Larval longevity and competency patterns of Caribbean reef-building corals
- Published
- Accepted
- Received
- Academic Editor
- Owen Wangensteen
- Subject Areas
- Conservation Biology, Ecology, Marine Biology, Zoology
- Keywords
- Broadcast spawning, Florida, Life history, Endangered species, Acropora palmata, Acropora cervicornis, Orbicella faveolata, Connectivity, Larvae
- Licence
- This is an open access article, free of all copyright, made available under the Creative Commons Public Domain Dedication. This work may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose.
- Cite this article
- 2020. Larval longevity and competency patterns of Caribbean reef-building corals. PeerJ 8:e9705 https://doi.org/10.7717/peerj.9705
Abstract
The potential for long-distance larval dispersal depends on the longevity of planktonic, free-swimming larvae and their capacity to successfully recruit to reef habitat. We present multi-year laboratory observations of the persistence of planular larvae and settlement competency over time for cohorts derived from the same parental populations of the most important Caribbean reef building coral species, Orbicella faveolata and Acropora spp. Despite variability among years/cohorts, larvae of both species display capacity for extended longevity (up to 83 d) and competency (demonstrated at up to 48 d). Both species also displayed significantly reduced survivorship and lower realized settlement under elevated temperatures. Although the observed levels of settlement in 24 h competency assays was extremely variable, the timing of onset of competence were highly consistent among years/cohorts but distinct between species. Orbicella faveolata displayed onset of competence during day 3–5 or 4–7 (with or without exposure to positive settlement cue) after spawning; whereas, onset for Acropora spp. was day 7–8 or day 10–11 (with or without cue, respectively). This longer pre-competency period for Acropora spp. nonetheless corresponded to a greater persistence of A. palmata larvae to this age of competence (71–83% of initial cohort compared to 54–55% for O. faveolata). Such life history variation implies meaningful differences in likely dispersal potential between these imperiled reef-building species.
Introduction
Connectivity of broadcast spawning, reef-building coral populations depends upon the dispersal of minute pelagic larvae. Due to their inapparency in nature and challenge to culture, these dispersive life history phases remain poorly characterized, especially for Caribbean species. Meanwhile, the imperiled status of Caribbean reef-building coral species (especially Acropora spp. and Orbicella spp.) highlights the importance of connectivity in allowing for long-distance recruitment to replenish locally depauperate stands. Improved understanding of early life history stages can yield improved prediction of connectivity and recovery potential for specific reef populations.
For example, larval longevity and settlement competence are important determinants of dispersal potential directly affecting population connectivity and recovery potential. While an increasing number of studies on coral larvae have been documented, numerous questions remain regarding factors and mechanisms involved with larval longevity and settlement (Gleason & Hofmann, 2011). Currently, several published studies have documented these important life history characteristics via empirical laboratory observations for Pacific spawning corals (Connolly & Baird, 2010; Moneghetti et al., 2019; Nozawa & Harrison, 2008; Tay et al., 2011). However, such studies are extremely rare for Caribbean corals (Davies et al., 2017 being the notable exception).
Under laboratory conditions, larval longevity and competency have been shown to vary dramatically. Larval longevity for five Indo-Pacific species (Acropora latistella, Montastraea magnistellata, Pectinia paeonia, Favia pallida, and Goniastrea aspera) has been documented up to several hundred days (Graham, Baird & Connolly, 2008), while other observations show a much shorter larval duration of approximately 30–40 days for two other Pacific Acropora spp. (A. muricata and A.valida; Nozawa & Harrison, 2008). Settlement competency periods are generally shorter than maximum larval longevity with peaks occurring approximately 3–10 days post fertilization (Nozawa & Harrison, 2008; Connolly & Baird, 2010; Tay et al., 2011). However, Orbicella franksi demonstrated an exception with a peak range of settlement response at 75–120 days post fertilization (Davies et al., 2017). These studies highlight the interspecific variability in phenotypic life history traits that may lead to underestimates in connectivity modelling projections. Parameter estimates typically used in published dispersal models are for a larval duration of 10–40 days (Baums, Paris & Cherubin, 2006; Holstein, Paris & Mumby, 2014; Indrayanti et al., 2019; Schill et al., 2015) and a competency period of 30 days or less (Drury et al., 2018; Holstein, Paris & Mumby, 2014; Indrayanti et al., 2019; Kool et al., 2011). While variation among individuals and cohorts may also be important both in modelling and in the growing implementation of larval rearing for coral restoration (Randall et al., 2020), most empirical studies characterize only a single cohort of larvae.
In this study, we focus on the primary Caribbean reef-building corals, Orbicella faveolata and Acropora palmata, both of which, to our knowledge, lack published documentation of longevity or competency periods. We conducted two types of laboratory observations to document (1) larval longevity (i.e., persistence of swimming larvae over time) as affected by temperature and in the absence of positive settlement cues, and (2) larval competency dynamics over time, when presented with a positive settlement cue. Additionally, these assays were performed over multiple years with each species to document variation in these parameters among years or cohorts.
Methods
Two separate lab studies are reported here, and referred to as the ‘larval longevity’ experiments (incorporating a temperature treatment) and the ‘competency assays.’ Competency assays were conducted with individual cohorts of larvae of available species during the spawning season each year from 2012 to 2017. The longevity studies were conducted the latter three years (2015–2017). Acropora palmata and Orbicella faveolata were the primary target species, and were attempted for both types of experiments in all years; however, each species was not always available due to inconsistencies in spawning, fertilization, and larval condition. Acropora cervicornis was targeted only in 2017. The general procedure for these two experiment types is described here with specific details and deviations for each year given in Table 1.
| Year | Species | Spawn location | Number of parents | Spawn date | Expt dates | Mean (±1SD) culture temperature | NOTES |
|---|---|---|---|---|---|---|---|
| 2012 | Ofav | Horseshoe | unk | 8 Aug | 21 Aug–25 Sept | 29.1 ± 1.00 | -Competency assays not begun until 14 d AS |
| 2013 | Apalm | Elbow | 6 | 24 Aug | 28 Aug–6 Sept | 29.2 ± 0.65 | |
| Ofav: Cohort a | Sand Island | 2 | 26 Aug | 28 Aug–25 Oct | |||
| Ofav: Cohort b | Grecian Rocks | <8 | 27 Aug | 6 Sept–4 Oct | |||
| 2014 | Apalm | Molasses and Elbow | 7 | 15 Aug | 18 Aug–1 Sept | 28.5 ± .051 | -Ceramic plug chips conditioned ∼2 mos substituted for natural rubble chip in competence assays |
| Ofav | Horseshoe and Grecian Rocks | unk | 17 Aug | 20 Aug–14 Sept | |||
| 2015 | Apalm | Elbow and Sand Is. | 5 | 4 Aug | LONG: 6 Aug–6 Oct | 29.5 ± 0.59 | -Glass inserts begun for competency assays |
| Ofav | Horseshoe | >8 | 6 Aug | LONG: 7 Aug–27 Oct | 30.6 ± 0.48 | -Insufficient Apalm to run competency assays | |
| COMP: 9–25 Aug | |||||||
| 2016 | Apalm | Elbow | 5 | 21 Aug | LONG: 22 Aug–31 Oct | 29.9 ± 0.15 | -Settlement assay done 25–26 Sept with remaining ‘warm’ dish larvae |
| COMP: 25 Aug–4 Sept | 31.0 ± 0.24 | -No Ofav larvae available | |||||
| 2017 | Apalm | Elbow and Horseshoe | 4 | 10 Aug | LONG: 11 Aug–6 Sept | 29.5 ± 0.64 | -Larvae from LONG pooled for final settlement assays 6 Sept prior to Hurricane evacuation |
| Acerv | CRF coral nursery | unk | 10 Aug | LONG: 11 Aug–6 Sept | 30.9 ± 0.84 | ||
| COMP: 14–25 Aug | |||||||
| Ofav | Horseshoe | >8 | 13 Aug | LONG, COMP: 15 Aug–6 Sept | -Insufficient Apalm to run competency assays |
Notes:
- Ofav
-
Orbicella faveolata
- Aplam
-
Acropora palmata
- Acerv
-
Acropora cervicornis
Horseshoe 25.140° N 80.294° W; Elbow 25.143° N 80.258° W; Grecian Rocks 25.110° N 80.306° W; Sand Island 25.018° N 80.368° W; Molasses 25.010° N 80.375° W.
Generally, gamete bundles were collected from reefs near Key Largo, Florida, USA on predicted spawning nights for each species using tent-shaped, collectors constructed of fine insect screen (so-called ‘noseeum netting’) with an inverted jar at the top for bundle collection. Bundles were returned to the boat and combined immediately for cross-fertilization. After returning to a field lab (approximately 1.5 h fertilization duration), excess sperm were rinsed away and embryos were left in filtered seawater overnight with very low aeration.
For the longevity studies, replicate aliquots of embryos (100 or 120 for Acropora spp; 200 for O. faveolata) were counted out either on day 1 or day 2 after spawn (dAS) and placed in glass bowls with approximately 400 ml filtered seawater (either 1um or 5um filtration) collected from offshore reef environments. These dishes were then divided between two outdoor, recirculating water baths providing two temperature treatments (n = 5). These temperature treatments were somewhat different in both mean and variability between years (Table 1), but the ‘cool’ treatment was targeted in the range of 29.0–30.0 °C while the ‘high’ treatment was in the range of 30.5–31.5 °C during different years. The ambient surface temperature over upper Florida Keys reefs in August is approximately 30 °C in modern times, approximately 1 °C higher than at the turn of the 20th century (Kuffner et al., 2015).
Water changes (approximately 75% volume) in each dish were conducted every other day with filtered reef water. Full water changes were conducted during counts of the larvae remaining in each dish, conducted every other day for the first two weeks and then less frequently (approximately weekly) thereafter. These counts scored organisms as pelagic/swimming larvae, settled polyps, or MUPs (metamorphosed, unattached polyps). Larvae that metamorphosed in the water column (MUPs) were never observed to attach so their viability as recruits is unclear, but we chose to exclude them from both estimates of larval supply and mortality. Settled polyps remained in the bowls in 2015, but during 2016 and 2017, all settlers were removed during each count. Data are reported as larval supply (remaining swimming planulae larvae) over time (i.e., age) and mortality over time (initial # minus remaining swimming planulae minus cumulative # settlers minus cumulative # MUPs).
There was a discontinuity during the 2015 and 2016 experiments at the completion of our field expedition (approximately 1 month following spawning). The individual dishes were lidded and transported in a dry cooler from the field lab in Key Largo back to the Southeast Fisheries Science Center in Miami FL. In 2015, the recirculating temperature-controlled baths were re-assembled, and the temperature treatments were maintained until all larvae expired or settled. In 2016, the warm treatment was terminated when this move was made, and only a single water bath for the cool treatment was carried on. In 2017, all experiments were curtailed at 23–25 dAS due to forced evacuation before Hurricane Irma.
At the conclusion of the experiment, larval longevity under the temperature treatments was analyzed using the “survival” (Therneau, 2020) and “survminer” (Kassambara et al., 2019) packages in R, with replicate dish as a random factor. Kaplan–Meier survival curves were estimated for each treatment within a cohort and compared using a log rank test.
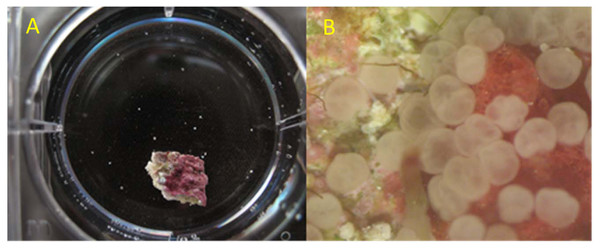
For the competency assays, larger batch cultures of larvae were maintained in round culture chambers that had a 100-µm mesh bottom and a continuous, recirculating slow drip around its walls. Culture chambers were housed in outdoor recirculating seawater systems containing reef-collected seawater with temperature control, salinity maintained at 35–37 ppt by addition of distilled water to compensate for evaporation, a UV sterilizer, and 5µm filtration. Partial exchange with new reef water was conducted periodically to maintain water quality. No effort was made to deprive these larvae of settlement cues since raw reef water was exchanged periodically and other tanks in the recirculating seawater system contained natural reef rubble. Beginning two to three days after spawn, replicate aliquots of larvae were counted out (10 for Acropora spp.; 20 for O. faveolata) into individual wells in two 6-well polystyrene culture plates along with a fragment of substrate with crustose coralline algae to provide positive settlement cues (n = 12; Fig. 1A). In most years, this substrate was a fragment chipped from a freshly collected piece of reef rubble, though in 2014 chips of artificial ceramic substrate that had been conditioned in local reef habitat for over two months were used. Rubble or ceramic chips were approximately 1 × 1 cm in size. Starting in 2015, glass inserts were placed inside the polystyrene 6-well plates such that settlement took place in the absence of plastic.
Figure 1: Illustration of one replicate of the competency assay.
(A) Small chip of reef rubble providing benthic settlement cues and O. faveolata larvae (small white dots) in one well of a 6-well culture dish. (B) Appearance of O. faveolata larvae scored as successful settlement with attached, flattened appearance, and substantial progress of metamorphosis.Generally, competency assays were established in late afternoon and scored approximately 24 hr later. Settlement of larvae (i.e., firmly attached and beginning to flatten; settlement, sensu Miller & Mundy (2003; Fig. 1B)) was scored under a fluorescent dissecting microscope. After each scored assay, larvae were discarded and a fresh selection of larvae and fresh rubble chips were used for each subsequent assay. These assays were initially conducted daily, with reduced frequency to every other day after about 2 weeks and were continued as long as healthy-looking larvae (i.e., elongated and swimming) were available from the initial batch culture with the following exceptions. In the first year of the study (2012), the competency assays were not initiated until day 14 and were conducted less frequently. An additional competency assay for Acropora spp was performed at a later date (after the initial batch culture was expended) using remaining larvae from the longevity assays in 2016 (the discontinued ‘warm’ treatment larvae when the experiment was moved back from the field lab) and in 2017 (all larvae from the longevity assays prior to required hurricane evacuation). Lastly, in 2013, two separate cohorts (termed a and b) collected on sequential nights from different sites were tested separately.
All collections were permitted by the Florida Keys National Marine Sanctuary (Permits # FKNMS-2012-101, FKNMS-2014-047, and FKNMS-2016-047-A1).
Results
Both experiments were conducted under generally consistent procedures (exceptions noted in Table 1) with larvae derived from the same general parent population (i.e., collected from 2–3 sites within ∼12 km distance), yet overall settlement rates and competency patterns over time were extremely variable. In contrast, onset of competency was quite consistent among experimental trials and cohorts within a species, while being distinct between species.
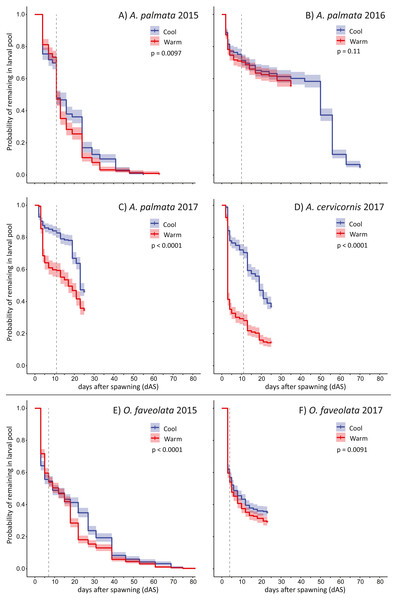
The pattern of larval supply (i.e., remaining swimming planula) over time for each trial is shown in Fig. 2. The maximum larval longevity (i.e., the persistence of swimming planula larvae) we observed was 83 days for O. faveolata (2015) and 70 days for A. palmata (2016). The first observation of metamorphosis in these experiments (no cue) was on either 10 or 11 dAS for four separate trials with Acropora spp (A. palmata in 2015, 2016, and 2017 plus A. cervicornis in 2017) and for O. faveolata, day 7 in 2015 and day 4 in 2017 (Fig. 2). The patterns of larval mortality are quite consistent with high mortality up to 4 or 5 dAS, after which mortality rate is greatly moderated in O. faveolata, and largely flat in Acropora spp. (Fig. S1).
Figure 2: Larval persistence under different temperatures.
Kaplan-Meier curves for swimming pelagic larvae in two temperature treatments (described in Table 1) for Acropora spp. (A–D) and Orbicella faveolata (E–F) cohorts. This shows the probability of remaining as swimming pelagic larvae over time; reduced by both mortality and metamorphosis (in the absence of specific settlement cues). Dashed line shows the time when first metamorphosis was observed in each trial. P-values show significance of difference between the two curves via log rank test.In five out of six trials, the warmer temperature treatments had mild but significant effects on larval supply over time (exception: 2016 A. palmata) and resulted in substantially fewer observed settlers for both species in 2015 and 2016 when the mean treatment increment was approximately 1 °C (Table 1, Figs. 2A, 2B and 2E). This effect was much larger for both Acropora spp. in the 2017 experiment when the mean treatment increment was approximately 1.5 °C and more variable (Table 1, Figs. 2C and 2D), though the effect of this more extreme temperature exposure on O. faveolata remained mild (Fig. 2F). Specifically, at the time of first observed metamorphosis (no cue), on average, ∼40% as many larvae remained in the warm treatment relative to the cool for A. cervicornis (at 11 dAS), ∼71% as many A. palmata (at 11 dAS), and ∼90% as many O. faveolata (at 4 dAS; Figs. 2C, 2D and 2F; Table 2).
| Year | Species | Experiment duration (days) | Mean cumulative % settlement | Proportion fewer settlers in Warm | First observed metamorphosis (dAS) | Mean % larvae remaining at first observed metamorphosis | ||
|---|---|---|---|---|---|---|---|---|
| Warm | Cool | Warm | Cool | |||||
| 2015 | O. faveolata | 83 | 8 | 13 | 0.39 | 7 | 54 | 54 |
| 2015 | A. palmata | 63 | 29 | 38 | 0.24 | 11 | 70 | 73 |
| 2016 | A. palmata | 70 | NA | 21 | NA | 10 | 70 | 71 |
| 2017 | A. palmata | 25a | 9 | 27 | 0.67 | 11 | 59 | 83 |
| 2017 | A. cervicornis | 25a | 8 | 23 | 0.64 | 11 | 28 | 71 |
| 2017 | O. faveolata | 23a | 2 | 2 | 0 | 4 | 55 | 58 |
Notes:
- dAS
-
days after spawn
Generally, the batch cultures used for the competency assays persisted longer for O. faveolata, with competency assay data available over 25–40 dAS, whereas for Acropora spp. most of the batch cultures had expired in less than 15 days, often due to spontaneous metamorphosis in the water column. There was remarkable variability between years in the overall settlement rates with the highest observed daily mean for O. faveolata ranging from 21% (2014) to over 50% (2015) and for A. palmata the highest daily mean settlement rate ranging from below 10% to 80% (Figs. 3 and 4).
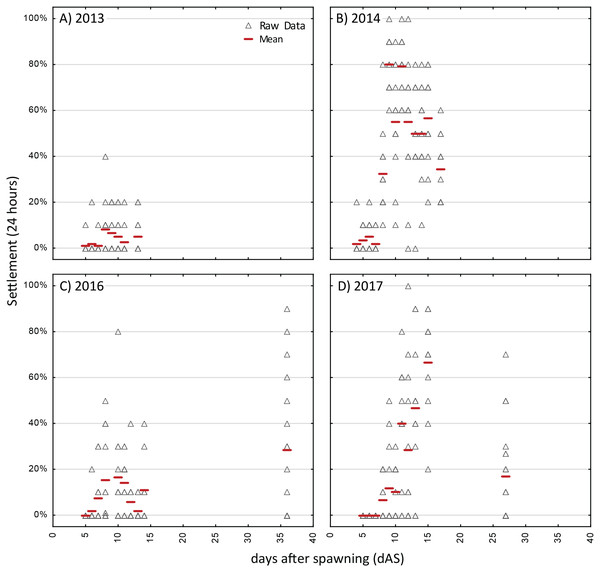
Figure 3: Competency assays—Acropora spp.
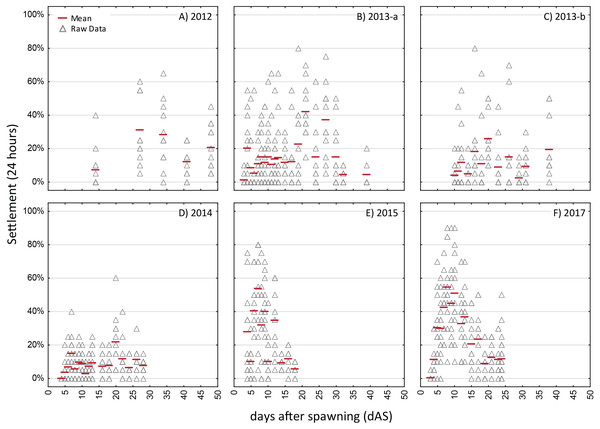
Settlement in sequential, 24-h assays with four different cohorts of larvae of Acropora spp. (A)–(C) are A. palmata while (D) is A. cervicornis. A separate aliquot of larvae was used for each trial. Triangles show raw data for n = 12 replicates on each occasion. Red dash shows the mean.Figure 4: Competency assays—Orbicella faveolata.
Settlement in sequential, 24-h assays with six different cohorts of larvae of Orbicella faveloata over five years (A–F). A separate aliquot of larvae was used for each trial. Triangles show raw data for n = 12 replicates on each occasion. Red dash shows the mean.The strongest pattern in all of the competency assays is the observation of higher variability among replicates within each trial run than among ages. Virtually all trials of these assays had individual replicates with zero settlement, simultaneous with other replicates with high settlement (maximum levels of 80–100% in 24 h; Figs. 3 and 4), thus, obscuring any clear patterns in competency with age such as hypothesized patterns of senescence. We also calculated the time windows where highest settlement occurred for each trial (i.e., settlement values within 25% of the maximum settlement observed for a cohort; Figs. S2 and S3). For O. faveolata, highest settlement occurred during days 6-10 (2015 and 2017) or days 20–27 (2012–2014). Acropora spp. settlement peaked at days 8-15 for the 2013, 2014, and 2017 cohorts (Fig. S2). However, in 2016 the maximum mean A. palmata settlement of 28% was observed at 35 dAS (Fig. 3C), the latest age for which a full competency assay was run for this species. The latest competency was demonstrated at 48 dAS for O. faveolata (2012; mean 20% settlement per 24 h, Fig. 4A). Meanwhile, some cohorts/years did show marked declines in competence with age consistent with senescence (e.g., O. faveolata in 2015; Fig. 4E).
Although the initial observations of settlement in the competency assays was at day 3 or 4 for all species, the onset of substantial settlement response at the population level was consistently earlier for O. faveolata, with mean settlement rate increasing during the range of 3–5 dAS whereas in Acropora spp, this increase of competency was not observed until day 6–8 (Figs. 3 and 4).
Discussion
This study is among the first to empirically document pre-competency, competency, and dynamics of larval supply over multiple years and cohorts in Caribbean spawning corals. A few studies have documented extended larval duration for Indo-Pacific broadcast spawning corals as well as timing and pattern of settlement. Connolly & Baird (2010) characterize both larval survivorship and competence in five species, all of which showed a peak in settlement competence in the range of 4–13 days with an asymptotic decline thereafter. Although all five species showed larvae remaining up to 90-120 dAS, after 30 days, four of the five species showed a settlement response of less than 10% (and rapidly declined) in 72-hour assays, and settlement was not observed after 36 days in three of the species. All five species showed a post-competence (senescent) period wherein surviving larvae failed to settle in standard assays. In contrast, while our results show larval longevity of up to 60–70 dAS for Acropora palmata and 83 dAS for Orbicella faveolata, there is no clear pattern of senescence. For example, several of the highest mean settlement responses were observed at 35 dAS (e.g., 2016 for A. palmata; 2012 and 2013b for O. faveolata; Figs. 3 and 4). Davies et al. (2017) similarly showed no pattern of senescence over more than 120 dAS for Orbicella franksi. However, the overall longevity of the batch cultures used for our competency assays, especially for A. palmata, was compromised as most of these larvae metamorphosed spontaneously (MUPs) in these batch cultures within 2 to 3 weeks. Metamorphosis without attachment has been demonstrated to result from a specific inducer derived from bacterial isolates of crustose coralline algae (Tebben et al., 2011), but is commonly observed in high-density culture conditions (M Miller, 1996–2017, Pers. Obs.; J Figueiredo, R Ritson-Williams, C Page, 2019, Pers. Comm.). Thus, the duration of availability of cultured Acropora palmata larvae in our competency study likely yielded an underestimate of the duration of the competence period for this species (i.e., it is likely substantially longer than 35 dAS).
| Orbicella faveolata | Acropora palmata | Acropora cervicornisa | |
|---|---|---|---|
| Maximum longevity (days) | 83 | 70 | N/A |
| First observed metamorphosis (dAS; without cue) | 4–7 | 10–11 | 11 |
| % larvae alive at first metamorphosis (cool treatment) | 54–58% | 71–83% | 71% |
| Onset of competency (dAS; with cue) | 3–5 | 6–8 | 8 |
| Maximum mean settlement 24-h (with cue) | 55% | 83% | 67% |
Notes:
- dAS
-
days After Spawn
Connectivity models consider several contributing processes to coral abundance, including spawning output, dispersal, larval behavior, predation, habitat, and post-settlement processes (Cowen & Sponaugle, 2009). Our results highlight the intra- and inter-cohort variability within spawning output, potential for dispersal, and larval behavior which may pose additional modelling challenges, and should be considered when estimating parameters for these models. The dominant pattern in the competency assays is of high variability both between years/cohorts and among replicates within individual trials. Some variation in settlement response is likely due to variation in the quality or nature of the positive settlement cue present on the field-conditioned substrate provided in each replicate of our experiment. It was only possible to standardize these based on rough visual appearance, whereas microscopic biofilms and subtle taxonomic distinctions of crustose coralline algae can profoundly affect settlement induction (Harrington et al., 2004; Ritson-Williams et al., 2014; Tebben et al., 2015). There is also evidence of a strong genetic component to settlement patterns, which can account for differences between cohorts. For example, Kenkel et al. (2011) showed settlement response varying from 37–94% (during a settlement period between 5–8 dAS) among full-sib families of Acropora millepora. These different potential mechanisms of variability in overall settlement success may yield somewhat different implications, not only for predicted connectivity but also in terms of potential remedies. For example, a rehabilitation strategy for poor settlement habitat (i.e., providing inadequate settlement cues) is different than a restoration strategy involved in selecting parents for larval propagules that can be successful settlers. Indeed, both types of strategies may be needed on modern, depauperate reefs.
Though the settlement response is highly variable, some characteristics are remarkably consistent between years, though contrasting between species (summarized in Table 3). For example, the initial observation of metamorphosis in the longevity studies (i.e., in absence of settlement cue) was either 10 or 11 dAS for both Acropora species over three years and either day 4 or 7 for O. faveolata. Given these different ages of competency, the percent of larvae remaining at this point in time was substantially greater for Acropora spp (71–83% on day 10–11) than for O. faveolata (55–58% on day 4–7) in the longevity study under the more benign (cool) temperature treatments (Table 2, Fig. 2). Knowledge of these consistent, species-specific characteristics can be leveraged in developing and optimizing production pipelines for larval propagules to meet the needs for large scale coral restoration (Baums et al., 2019; Randall et al., 2020).
Our temperature exposure treatments averaged about 1 °C difference in 2015 and 2016. This small temperature difference yielded subtle but significant differences in larval survivorship for both species. In 2017, we implemented an average 1.5 °C temperature increment between treatments (with maximum exposures in the high treatment reaching slightly over 32 °C for a short period early in the experiment). This more extreme temperature regime caused more dramatic mortality, especially for Acropora spp. Nonetheless, even the less extreme ‘warm’ treatments, averaging 30.6−31°C (2015 and 2016) resulted in 24–39% fewer cumulative settlers over the entire course of larval duration (Table 2). Current mean monthly temperatures during the spawning season for these species, August through September, in the upper Florida Keys are already over 30 °C (Kuffner et al., 2015; Manzello, 2015), similar to our ‘cool’ treatment in 2016, but warmer than our cool treatment in 2015 and 2017.
Surprisingly, we did not observe more rapid onset of metamorphosis in the warm treatments of the longevity experiments (no cue) as several other studies have documented accelerated development timelines in warmer temperatures (Baums et al., 2013; Randall & Szmant, 2009). In all cases but one, the first metamorphosis was observed on the same day in both temperature treatments. The exception was O. faveolata in 2015, in which case first metamorphosis was observed on day 7 in the warm and day 9 in the cool treatments. It may be that the previously documented acceleration of development rate in warmer temperatures results in differences in competency on the scale of hours (rather than days) and was thus not detectable at the temporal resolution of our observations (1–2 days).
Conclusion
These results provide a more solid basis for predicting dispersal of the key reef-building Caribbean corals than has been previously available and, hence, the connectivity potential of metapopulations of these foundation species. However, the realization of this potential is predicated on successful larval production and successful recruitment into the receiving habitat (i.e., settlement and survivorship to maturity). There is evidence that the latter process is failing in many reefs (Hughes & Tanner, 2000; Van Woesik, Scott & Aronson, 2014; Vermeij et al., 2011) and growing suspicion that the former may also be impaired in areas where abundance and genotypic diversity of adult populations is reaching depensatory levels (Hughes et al., 2019; NMFS, 2015; Williams, Nedimyer & Miller, 2020). Models, even with improved parameter estimates provided by studies such as this one, should not be interpreted as assuring connectivity. Restoration and other management actions supporting successful coral sexual reproduction are also needed (Baums et al., 2019; National Academies of Sciences, 2019).
Supplemental Information
Raw data for competency assays
The number of settled larvae in subsequent 24h assays performed over time for each cohort of larvae.
Raw data for larval longevity studies
Counts of swimming larvae, metamorphosed but unattached polyps, and settled polyps in each dish over time.