and TNF-α in the intestine
Intestinal sections were fixed in 10% neutral buffered formalin, cast in paraffin, sliced into 4 µm sections and placed onto microscope slides. After deparaffinization, slides were immersed in PBS. Then, sections were stained with anti-IL-6 (sc-57315, 1:100, Santa Cruz Biotechnology, Dallas, TX) and anti-TNF-α (sc-52746, 1:100, Santa Cruz Biotechnology, Dallas, TX) at 4 °C overnight. Slides were then incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000, Santa Cruz Biotechnology, Dallas, TX) for 1 h at room temperature. Immunolabeling was visualized with 0.05% diaminobenzidine (DAB). A Nikon 80i microscope (Nikon) was used to capture the images, and Nikon Elements (Nikon) software was used for image processing. Three slides were analyzed for each rat, and eight rats were included in each group.
Statistical analysis
Data are shown as the mean ±SD. Statistical analyses were calculated with Student’s t-test for the difference between two groups and with one-way ANOVA followed by Tukey’s post hoc test for the difference among groups. GraphPad Prism 6 (GraphPad Software Inc., CA, USA) was used for data analysis. P < 0.05 was defined as significant.
Results
The effect of sitagliptin intervention on food intake, energy intake, body weight and blood glucose in pregnancy and lactation on dams
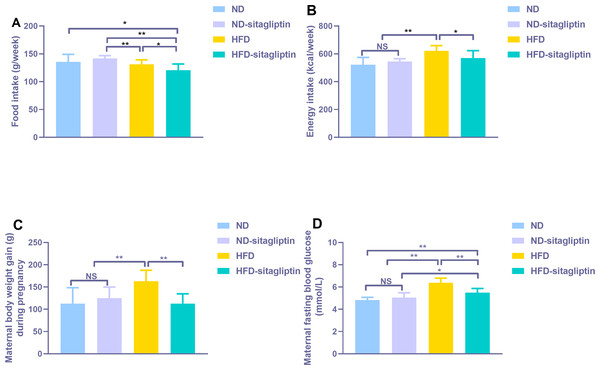
Food intake from HFD and HFD-sitagliptin reduced compared with ND-sitagliptin (p < 0.01, Fig. 2A) during pregnancy. However, energy intake of HFD dams was higher than that of the ND and ND-sitagliptin dams (p < 0.01, Fig. 2B). Accordingly, HFD dams gained significantly more weight than ND and ND-sitagliptin dams during pregnancy (p < 0.01, Fig. 2C). Sitagliptin intervention reduced dam food intake, energy intake and weight gain of HFD dams during pregnancy (p < 0.05or0.01, Figs. 2A, 2B, 2C). HFD dams had higher fasting blood glucose than ND and ND-sitagliptin dams at weaning time (p < 0.01, Fig. 2D); however, sitagliptin intervention reduced fasting blood glucose of HFD dams at weaning (p < 0.01, Fig. 2D).
Figure 2: Effect of sitagliptin on maternal food intake (A), energy intake (B), body weight gain (C) and blood glucose (D).
(A) Maternal food intake during pregnancy. (B) Maternal energy intake during pregnancy. (C) Maternal body weight gain during pregnancy. (D) Maternal fasting blood glucose at weaning time. Values are mean ± S.D. (n = 8). * p < 0.05, ** p < 0.01, ns not significant. ND: normal diet; HFD: high fat diet.The effect of maternal sitagliptin intervention on metabolism in offspring
No difference in birth weight was observed among pups from ND, ND-sitagliptin, HFD, and HFD-sitagliptin dams (p > 0.05, Table 2). Body weight at 3 weeks of age was higher in both of male and female offspring from HFD dams than those from ND and ND-sitagliptin dams (p < 0.05 or 0.01, Table 2). Notably, maternal sitagliptin intervention reduced offspring body weight at 3 weeks of age (p < 0.01, Table 2). Only male pups from HFD dams had higher fasting blood glucose, blood glucose after oral glucose load, and area under the curve (AUC) of blood glucose than those from ND and ND-sitagliptin dams (p < 0.01, Table 2). Maternal sitagliptin intervention reduced fasting blood glucose, blood glucose after oral glucose load and AUC of blood glucose in male offspring from HFD dams (p < 0.01, Table 2). Additionally, the serum fasting insulin concentration and HOMA-IR index in pups from HFD dams were higher than those in male pups from ND and ND-sitagliptin dams (p < 0.01, Table 2), and maternal sitagliptin intervention reduced serum fasting insulin levels and the HOMA-IR index (p < 0.01, Table 2). There was no significant difference in fasting blood glucose, AUC of blood glucose, serum insulin and HOMA-IR among ND, ND-sitagliptin and HFD groups in female offspring (p > 0.05, Table 2). Female offspring in HFD-sitagliptin group had a slight increase than those from ND group in fasting blood glucose, AUC of blood glucose and HOMA-IR (p < 0.05, Table 2).
| Biochemical parameters | Offspring gender | ND | ND-sitagliptin | HFD | HFD-sitagliptin |
|---|---|---|---|---|---|
| birth weight (g) | Male | 6.85 ± 0.51 | 6.71 ± 0.54 | 7.08 ± 0.43 | 7.20 ± 0.58 |
| Female | 6.70 ± 0.28 | 6.88 ± 0.34 | 6.96 ± 0.15 | 6.99 ± 0.40 | |
| body weight at weaning time (g) | Male | 51.25 ± 4.53 | 53.75 ± 6.07 | 60.13 ± 4.16∗∗# | 51.38 ± 4.21&& |
| Female | 48.13 ± 5.19 | 50.13 ± 4.70 | 61.50 ± 3.20∗∗## | 48.50 ± 2.82&& | |
| Fasting blood glucose (mmol/L) at weaning time | Male | 4.94 ± 0.40 | 5.10 ± 0.69 | 6.23 ± 0.60∗∗## | 5.03 ± 0.45&& |
| Female | 4.54 ± 0.53 | 4.70 ± 0.32 | 4.81 ± 0.20 | 5.00 ± 0.42∗ | |
| AUC (mmol/L/h) | Male | 12.58 ± 0.67 | 12.98 ± 1.07 | 18.78 ± 0.63∗∗## | 14.98 ± 0.98∗∗##&& |
| Female | 13.25 ± 0.81 | 14.11 ± 1.32 | 14.64 ± 1.78 | 15.05 ± 2.10∗ | |
| Insulin (ng/mL) | Male | 0.66 ± 0.15 | 0.57 ± 0.08 | 1.26 ± 0.10∗∗## | 0.71 ± 0.08##&& |
| Female | 0.51 ± 0.07 | 0.52 ± 0.11 | 0.56 ± 0.10 | 0.60 ± 0.07 | |
| HOMA-IR | Male | 3.05 ± 0.74 | 2.75 ± 0.65 | 7.41 ± 1.13∗∗## | 3.40 ± 0.48##&& |
| Female | 2.31 ± 0.46 | 2.35 ± 0.60 | 2.53 ± 0.60 | 2.83 ± 0.37∗ |
Notes:
Values are mean ± S.D. (n = 8). *p < 0.05, **p < 0.01 vs ND group, #p < 0.05, ##p < 0.01 vs ND-sitagliptin group, &&p < 0.01 vs HFD group. ND, normal diet; HFD, high fat diet.
The effect of maternal early sitagliptin intervention on gene expression in male offspring intestine from gene array results and pathways
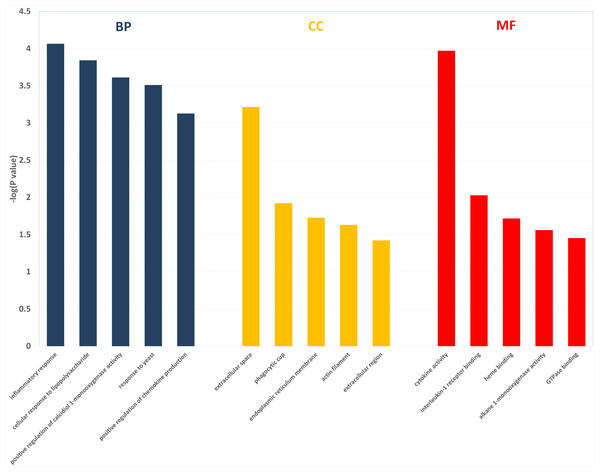
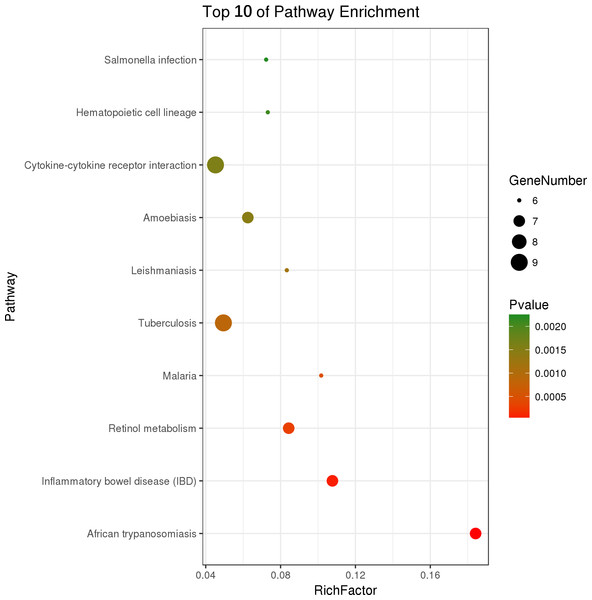
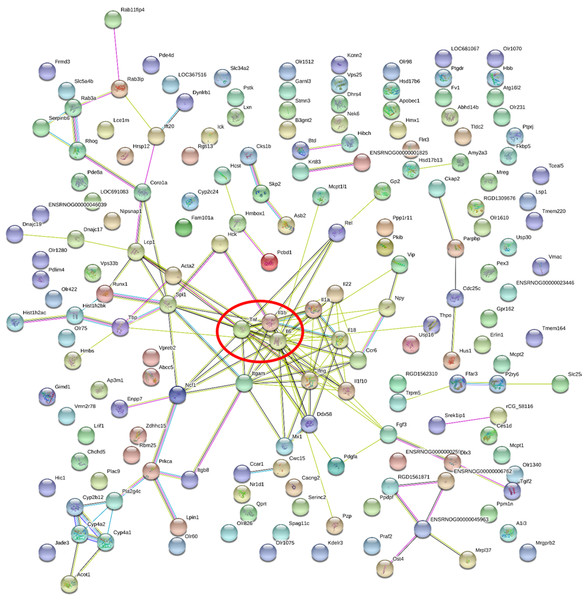
Two hundred and one genes were differentially expressed in the intestines of pups from HFD-sitagliptin dams compared with those of pups from HFD dams (>1.50-fold, p < 0.05; 119 upregulated and 82 downregulated). To investigate the possible regulatory mechanisms of genes affected by maternal early sitagliptin intervention on offspring intestine, we analyzed biological processes, molecular functions, and cellular components through Gene Ontology and enriched KEGG pathways by DAVID. The results showed that differentially expressed genes between the HFD and HFD-sitagliptin groups were enriched in the following biological processes: inflammatory response, cellular response to lipopolysaccharide, positive regulation of calcidiol 1-monooxygenase activity, response to yeast, and positive regulation of chemokine production (p < 0.0001, Fig. 3, Table 3). They are mainly enriched in the following molecular functions: cytokine activity, interleukin-1 receptor binding, heme binding, alkane 1-monooxygenase activity, and GTPase binding (p < 0.05, Fig. 3, Table 3). Significant cellular components were extracellular space, phagocytic cup, endoplasmic reticulum membrane, actin filament, and extracellular region (p < 0.05, Fig. 3, Table 3). Enriched KEGG pathways by DAVID displayed in the KEGG pathway database showed that genes affected by maternal sitagliptin intervention on offspring intestine were significantly enriched in African trypanosomiasis, inflammatory bowel disease, retinol metabolism, malaria, tuberculosis, leishmaniasis, amoebiasis, cytokine-cytokine receptor interaction, hematopoietic cell lineage, and salmonella infection (p < 0.005, Fig. 4, Table 4). In STRING analysis, all differentially expressed genes were mapped in one network. In this network figure, Il1b, Il6, and Tnf were in the center of the differentially expressed gene network (Fig. 5, Table 5).
Figure 3: The enriched GO terms with differentially expressed genes between HFD group and HFD-sitagliptin group.
Top five terms in biological process (BP), cellular component (CC) and molecular function (MP).| Term ID | Term name | Count | p value | Fold enrichment | Catalog |
|---|---|---|---|---|---|
| GO:0006954 | inflammatory response | 12 | 8.59 × 10−5 | 4.42 | biological processes |
| GO:0071222 | cellular response to lipopolysaccharide | 9 | 1.44 × 10−4 | 5.90 | biological processes |
| GO:0060559 | positive regulation of calcidiol 1-monooxygenase activity | 3 | 2.44 × 10−4 | 109.59 | biological processes |
| GO:0001878 | response to yeast | 4 | 3.07 × 10−4 | 29.22 | biological processes |
| GO:0032722 | positive regulation of chemokine production | 4 | 7.45 × 10−4 | 21.91 | biological processes |
| GO:0005615 | extracellular space | 24 | 6.08 × 10−4 | 2.16 | cellular components |
| GO:0001891 | phagocytic cup | 3 | 1.19 × 10−2 | 17.80 | cellular components |
| GO:0005789 | endoplasmic reticulum membrane | 11 | 1.87 × 10−2 | 2.34 | cellular components |
| GO:0005884 | actin filament | 4 | 2.33 × 10−2 | 6.50 | cellular components |
| GO:0005576 | extracellular region | 12 | 3.77 × 10−2 | 1.99 | cellular components |
| GO:0005125 | cytokine activity | 9 | 1.07 × 10−4 | 6.15 | Molecular function |
| GO:0005149 | interleukin-1 receptor binding | 3 | 9.36 × 10−3 | 20.14 | Molecular function |
| GO:0020037 | heme binding | 6 | 1.91 × 10−2 | 3.88 | Molecular function |
| GO:0018685 | alkane 1-monooxygenase activity | 2 | 2.74 × 10−2 | 71.61 | Molecular function |
| GO:0051020 | GTPase binding | 3 | 3.51 × 10−2 | 10.07 | Molecular function |
Figure 4: Top ten KEGG pathways enrichment point diagram in HFD-sitagliptin group compared with HFD group.
The vertical axis represents the pathway name, the horizontal axis represents the Rich factor, the size of the dot indicates the number of genes expressed in the pathway, and the color of the dot corresponds to the different p value.| Pathway ID | Pathway term | Count | Fold enrichment | p value | Involved genes |
|---|---|---|---|---|---|
| rno05143 | African trypanosomiasis | 7 | 16.40 | 3.25 × 10−6 | PRKCA, IL6, TNF, IL18, IFNG, IL1B, HBB |
| rno05321 | Inflammatory bowel disease (IBD) | 7 | 9.59 | 7.67 × 10−5 | IL6, TNF, IL18, IFNG, IL1B, IL22, IL1A |
| rno00830 | Retinol metabolism | 7 | 7.51 | 2.99 × 10−4 | CYP4A2, CYP4A1, DHRS4, LOC100362350, HSD17B6, CYP2C24, CYP2B12 |
| rno05144 | Malaria | 6 | 9.05 | 4.68 × 10−4 | IL6, TNF, IL18, IFNG, IL1B, HBB |
| rno05152 | Tuberculosis | 9 | 4.40 | 8.83 × 10−4 | LSP1, CORO1A, IL6, TNF, IL18, IFNG, IL1B, IL1A, ITGAM |
| rno05140 | Leishmaniasis | 6 | 7.42 | 1.16 × 10−3 | TNF, NCF1, IFNG, IL1B, IL1A, ITGAM |
| rno05146 | Amoebiasis | 7 | 5.56 | 1.46 × 10−3 | PRKCA, IL6, TNF, SERPINB6, IFNG, IL1B, ITGAM |
| rno04060 | Cytokine-cytokine receptor interaction | 9 | 4.02 | 1.56 × 10−3 | IL6, TNF, CCR6, IL18, IFNG, IL1B, IL22, IL1A, THPO |
| rno04640 | Hematopoietic cell lineage | 6 | 6.51 | 2.09 × 10−3 | IL6, TNF, IL1B, IL1A, ITGAM, THPO |
| rno05132 | Salmonella infection | 6 | 6.43 | 2.20 × 10−3 | IL6, IL18, IFNG, IL1B, IL1A, RHOG |
Figure 5: Gene-gene interaction network in HFD-sitagliptin group compared with HFD group.
The nods stand for differentially expressed genes in HFD-sitagliptin group compared with HFD group. The lines stand for the interactions between two proteins.| Gene accession | Gene symbol | Gene name | Degree |
|---|---|---|---|
| NM_012589.2 | Il6 | interleukin 6 | 22 |
| NM_012675.3 | Tnf | tumor necrosis factor | 17 |
| NM_031512.2 | Il1b | interleukin 1 beta | 13 |
| NM_012711.1 | Itgam | integrin subunit alpha M | 12 |
| NM_138880.2 | Ifng | interferon gamma 1 | 11 |
| NM_019165.1 | Il18 | interleukin 18 | 10 |
| NM_001005892.2 | Spi1 | Spi-1 proto-oncogene | 10 |
The effect of maternal sitagliptin intervention on gene expression in male offspring intestine from real-time PCR
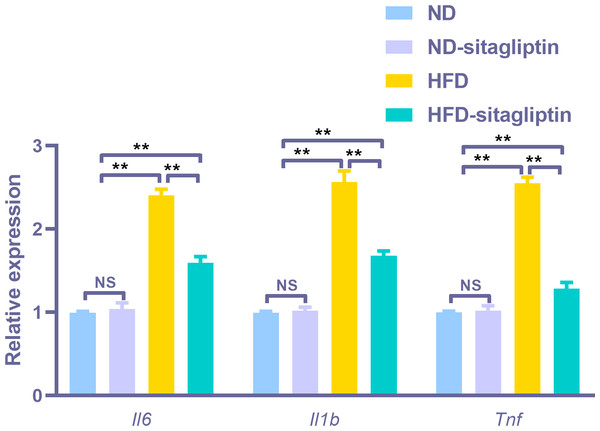
To assess the reliability of the array hybridization results, three differentially expressed genes were quantified using real-time PCR. Gene array GO analysis showed that the inflammatory response was among the top biological processes. Moreover, cytokine-cytokine receptor interactions were among the top ten KEGG pathways. Proinflammatory markers Il6, Il1b and Tnf were in the center of the differentially expressed gene network. Thus, we selected these cytokines to validate the gene array results. Il6, Il1b, and Tnf expression levels were increased in the intestines of male offspring from HFD dams compared to those from ND and ND-sitagliptin dams (p < 0.01, Fig. 6). Interestingly, maternal sitagliptin intervention reduced Il6, Il1b, and Tnf expression levels in male offspring intestines form HFD dams (p < 0.01, Fig. 6).
Figure 6: Confirmation of three representative differentially expressed genes (Il6, Il1b, Tnf) by qPCR.
Values are mean ± S.D. (n = 8). ** p < 0.01, ns not significant. ND: normal diet; HFD: high fat diet.The effect of maternal sitagliptin intervention on proinflammatory markers IL-6 and TNF-α protein expression in male offspring intestines by immunohistochemical staining
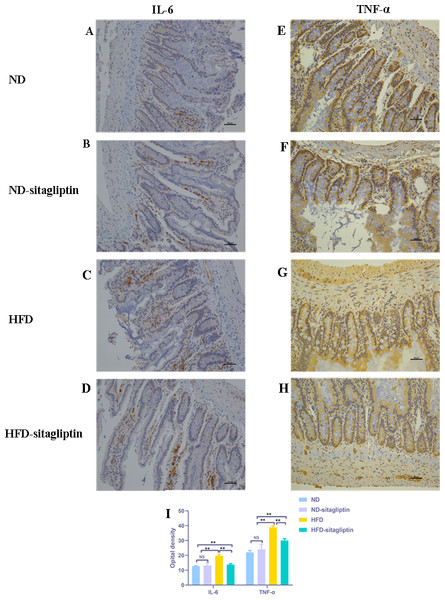
Consistent with the findings by real-time PCR, IL-6 and TNF-α expression in male offspring intestines from HFD dams was increased, compared with those from ND and ND-sitagliptin dams (p < 0.01, Fig. 7). However, maternal sitagliptin intervention reduced the immunoreactivity of IL-6 and TNF-α in male offspring from HFD dams (p < 0.01, Fig. 7).
Figure 7: Effect of maternal sitagliptin on intestinal IL-6 and TNF-α expression in male offspring.
(A–D) Immunostaining for IL-6 (200X) in ND, ND-sitagliptin, HFD, and HFD-sitagliptin group, (E–H) Immunostaining for TNF-α (200X) in ND, ND-sitagliptin, HFD, and HFD-sitagliptin group, (I) Optical density of IL-6 and TNF-α in intestine. Values are mean ± S.D. (n = 8). ** p < 0.01, ns not significant. ND: normal diet; HFD: high fat diet.The effect of maternal sitagliptin intervention on serum proinflammatory cytokines IL-6, TNF-α and anti-proinflammatory cytokine IL-10 in male offspring
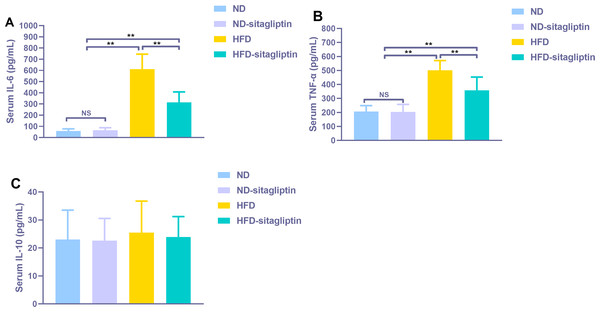
Serum proinflammatory cytokines IL-6 and TNF-α increased significantly in male rat offspring from HFD dams (p < 0.01, Fig. 8), Maternal sitagliptin treatment reduced serum IL-6 and TNF-α levels in male offspring from HFD dams (p < 0.01, Fig. 8). However, there was no difference in serum anti-proinflammatory cytokine IL-10 levels among four groups (p > 0.05, Fig. 8).
Figure 8: Effect of maternal sitagliptin on serum pro-inflammatory cytokines IL-6, TNF-α and anti-inflammotory cytokine IL-10 in male offspring.
(A) Serum IL-6, (B) Serum TNF-α, (C) Serum IL-10. Values are mean ± S.D. (n = 8). **p < 0.01, nsnot significant. ND: normal diet; HFD: high fat diet.Discussion
Our results showed that sitagliptin had no significant effect on body weight and glucose metabolism of mother rats with normal diet and their pups. HFD increased energy intake and body weight gain during pregnancy, whereas sitagliptin intervention significantly reduced energy intake and body weight gain during pregnancy. A previous study also provided evidence that preconception sitagliptin treatment reduced body weight gain during pregnancy in a HFD-induced obese rodent model (Dennison, Eslinger & Reimer, 2017). In addition, we found that sitagliptin intervention improved maternal glucose homeostasis. One pilot study evaluated sitagliptin-metformin combined therapy in glucose-impaired women with a history of gestational diabetes mellitus (GDM) (Elkind-Hirsch et al., 2018). Sitagliptin-metformin is superior to metformin alone in improving glycemia in this prediabetic gestational female population (Elkind-Hirsch et al., 2018). Another clinical trial administered sitagliptin to Chinese GDM patients in their 2nd trimester (Sun et al., 2017). Sixteen weeks of sitagliptin administration significantly lowered fasting plasma glucose levels (Sun et al., 2017).
In addition, our findings revealed that a maternal HFD increased male and female offspring body weight at weaning. Consistent with our findings, other researchers also found that maternal high-fat diet exposure did not change birth weight but increased weaning weight in both female and male offspring (Ribaroff et al., 2017). However, our result showed that only male offspring from HFD dams had higher fasting blood glucose, glucose intolerance and insulin resistance. Previous paper also indicated the impact of offspring sex on blood glucose and insulin level is conflicting (Aldhous et al., 2015; Zheng et al., 2016). The potential mechanism lies in different responses to maternal diet, such as hormone release (Aiken & Ozanne, 2013). Next, we evaluated the effect of maternal sitagliptin intervention on offspring glucose metabolism. We found that maternal sitagliptin intervention attenuated glucose metabolism and insulin resistance in male offspring at weaning. However, intervening before pregnancy with sitagliptin did not have a significant effect on glucose metabolism (Dennison, Eslinger & Reimer, 2017). These different results may be due to differences in sitagliptin treatment time and duration.
Increasing evidence has revealed that maternal diet significantly affects infant microbiota composition (Nash, Frank & Friedman, 2017). A maternal HFD reduced the relative composition of Campylobacter, Helicobacter, and Bacteroidetes (Ma et al., 2014) and increased Lachnospiraceae and Clostridiales (Myles et al., 2013) in offspring. Importantly, changing to a control diet cannot completely reverse the key bacterial composition in pups (Ma et al., 2014). Bacterial composition disorders may affect biological processes in the gut. Currently, the gut is considered an important organ for whole body immune/metabolic health. Our results are the first to evaluate the effects of maternal sitagliptin intervention on offspring intestines. We observed that maternal sitagliptin intervention increased the expression of 119 genes and reduced the expression of 82 genes in male offspring intestine. Abundant studies have revealed that exposure to a high-fat diet causes significant alterations in intestinal gene expression (De Wit et al., 2011; Desmarchelier et al., 2012; Steegenga et al., 2012; Tremblay et al., 2013; Steegenga et al., 2017) found that maternal exposure to a Western-style (WS) diet during the perinatal period altered intestinal gene expression. These differentially expressed genes play an important role in intestinal development and functioning.
Interestingly, our findings show that maternal sitagliptin intervention mainly affected the inflammatory response biological process and cytokine-cytokine receptor interaction pathway. We observed dysregulated Il6, Il1b and Tnf gene expression in the intestines of male offspring in the HFD group at weaning, and remarkably, intestines of male offspring in the HFD-sitagliptin group exhibited reduced Il6, Il1b, and Tnf gene expression. Correspondingly, immunohistochemistry results also showed that maternal sitagliptin intervention reversed increased IL-6 and TNF-α levels in male offspring intestines affected by a maternal HFD. More interesingly, maternal sitagliptin intervention reduced serum IL-6 and TNF-α in male rat offspring. However, maternal sitagliptin did not affect offspring serum IL-10 levels. T2DM has been considered a disease condition with a low-grade inflammation state (Wellen & Hotamisligil, 2005). Glucose metabolism and food intake content are related to inflammatory status (Greco et al., 2014; Silveira et al., 2018), such as hypoglycemia and high fat diet (Arya et al., 2010). Proinflammatory pattern of upregulated IL-6 was observed in adipose tissue from GDM women (Lorenzo-Almoros et al., 2019; Wolf et al., 2004). In an animal model, a maternal high-fat diet not only induces cytokines in maternal serum and placenta involving IL-1β, TNF-α and MCP-1 (Ashino et al., 2012; Frias et al., 2011) but also enhances the level of TNF-α in adipocytes (Murabayashi et al., 2013) and increases IL-6 and TNF-α levels in the liver (Bruce et al., 2009; Oben et al., 2010). Adipose derived cytokines such as IL-6 and TNF-α elevated in obese adolescents and children (Syrenicz et al., 2006). The accumulation of activated macrophages within adipose tissue enlarged adipocytes of obese animals and humans (Weisberg et al., 2003; Xu et al., 2003). IL-6 and TNF-α may interact with each other. TNF-α functions at adipose perhaps stimulate the secretion of IL-6 (Kern et al., 2001). We found the reduced body weight in sitagliptin treated mother rats and their offspring. Thus, sitagliptin may inhibit low-grade chronic inflammation through reducing adipose tissue mass. In addition to the liver and adipose tissue (Hotamisligil, 2006), the gut may show dysbiosis and a certain degree of inflammation involved with gut barrier function disorder and hyperpermeability (Slyepchenko et al., 2016). For instance, gut-derived inflammation is involved with intestinal lipopolysaccharide (LPS) hyperpermeability (Lucas & Maes, 2013). Some evidence shows that a HFD increases proinflammatory cytokine expression in the intestine (Ding et al., 2010; Kim et al., 2012). Maternal HFD increased offspring serum IL-6 and TNF-α levels, even switch to normal diet after weaning in offspring (Kačarević et al., 2017; Peric Kacarevic et al., 2016). This indicates that inflammatory response was provoked in offspring by prenatal HFD. And HFD also elevated the concentration of LPS which can induce insulin resistance (Cani et al., 2007). Antibiotic treatment can lower inflammatory markers, decrease glucose intolerance and caecal LPS levels in obese mice (Cani et al., 2008). Moreover, HFD-induced inflammatory markers are directly transferred to the offspring via dam circulation or alter the quality and quantity of milk production during lactation (Bautista et al., 2016; Saben et al., 2014). Therefore, maternal sitagliptin intervention may inhibit proinflammatory cytokines IL-6 and TNF-α expression in the intestine to moderate intestinal permeability to LPS, leading to attenuate glucose dysmetabolism in offspring.
There is still some limitations of this study need to be stated. Nutrient composition of milk in dams during lactation may also play a role in offspring health. The milk of obese rats contained more energy than that from lean rats (Rolls et al., 1986). The effect of maternal HFD during lactation on milk composition is conflicting, because of different milk collection time-point. Butruille et al. reported that maternal HFD during lactation had no effect on rat global breast milk composition (Butruille et al., 2019). However, others found that milk from HFD dams had higher fat content at postnatal day (PND) 10 and 21(Purcell et al., 2011). And pups from HFD dams consumed more than pups from normal control diet dams (Purcell et al., 2011). For technical limitation, we did not test breast milk composition, consumption and neonatal rat blood glucose. Thus, we cannot eliminate the effect of maternal sitaglition on breast milk composition and consumption.
Conclusions
In conclusion, we demonstrated that maternal sitagliptin intervention provides protection to offspring glucose metabolism and that improvement of intestinal proinflammatory cytokines IL-6 and TNF-α expression may be involved in the mechanism. The intestinal proinflammatory cytokines IL-6 and TNF-α pathway may be a novel target for reversing offspring glucose abnormalities affected by in utero overnutrition.