Retinal Circuits: How we see the forest and the trees
Imagine you are walking through an alpine forest on a beautiful fall day, passing a stand of aspen trees with their thin trunks forming a vertical grid before a brilliant backdrop of autumn color. A closer look reveals the horizontal striations in their white bark (Figure 1A,B). This simple, sylvan example highlights how our visual system seamlessly shifts its attention across the broad range of spatial frequencies in the natural world: it can report global shapes, patterns and motion, and also encode fine details, enabling us to see the forest – and the trees.
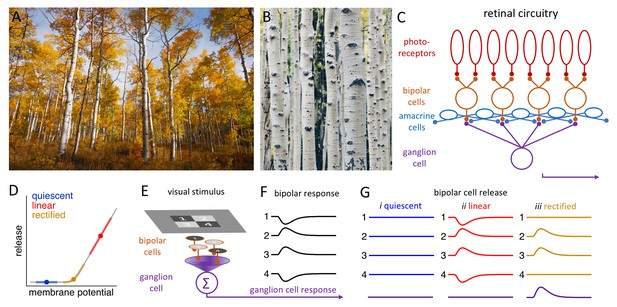
Processing visual information in the retina.
(A) A stand of aspen trees, seen from a distance, presents primarily vertical lines (Image credit: John Price). (B) Closer inspection reveals primarily horizontal features in the bark of individual trees (Image credit: Peng Chen). (C) Simplified schematic of the retinal circuitry showing the synapses between photoreceptors (top) and bipolar cells, and between bipolar cells and a single ganglion cell. The amacrine cells influence the behavior of the bipolar cells (and the ganglion cells). (D) Neurotransmitter release by bipolar cells (y-axis) versus the membrane potential of these cells. Bipolar cells inhabit one of three release regimes: quiescent (blue), when visual stimulation is insufficient to evoke release; linear (red), when release is proportional to the stimulus; and rectified (gold), when only positive stimuli evoke release. (E) Schematic showing a checkerboard stimulus presented to a 2 × 2 array of bipolar cells. (F) The change in the membrane potential (y-axis) over time (x-axis) of each bipolar cell depends on whether it receives a positive stimulus (2 and 3) or a negative stimulus (1 and 4) from the checkerboard. (G) The release of neurotransmitters from the four bipolar cells and the resulting response in the ganglion cell (bottom) depend on the release regime occupied by the bipolar cell (see main text).
One might expect that such a sophisticated system would require this information to be sent to 'higher' visual centers in the brain for processing. However, much of this processing is actually carried out at a relatively 'low' level by the neurons in the retina (Hochstein and Shapley, 1976; Demb et al., 1999; Schwartz et al., 2012; Grimes et al., 2014; Turner and Rieke, 2016). Now, in eLife, Maxwell Turner and Fred Rieke of the University of Washington, and Gregory Schwartz of Northwestern University, report how circuits in the retina fine-tune their spatial sensitivity in response to the surrounding visual world (Turner et al., 2018).
Neurons communicate with each other by releasing signaling molecules called neurotransmitters into the synaptic gaps between them. In the retina, visual signals in the form of slow, graded changes in membrane potential are transmitted from photoreceptors (the cells that actually detect the light we see) to bipolar cells and then to ganglion cells (Figure 1C). The release of neurotransmitters from bipolar cells into a synapse depends on the value of the membrane potential of the neuron relative to the activation range of the calcium ion channels that trigger the release (Figure 1D). There are three different regimes: the 'quiescent' regime, in which only a very strong positive stimulus will evoke release; the 'rectified' regime, in which a positive stimulus will evoke release, but a negative stimulus will not; and the 'linear' regime, in which a positive stimulus will lead to an increase in release, and a negative stimulus will lead to a decrease. Many of the synapses formed by bipolar cells operate in the 'rectified' regime.
Turner et al. studied how visual signals are transmitted from a number of bipolar cells to a single ganglion cell. This transmission depends on which regime the bipolar cells are in, particularly when the intensity of the visual image being transmitted varies across the receptive field of the ganglion cells (Croner and Kaplan, 1995).
Suppose that the bipolar cells in a 2 × 2 array are stimulated independently by a checkerboard image, with two cells receiving a positive stimulus and two receiving a negative stimulus (Figure 1E,F). If the bipolar cells are quiescent, the stimuli will not evoke a release from any of the four cells, and hence no signal will be transmitted to the ganglion cell. Likewise, if the bipolar cells are in the 'linear' regime, the release of neurotransmitters from two of the cells will increase, and the release from two will decrease, thus cancelling each other out, so the signal being transmitted to the ganglion cell will not change. Linear responses can, therefore, diminish the responses of the ganglion cells to higher spatial frequencies. However, if the bipolar cells are in the 'rectified' regime, only the two positively stimulated bipolar cells will release a neurotransmitter, enabling the ganglion cells to respond (Figure 1G; Enroth-Cugell and Robson, 1966).
Another set of cells in the inner retina, the amacrine cells, are also involved regulating the release of neurotransmitters by bipolar cells and thus fine-tuning the information transferred to ganglion cells. In particular, the amacrine cells contribute to the 'center-surround' organization of the receptive fields of ganglion cells: put simply, this means that if a ganglion cell is excited by a stimulus in the center of its receptive field, a similar stimulus in the surrounding area will be inhibitory.
Turner et al. show that ‘surround inhibition’ can influence the spatial sensitivity of the ganglion cells by shifting the bipolar cells from one release regime to another. Strong surround inhibition pushes bipolar cells toward quiescence, limiting responses to center stimuli. Conversely, surround stimuli of the opposite polarity to that of the center decreases inhibition in the surround, pushing the bipolar cells into their linear regime. As a result, contrasting details in the center cancel each other, reducing the ganglion cells’ spatial sensitivity. This proves useful when visual features change abruptly on a larger spatial scale, and encoding global contrast or motion takes temporary precedence over the finer details.
In our spatially correlated natural world, however, the luminance of the center and surround are often similar, so that bipolar cells occupy their rectified regime, thereby maximizing the sensitivity of the ganglion cells to higher spatial frequencies (Field, 1987). Notably, these changes can occur quickly, enabling the circuit to adapt in real time to changing visual conditions.
The work by Turner et al. and others has certainly expanded our appreciation for the remarkable versatility and computational power of this thin, transparent sheet of neurons that lines the back of the eye (Gollisch and Meister, 2010). The retinal circuitry has revealed itself as a dense forest of connected trees that holds many more secrets yet to be discovered.
References
-
Functional circuitry of the retinal ganglion cell's nonlinear receptive fieldJournal of Neuroscience 19:9756–9767.https://doi.org/10.1523/JNEUROSCI.19-22-09756.1999
-
The contrast sensitivity of retinal ganglion cells of the catThe Journal of Physiology 187:517–552.https://doi.org/10.1113/jphysiol.1966.sp008107
-
Relations between the statistics of natural images and the response properties of cortical cellsJournal of the Optical Society of America A 4:2379–2394.https://doi.org/10.1364/JOSAA.4.002379
-
Linear and nonlinear spatial subunits in Y cat retinal ganglion cellsThe Journal of Physiology 262:265–284.https://doi.org/10.1113/jphysiol.1976.sp011595
-
The spatial structure of a nonlinear receptive fieldNature Neuroscience 15:1572–1580.https://doi.org/10.1038/nn.3225
Article and author information
Author details
Publication history
- Version of Record published: October 12, 2018 (version 1)
Copyright
© 2018, Diamond
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,083
- views
-
- 186
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Nociceptive sensory neurons convey pain-related signals to the CNS using action potentials. Loss-of-function mutations in the voltage-gated sodium channel NaV1.7 cause insensitivity to pain (presumably by reducing nociceptor excitability) but clinical trials seeking to treat pain by inhibiting NaV1.7 pharmacologically have struggled. This may reflect the variable contribution of NaV1.7 to nociceptor excitability. Contrary to claims that NaV1.7 is necessary for nociceptors to initiate action potentials, we show that nociceptors can achieve similar excitability using different combinations of NaV1.3, NaV1.7, and NaV1.8. Selectively blocking one of those NaV subtypes reduces nociceptor excitability only if the other subtypes are weakly expressed. For example, excitability relies on NaV1.8 in acutely dissociated nociceptors but responsibility shifts to NaV1.7 and NaV1.3 by the fourth day in culture. A similar shift in NaV dependence occurs in vivo after inflammation, impacting ability of the NaV1.7-selective inhibitor PF-05089771 to reduce pain in behavioral tests. Flexible use of different NaV subtypes exemplifies degeneracy – achieving similar function using different components – and compromises reliable modulation of nociceptor excitability by subtype-selective inhibitors. Identifying the dominant NaV subtype to predict drug efficacy is not trivial. Degeneracy at the cellular level must be considered when choosing drug targets at the molecular level.
-
- Neuroscience
Despite substantial progress in mapping the trajectory of network plasticity resulting from focal ischemic stroke, the extent and nature of changes in neuronal excitability and activity within the peri-infarct cortex of mice remains poorly defined. Most of the available data have been acquired from anesthetized animals, acute tissue slices, or infer changes in excitability from immunoassays on extracted tissue, and thus may not reflect cortical activity dynamics in the intact cortex of an awake animal. Here, in vivo two-photon calcium imaging in awake, behaving mice was used to longitudinally track cortical activity, network functional connectivity, and neural assembly architecture for 2 months following photothrombotic stroke targeting the forelimb somatosensory cortex. Sensorimotor recovery was tracked over the weeks following stroke, allowing us to relate network changes to behavior. Our data revealed spatially restricted but long-lasting alterations in somatosensory neural network function and connectivity. Specifically, we demonstrate significant and long-lasting disruptions in neural assembly architecture concurrent with a deficit in functional connectivity between individual neurons. Reductions in neuronal spiking in peri-infarct cortex were transient but predictive of impairment in skilled locomotion measured in the tapered beam task. Notably, altered neural networks were highly localized, with assembly architecture and neural connectivity relatively unaltered a short distance from the peri-infarct cortex, even in regions within ‘remapped’ forelimb functional representations identified using mesoscale imaging with anaesthetized preparations 8 weeks after stroke. Thus, using longitudinal two-photon microscopy in awake animals, these data show a complex spatiotemporal relationship between peri-infarct neuronal network function and behavioral recovery. Moreover, the data highlight an apparent disconnect between dramatic functional remapping identified using strong sensory stimulation in anaesthetized mice compared to more subtle and spatially restricted changes in individual neuron and local network function in awake mice during stroke recovery.






