INTRODUCTION
Population explosion is one of the major problems in developing countries like India that would bump up to about 9.2 billion by the year 2050 (Solakhia et al., 2019). Therefore, measures have been taken to overcome this problem by developing antifertility agents called contraceptives. These are chemical substances which are used to check pregnancy. A good number of synthetic contraceptives are available in the market, but due to the adverse effects produced by synthetic steroidal contraceptives, herbal medicine has been gaining ground faster during the last few decades that are cheap, potential, efficient, and have lesser side effects (Bala et al., 2014).
Plants have been the basis for medicinal practice since prehistoric times and are still widely used (Chevallier, 1996; Fomina, 2014). Many plants or their extracts have been used traditionally by the local tribes as antifertility agents. Persicaria hydropiper (L.) Delarbre is one such plant used as antifertility agents. Persicaria hydropiper is a member of the family Polygonaceae. It is a widely grown weed in the northeastern states of India. It is commonly known as water pepper and locally known as Patharua bihalagani in Assam, a state of India (P. hydropiper 2009). It is an annual herb with an upright stem and grows to a height of 20–70 cm (USDA, 2020). The leaves are alternate and almost stalkless (USDA, 2020). It is a warm or wet season annual herb, occurring wherever there is moist soil or standing water. It grows on poorly drained agricultural land, ditches, puddles, along creeks, rivers, canals, and stream banks, in marshes and swamps, in poorly drained hollows, and in seasonally flooded areas. Persicaria hydropiper is globally distributed through many parts of Europe, the Mediterranean, temperate and tropical Asia, and Australia (USDA, 2020). In Assam, P. hydropiper is commonly found in both the plains and hilly areas.
Persicaria hydropiper has been traditionally used for its medicinal purposes. In Europe, P. hydropiper has been used to treat kidney problem and to regulate menstrual cycle irregularities (Huq et al., 2014). The dried powder of the P. hydropiper root is used by the Mishing women of Assam to prevent pregnancy (Hazarika and Sarma 2006a, 2006b). If this plant extract is used continuously for a longer period, it might cause permanent sterility in women (Hazarika and Sarma 2006a, 2006b). The leaf juice of P. hydropiper is also used in curing uterine disorders by the people of Lakhimpur, Assam, India (Choudhary et al., 2011). In Bangladesh, Garo tribes use the juice of the leaf for the treatment of menstrual pain and the paste of the leaf is used to stop the bleeding (Hasan et al., 2017). The whole plant, either alone or mixed with other herbs, is also used for the treatment of diarrhea, skin itching, heavy, painful menstruation, and hemorrhoids (Jan et al., 2020). Although the plant has medicinal value along with its traditional use in controlling pregnancy, there is little knowledge and scientific data on the effect of this plant in Assam. However, a detailed study on the effect of this traditional herbal extract on reproductive physiology is yet to be carried out. The present work investigates the effect of methanolic root extract of P. hydropiper on the weight and histoarchitecture of the ovary and uterus of albino mice. This study aims to investigate in detail about the ovarian follicular count, endometrial thickness, luminal diameter, and number of endometrial glands to know the antifertility efficacy of the P. hydropiper plant.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals and reagents used in the current study were highly purified and purchased from different reputed chemical companies. 17β-estradiol (molecular formula: C18H24O2; CAS No. 50-28-2) was purchased from Merck Limited, Mumbai, India, and sesame oil (CAS No. 8008-74-0, Cat No. S3547) was obtained from Sigma Aldrich, USA. All other chemicals used in the histological preparation were purchased from North East Chemicals Corporation, Guwahati, Assam, India.
Collection of plant material and preparation of the extract
The plant was identified and authenticated as P. hydropiper (L.) Delarbre (specimen voucher number: BSI/ERC/Tech/2018/229) by the Botanical Survey of India, Shillong. The roots of P. hydropiper were collected from Changsari area of Assam, India, in May 2017. The roots of P. hydropiper, after collection and proper washing, were cut into small pieces, shade-dried, and moderately powdered using a mixer grinder. The powdered roots (75 g) were macerated with methanol (300 ml) for 72 hours at room temperature. The resulting solution was then filtered with Whatman number 1 filter paper and a semisolid mass was obtained using a rotary evaporator at 40°C–50°C (yield = 2.9%). This semisolid mass was then reconstituted in distilled water so as to achieve the required concentration for carrying out all the pharmacological tests. The concentrate dissolved in distilled water, used for this study, was referred as methanolic P. hydropiper extract (MPHE).
Determination of doses
Two doses (300 and 600 mg/kg bwt/day) were administered to mice. A low dose of 300 mg/kg bwt/day and a high dose 600 mg/kg bwt/day were administered for a short (7 days) and long (15 days) duration. The dose selection was based on preliminary investigation and available literature (Hazarika and Sarma 2006a, 2006b; Maurya et al., 2004). The higher dose of 600 mg/kg bwt/day was selected in our study to evaluate a dose-dependent antifertility efficacy of P. hydropiper as minimum changes were observed in ovarian and uterine histology in the initial days of treatment. Akhter et al. (2013) reported that the oral administration of methanolic extract of P. hydropiper leaves up to 4,000 mg/kg bwt/day did not show any visible sign of toxicity or mortality in the animals. Furthermore, serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase activities were studied in the extract-treated group (MPHE300 and MPHE600) in our study following the method of Reitman and Frankel (1957) and no significant differences of activity of these liver enzymes were found as compared to the control animals. Along with the treated groups, a positive control group was also maintained to compare the results. The positive control group received 17β-estradiol (E2) (1 μg/kg bwt) for 7 and 15 days (Shah and Jhade 2018). E2 was dissolved in sesame oil and injected subcutaneously (Hazarika and Sarma, 2006a). The vehicle control group received sesame oil only.
Experimental animals
Laboratory-bred adult female mice (Mus musculus) aged 80–100 days, weighing between 25 and 28 gm, were used for the study. Necessary approval and ethical clearances were taken from the Institutional Ethical Committee of our university (Ref: IAEC/Per/2018/PP-IAEC/2018-50). The procedures for handling and care of animals comply with the Guide for the Care and Use of Laboratory Animals (National Research Council) and Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines. The animals were housed in polypropylene cages, bedded with paddy husk, and fed pelleted food and water ad libitum and were acclimatized to laboratory conditions before performing the experiment.
Study design
Animals were divided into eight groups consisting of five animals in each group (n = 5). The methanolic root extract of P. hydropiper (MPHE) was administered orally using orogastric tube to adult female mice at a dose of 300 mg/kg body weight/day and 600 mg/kg body weight/day for 7 days and 15 days. The control group received water and the vehicle control group received sesame oil only.
- Groups 1 and 2: Control and vehicle control groups;
- Group 3: Females receiving MPHE300 (300 mg/kg bwt) for 7 days;
- Group 4: Females receiving MPHE600 (600 mg/kg bwt) for 7 days;
- Group 5: Females receiving MPHE300 (300 mg/kg bwt) for 15 days;
- Group 6: Females receiving MPHE600 (600 mg/kg bwt) for 15 days;
- Group 7: Females receiving 17β-estradiol (1 μg/kg bwt/day) for 7 days;
- Group 8: Females receiving 17β-estradiol (1 μg/kg bwt/day) for 15 days.
Effect on organs and body weight
Individual weights of the animals were recorded throughout the study period. Animals were sacrificed by cervical dislocation. After sacrificing the animals, the uteri (with its luminal fluid intact) and the ovaries were dissected out, free of adhering fat, and were weighed using a weighing balance. The relative weights of the ovary and uterus were calculated for each animal as follows: [organ weight / body weight] × 100, as described by Kume et al. (2000).
Ovarian histology and ovarian follicular count
The histological study of ovarian tissue was carried out during the study to observe histological changes. The fixation of ovary tissue was done in 10% neutral buffered formalin, dehydrated in different grades of alcohol, and cleared in xylene. The embedding was made in paraffin wax. Sections were cut at a thickness of 4–5 μ and stained with hematoxylin and eosin.
Follicles were enumerated and categorized as primary, secondary, tertiary or antral, and preovulatory or Graafian follicles, according to the morphological classifications by Pedersen and Peters (1968) and Myers et al. (2004). The mean percentage of healthy follicles and atretic follicles were counted.
Histology and histomorphometry of the uterus
After sacrificing the animals, the uterine horns were freed of adhering fats. The histological slides were prepared like the ovary tissue. The thickness of uterine endometrium and myometrium, height of luminal epithelial cells, diameter of the lumen, and number of endometrial glands were determined using Labomed Lx400 Trinocular digital microscope with camera and touchscreen monitor at 4x objective magnification. The average of each parameter was calculated from the data of five uterine cross-sections per animal. The average thickness of endometrium and myometrium was calculated following the methodology of Markey et al. (2001) and Rossi et al. (2002).
Statistical analysis
The results were analyzed using IBM SPSS Statistics V.28.0 and t-test was carried out to check significance level. Values were recorded as mean ± SEM. p-values of less than 0.05 were considered significant.
RESULTS
Effect on organs and body weight
No significant change was observed in the body weight of the plant extract administered mice as compared to the control group. However, treatment with P. hydropiper root extract showed a significant dose-dependent (p < 0.01) decrease in the absolute and relative ovarian weight as compared to control group (Fig. 1). Treatment with a lower dose of MPHE (300 mg/kg bwt) showed a significant duration-dependent (p < 0.05) decrease in the absolute and relative uterine weight. However, a nonsignificant increase in the relative uterine weight was observed in the mice treated with MPHE600 (600 mg/kg bwt) for 7 days; but when administered for 15 days, the uterine wet weight and uterine body weight ratio was significantly (p < 0.01) reduced (Fig. 1). The E2-treated group showed a significant increase in both the uterine wet weight and ovarian wet weight, as well as the uterine/body weight ratio and ovary/body weight ratio after 7 days and 15 days treatment as compared to the vehicle control group.
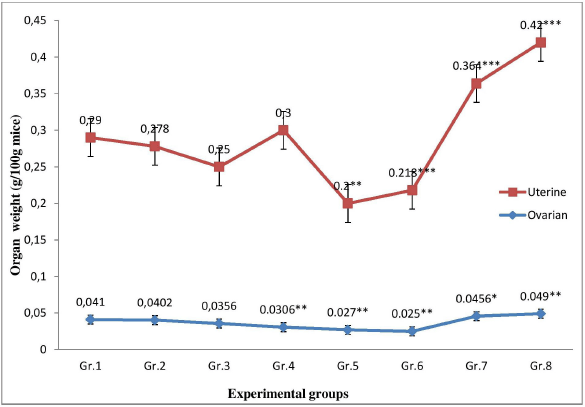 | Figure 1. Comparison of the ratio of uterine wet weight/body weight and ovarian wet weight/body weight in different experimental groups. [Click here to view] |
Effect on ovarian histology
The ovarian histology of control and vehicle control mice showed numerous healthy follicles at different stages of development. Healthy preantral, antral, Graafian follicles, fresh corpus luteum, and a few atretic follicles were present in the ovaries of the control group (Fig. 2A). However, mice treated with MPHE 300 mg/kg bwt/day showed degeneration of follicular cells, atrophied granulosa cells, atretic follicles, and cell debris in the antrum. These degenerative changes were more prominent in mice treated with MPHE300 for 15 days (Fig. 2C). The histological examination of the ovarian tissue sections of MPHE600-treated females (600 mg/kg bwt) showed a reduction in the number of ovarian follicles. Vacuolation of interstitial cells, luteal cells, and ooplasm in granulosa cells of pre-ovulatory follicle was observed (Fig. 2D and E). Granulosa cells of the preovulatory follicles were deeply stained and loosely connected to one another. Further changes include irregular stroma, disruption, and thinning of the zona pellucida. These results were more evident in the females treated with MPHE600 for 15 days.
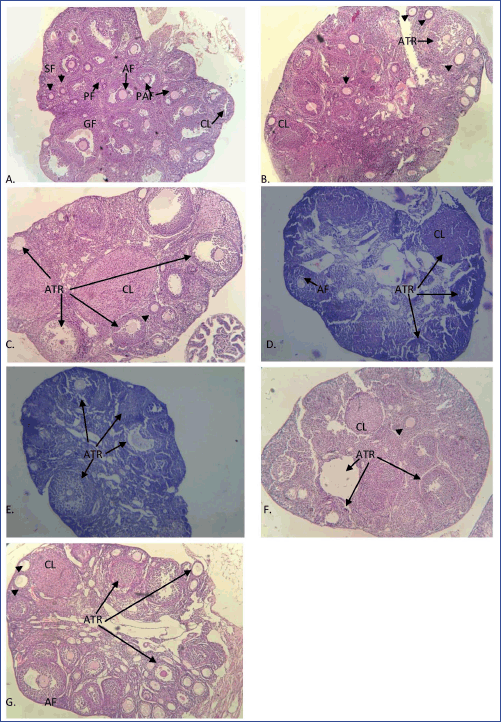 | Figure 2. Ovarian histological sections stained with hematoxylin and eosin in different groups: (A) control; (B) MPHE300 (7 days); (C) MPHE300 (15 days); (D) MPHE600 (7 days); (E) MPHE600 (15 days); (F) E2 (7 days); and (G) E2 (15 days) stained in H&E under 100× magnification showing primary follicle (PF; shown by arrow), secondary follicle (SF; shown by arrowhead), pre-antral follicle (PAF), antral follicle (AF), preovulatory (Graafian) follicle (GF), corpus luteum (CL), and atretric follicle (ATR). [Click here to view] |
The subcutaneous injection of E2 (1 μg/kg bwt) showed structural abnormalities in the ovarian histology of mice in comparison to the vehicle control and the root extract-treated animals (Fig. 2F and G). Degeneration of corpus luteum with frequent hemorrhage and cystic follicles was observed in these animals. E2 (1 μg/kg bwt daily) for 15 days caused the recruitment of follicles (Fig. 2G).
Effect on ovarian follicular count
Treatment with MPHE300 for 7 days (Group 3) showed an increase in the percentage of secondary follicles and atretic follicles with a decrease in the percentage of Graafian follicles (p<0.05) as compared to the control group (Table 1). Healthy primary and preantral follicles were seen frequently but antral follicles and Graafian follicles (p < 0.05) were less frequently observed in mice treated with MPHE300 for 7 days. In the mice treated with MPHE300 for 15 days (Group 5), the (PAFs) percentage of PAFs (p < 0.05), Graafian follicles (p < 0.01), fresh corpus luteum (p < 0.05), and total healthy follicles (p < 0.001) was significantly reduced, while the percentage of atretic follicles (p < 0.001) was significantly increased compared to the control and E2-treated groups. Mean percentage of primary follicles (p < 0.01), Graafian follicles (p < 0.01), fresh corpus luteum (p < 0.01), and total healthy follicles (p < 0.001) was significantly reduced in mice administered with MPHE600 for both 7 days and 15 days with a significant increase in the percentage of atretic follicles (p < 0.001) (Table 1).
In the E2-treated groups for 7 days (Group 7), the percentage of secondary follicles and atretic follicles was significantly increased with a decrease in the percentage of antral and Graafian follicles as compared to the vehicle control group. In the E2-treated groups for 15 days (Group 8), the percentage of secondary follicles and atretic follicles was significantly increased with a decrease in the percentage of Graafian and total healthy follicles (Table 1). The number of corpora lutea in the positive control group was significantly higher as compared to control and extract-treated groups.
Effect on histology and histomorphometry of the uterus
The histology of uterine tissue in control and vehicle control mice showed normal characteristics: single layered columnar epithelial cells lining the lumen, highly folded endometrial lumen, endometrium containing endometrial glands, and edematous stroma. The myometrium is composed of an inner circular smooth muscle (CM) and outer longitudinal smooth muscle layer (LM) and is covered by the perimetrium (Fig. 3A and B). Treatment with MPHE caused moderate to marked uterine atrophy in a dose-dependent manner that involved reduction in the height of luminal and glandular epithelial cells and thinning of the myometrium with reduction in the size of smooth muscle cells. Extensive folding of the lumen was absent in mice treated with MPHE300 for 15 days (Fig. 3E and F), MPHE600 for 7 days (Fig. 3G and H), and MPHE600 for 15 days (Fig. 3I and J). The vascular plexus between circular and longitudinal smooth muscle layer was also observed to be extensive. Endometrial glands forming conglomerates were observed in Groups 5 and 6. This was accompanied by decrease in the number of endometrial glands and presence of cystic glands. These changes were more prominent in mice administered with MPHE600 for 15 days. However, uterine histology of mice injected with E2 showed moderate to severe cystic endometrial hyperplasia characterized by an increased number of endometrial glands and dilation of endometrial glands, thereby increasing the thickness of the endometrial layer (Fig. 4). E2 administered for 15 days caused luminal dilation and hypertrophy of endometrial gland cell lining.
Histomorphometric study revealed a dose-dependent significant decrease in the thickness of CM, LM, and height of the luminal epithelium in all root extract-treated groups (Table 2). No significant changes were observed in the thickness of endometrium, CM, LM, and height of luminal epithelium, but the diameter of lumen was significantly decreased (p < 0.01) in Group 3. In Group 4, the thickness of the endometrium and diameter of the lumen were significantly increased as compared to control and other experimental groups. Animals in Groups 5 and 6 showed a dose-dependent significant reduction in the thickness of endometrium, smooth circular muscle, diameter of the lumen, and height of the luminal epithelium (Table 2). On the other hand, the administration of E2 (in Groups 7 and 8) caused a duration-based significant increase in the thickness of endometrium, diameter of the lumen, and height of luminal epithelial cell (Table 2) as compared to the vehicle control group (IB). However, these changes were more evident in Group 8.
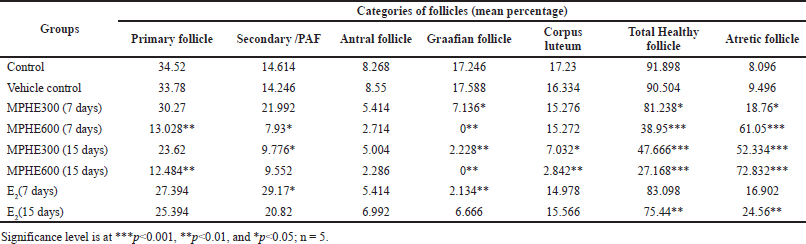 | Table 1. Follicular counts (in percentage) of different groups. [Click here to view] |
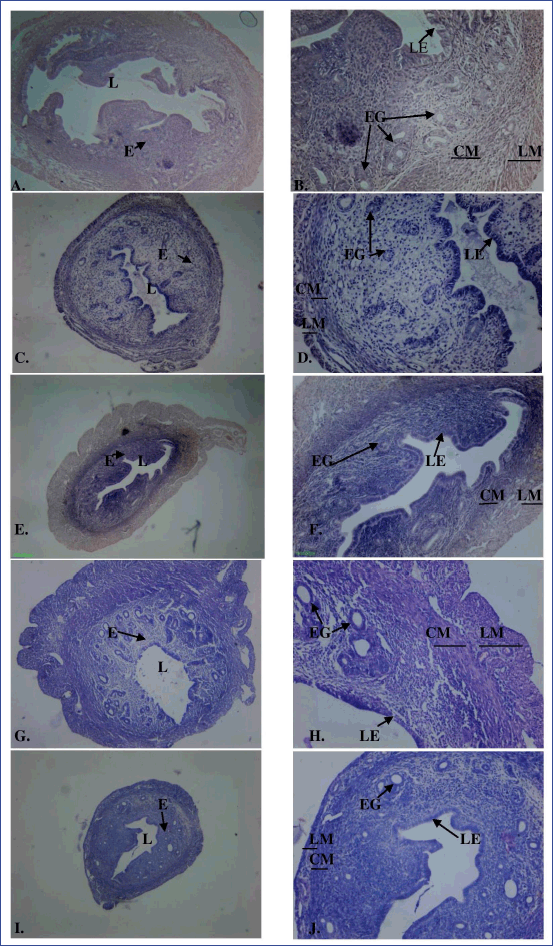 | Figure 3. Cross-section of the uterus of the control (A: 40× magnification; B: 100× magnification), MPHE 300 mg/kg body wt/day for 7 (C: 40× magnification; D: 100× magnification) and 15 days (E: 40× magnification; F: 100× magnification) and MPHE 600 mg/kg body wt/day for 7 (G: 40× magnification; H: 100× magnification) and 15 days (I: 40× magnification; J: 100x magnification) stained with hematoxylin and eosin, showing endometrium (E), circular smooth muscle (CM), longitudinal smooth muscle (LM), lumen (L), luminal epithelium (LE), and endometrial gland (EG). [Click here to view] |
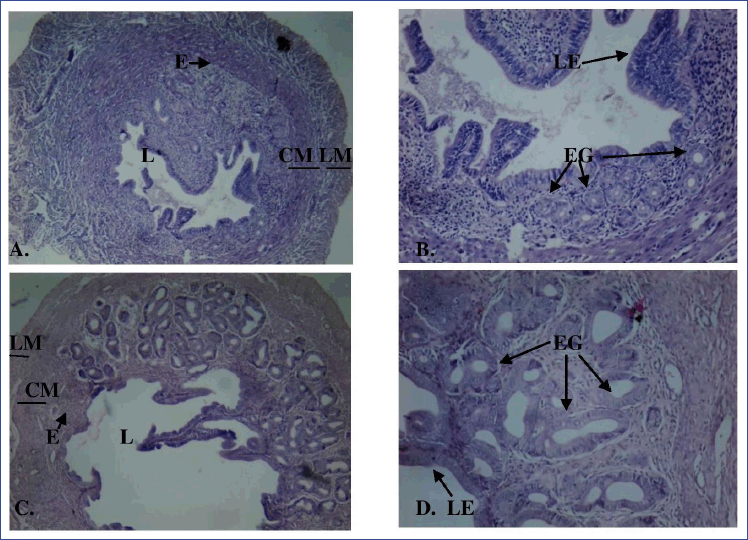 | Figure 4. Cross-section of the uterus of E2-injected mice for 7 days (A: 40× magnification; B: 100× magnification) and 15 days (C: 40× magnification; D: 100× magnification) showing endometrium (E), circular smooth muscle (CM), longitudinal smooth muscle (LM), lumen (L), luminal epithelium (LE), and endometrial gland (EG) stained with hematoxylin and eosin. [Click here to view] |
Results from Table 3 show a dose-dependent significant decrease (p < 0.05) in the number of endometrial glands in Groups 3–6 as compared to control and positive control groups. However, the number of endometrial glands in Groups 7 and 8 were significantly (p < 0.01) increased.
DISCUSSION
In this study, no significant change in the body weight of the mice was observed after the administration of methanolic root extract of P. hydropiper for 7 and 15 days at both doses. However, a significant reduction in ovarian and uterine weight was observed in extract-treated mice (at both low and high doses). Since the body weight of the extract-treated mice did not alter as compared to the controls, the histomorphological changes observed in the ovaries and uteri of the treated female mice may be attributed to the effect of the extract itself on the reproductive system. The dose-dependent significant decrease observed in the weight of the ovary and uterus after treatment of the female mice with P. hydropiper root extract at these dose levels may be due to the absence or reduced availability of ovarian hormones and gonadotropins (Sarita, 2018; Solomon et al., 2010). Also, the significant reduction in the uterine weight may be due to weak estrogenic and strong antiestrogenic effects (Sarita, 2018) of the extract at the doses used in the present study. The root extract either directly acts on the uterus as an estrogen antagonist or indirectly by disrupting the hormonal balance (Abdoon et al., 2014). Mutreja et al. (2008) reported a significant decrease in uterine and ovarian weight as a result of antiestrogenic activity of Nelumbo nucifera seed extract. However, a nonsignificant increase in the uterine weight was recorded in the mice treated with P. hydropiper root extract at high dose for 7 days. This may be due to an increase in the thickness of endometrium and dilation of the lumen. The uterine and ovarian wet weights were increased in the E2-treated groups. This may be due to the synergistic effect of exogenous estradiol.
The number of ovarian follicles in the ovary is an indication of the degree of ovarian activity (Ataman and Sakpa, 2017). The significant decrease in the number of corpus luteum in the MPHE-treated groups may be due to inhibition of the process of conversion of the preovulatory follicles into corpus luteum, thus arresting ovulation (Sarita, 2018). Solomon et al. (2010) reported a significant decrease in the number of corpus luteum as a result of antifertility effect of Rumex steudelii root extract. Atretic follicles are degenerating preovulatory follicles that take place due to disruption in the growth and differentiation of these follicles (Shivalingappa et al., 2002). The presence of numerous atretic follicles in the ovary of MPHE-treated mice is due to the disruption of growth and differentiation in the follicles. The disruption in the growth and differentiation of preovulatory follicles may be due to the nonavailability of either steroidal hormones or local estrogen produced by granulosa cells or due to imbalanced endogenous steroid (Monima et al., 2019; Shivalingappa et al., 2002). The decrease in the number of Graafian follicles and concomitant increased number of atretic follicles in the present study may be due to the nonavailability of required concentration of gonadotropins (Muchtaromah et al., 2015). This reduction in the number of Graafian follicle indicates that P. hydropiper, for both low and high doses, promotes degeneration of preovulatory follicles. Similar results were reported for Phyllanthus amarus and Caesalpinia bonducella (Ataman et al., 2017; Lilaram et al., 2012).
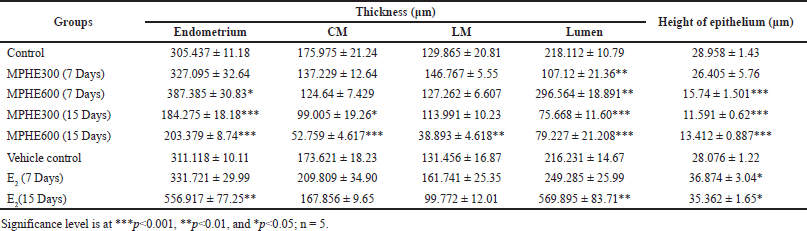 | Table 2. Thickness of the endometrium, CM, LM, lumen, and height of luminal epithelium (mean ± SEM). [Click here to view] |
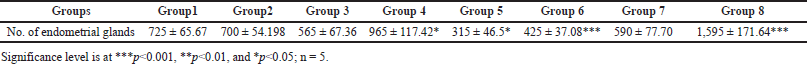 | Table 3. Effect of P. hydropiper methanolic root extract on a number of endometrial glands (mean ± SEM). [Click here to view] |
Uterine growth depends on the availability of ovarian steroid hormones, especially estrogens (Findlay, 1994; Hakameri et al., 2020; Sharanabasappa et al., 2002). In the present study, the root extract caused an atrophic effect on the uterine tissue as revealed by a significant reduction in endometrial thickness, luminal epithelial cell height, diameter of the lumen, disarrangement of the endometrium, and loss of endometrial glands. These uterine histomorphological changes observed in the mice administered with P. hydropiper may be due to inhibition of circulating endogenous estrogen (Dolatabad et al., 2014). The root extract either directly acts as an estrogen antagonist or indirectly acts by disrupting the hormonal balance (Abdoon et al., 2014). Mandal et al. (2007) reported a significant decrease in the height of luminal epithelium, number and size of uterine glands, and thickness of longitudinal and circular muscle layer as a result of antifertility effect of Melia azedarach Linn. seed extract in female albino rats. Similar results have been obtained with Plumeria rubra Linn. and Calina papaya leaf extracts (Dabhadkar and Zade 2012; Setiawan et al., 2021). Asuquo et al. (2013) reported distortion of the endometrial epithelium and reduction in the number of uterine glands in Wistar rats when treated with ethanolic leaf extract of Spondias mombin at a dose of 500 mg/kg body. In case of the mice treated with E2, the presence of irregular and cystic dilation of endometrial glands may be due to prolonged estrogen administration, which is indicated by increase in number of dilated or irregular cystic glands, which are lined by hyperchromatic cuboidal or columnar cells (Wangikar et al., 2011).
CONCLUSION
The present study concludes that the methanolic root extract of P. hydropiper may enact the antifertility effect by inhibiting ovarian function, reducing ovary and uterine weight, and disrupting ovarian folliculogenesis. Moreover, P. hydropiper may block ovulation by reducing the number of Graafian follicles and concomitantly increasing the number of atretic follicles. In addition, the extract has an anti-uterotrophic effect that caused the endometrium to be unfavorable for the implantation of the fertilized ovum, rendering an antifertility effect. The root extract is found to be safe at the effective doses (300 and 600 mg/kg bwt) used in this study. However, further research on the mechanism of action and isolation of the active compound(s) responsible for the antifertility effect needs to be conducted.
LIST OF ABBREVIATIONS
Bwt, body weight; CM, Circular muscle; E2, 17β-estradiol; LM, Longitudinal muscle; MPHE, Methanolic Persicaria hydropiper extract
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data or analysis, and interpretation of data; drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors guidelines.
ACKNOWLEDGMENTS
The authors are thankful to the Department of Zoology, Gauhati University and Institutional Biotech Hub of B. Borooah College for providing the necessary facilities for conducting the study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
Necessary approval and ethical clearances were taken from the Institutional Ethical Committee of our university (Ref: IAEC/Per/2018/PP-IAEC/2018-50) on the use and care of experimental animals before conducting any of the experiments. All experiments performed on animals complied with the Guide for the Care and Use of Laboratory Animals and ARRIVE guidelines.
FUNDING SOURCES
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdoon ASS, Abdel-Rahman HA, Kandil OM, Al-Sagair OA, Mohamed AA. Effect of Salvadora persica (Miswak) leaves and stem aqueous extracts on ovarian folliculogenesis and uterine histology in female albino rats. Int J Phytomed, 2014; 6(3):346–53.
Akhter R, Haque MA, Bhuiyan MA, Shahriar M. In vivo Pharmacological investigation of leaf of Polygonum hydropiper (L.). Dhaka Univ J Pharm Sci, 2014; 12(2):165–9. CrossRef
Asuquo OR, Oko OOK, Brownson ES, Umoetuk GB, Utin IS. Effects of ethanolic leaf extract of Spondias mombin on the pituitary-gonadal axis of female Wistar rats. Asian Pac J Reprod, 2013; 2(3):169–73. CrossRef
Ataman JE, Sakpa CL. Effects of ethanolic leaf extract of Phyllanthus amarus (Schum AandThonn) on ovarian morphology and reproductive parameters in wistar rats. Afr Sci, 2017; 18(4):245–51.
Bala K, Arya M, Katare DP. Herbal contraceptive: an overview. World J Pharm Pharm Sci, 2014; 3(8):1305–26.
Chevallier A. The encyclopedia of medicinal plants. Dorling Kindersley, London, UK, 1996.
Choudhary RK, Oh S, Lee J. An ethnomedicinal inventory of knotweeds of Indian Himalaya. J Med Plant Res, 2011; 5(10):2095–103.
Dabhadkar D, Zade V. Abortifacient activity of Plumeria rubra (Linn) pod extract in female albino rats. Indian J Exp Biol, 2012; 50(10):702–7.
Dolatabad SS, Rostami FF, Khaki A. Antifertility activity of methanolic extract of Gossypium herbaceum in female rats. Baltica, 2014; 27(1):307–15.
Findlay JK. Molecular biology of the female reproductive system. Academic Press, San Diego, CA, p 457, 1994.
Flowers of India- Persicaria hydropiper. Available via https://www.flowersofindia.net/catalog/slides/Water%20Pepper.html (Accessed 12 June 2020).
Fomina TN. Medicinal plants and their importance. Russian State Agrarian University, Moscow, Russia, 2014.
Hakameri CS, Tofrizal, Usman E. Effect of giving young Papaya (Carica papaya L.) fruit extract on endometrial histology of female rats (Rattus norvegicus). Sci Midwifery, 2020; 9(1), Oktober):181–6.
Hasan M, Habib SA, Alam J, Islam SMN, Farazi M, Khan HA, Rashid HA. Pharmacological and toxicological aspects of Persicaria hydropiper (L.) Delarbre of bangladeshi medicinal plant used by folk medicine practitioners. Int J Phytopharm, 2017; 8(1):1–7.
Hazarika A, Sarma HN. Polygonum hydropiper crude root extract mimics estrogenic properties in females: evidence of uterine protein profiles studied by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Reprod Med Biol, 2006b; 5:155–60. CrossRef
Hazarika A, Sarma HN. The estrogenic effects of Polygonum hydropiper Linn. root extract induces follicular recruitment and endometrial hyperplasia in female albino rat. Contraception, 2006a; 74(5):426–34. CrossRef
Huq AKMM, Jamal JA, Stanslas J. Ethnobotanical, Phytochemical, pharmacological, and toxicological aspects of Persicaria hydropiper (L.) Delarbre. Evid Based Complement Alternat Med, 2014; 782830:1–11. CrossRef
Jan M, Mir TA, Khare RK. Indigenous medicinal usage of family Solanaceae and Polygonaceae in Uri, Baramulla, Jammu and Kashmir. J Med Herbs Ethnomed, 2020; 6:086–9 CrossRef
Kume G, Yamaguchi A, Aoki I, Taniuchi T. Reproductive biology of the cardinalfish Apogon lineatus in Tokyo Bay, Japan. Fisheries Sci, 2000; 66:947–4. CrossRef
Lilaram, Ahmed RN. Effect of ethanolic seed extract of Caesalpinia bonducella on female reproductive system of albino rat: a focus on antifertilitefficacy. Asian Pac J Trop Dis, 2012; 2:S957–62. CrossRef
Mandal R, Dhaliwal PK. Antifertility effect of Melia azedarach Linn. (dharek) seed extract in female albino rats. Indian J Exp Biol, 2007; 45(10):853–60.
Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect, 2001; 109(1):55–60. CrossRef
Maurya R, Srivastava S, Kulshreshta DK, Gupta CM. Traditional remedies for fertility regulation. Curr Med Chem, 2004; 11(11):1431–50. CrossRef
Monima LA, Buhari M, Lawal S, Isaac E, Fred S, Elna O, Edmund B, Diaz ME, Bassey AV, Ikwap K. Effect of Cleome gynandra leaf extract on the estrous cycle and histology of the ovary and uterus of Wistar albino rats. Anat J Afr, 2019; 8(1):1385–94. CrossRef
Muchtaromah B, Romaidi R, Griani TP, Hasfita Y. Potencial antifertility of Centella asiatica leaf extract. Aust J Basic Appl Sci, 2015; 7(9):102–5.
Mutreja A, Agarwal M, Kushawaha S and Chauhan A. Effect of Nelumbo nucifera seeds on the reproductive organs of female rats. Iran J Reprod Med, 2008; 6(1):7–11.
Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction, 2004; 127(5):569–80. CrossRef
Pedersen T, Peters H. Proposal for a classification of oocyte and follicles in the mouse ovary. J Reprod Fert, 1968; 17(3):555–7. CrossRef
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 1957; 28(1):56–63. CrossRef
Rossi AGZ, Soares Jr.JM, Motta ELA, Simoes MJ, Oliveira-Filho RM, Haidar MA, Lima GRD, Baracat EC. Metoclopramide-Induced Hyperprolactinemia Affects Mouse Endometrial Morphology. Gynecol Obstet Invest, 2002; 54(4):185–90. CrossRef
Sarita M. Antifertility activity of Eugenia jambolana seed extract in female albino rat. Biochem Physiol, 2018; 7(2):237.
Setiawan H, Wulandari SW, Nurwidyantary FE, Dewantari I. The effects of Calina papaya leaf ethanol extract on estrus cycle and uterus morphology of wistar rats. Biosaintifika: J Biol Biol Educ, 2021; 13(3):305–12. CrossRef
Shah SK, Jhade DN. Evaluation of antifertility potential of Piper betle (Petiole) on female wistar rats “rising approaches of herbal contraception’’. Biochem Biophys Rep, 2018; 15:97–102. CrossRef
Sharanabasappa A, Vijaykumar B, Sarasweit B. Effect of Momordica charantia seed extracts on ovarian and uterine activities in albino rats. Pharm Biol, 2002; 40(7):501–7. CrossRef
Shivalingappa H, Satyanarayan ND, Purohit MG, Sharanabasappa A, Patil SB. Effect of ethanol extract of Rivea hypocrateriformis on the estrous cycle of the rat. J Ethnopharmacol, 2002; 82(1):11–7.
Solakhia R, Gupta J, Parashar B. Herbal contraceptives: anti-fertility activity of herbal plants. Int J Health Clin Res, 2019; 2(4):5–10. CrossRef
Solomon T, Largesse Z, Mekbeb A, Eyasu M, Asfaw D. Effect of Rumex steudelii methanolic root extract on ovarian folliculogenesis and uterine histology in female albino rats. Afr Health Sci, 2010; 10(4):353–61.
USDA, Agricultural Research Service, National Plant Germplasm System, 2020. Available via https://npgsweb.arsgrin.gov/gringlobal/taxon/taxonomydetail?id=400981 (Accessed 12 June 2020).
Wangikar P, Ahmed T, Vangala S. Toxicologic pathology of the reproductive system. In Gupta R (ed.). Reproductive and developmental toxicology, Elsevier Inc., Amsterdam, The Netherlands, pp 1003–26, 2011. CrossRef