INTRODUCTION
Andrographolide (ANDR), having the chemical structure as shown in Figure 1, is a member of diterpenoid compounds, mainly isolated from A. paniculata belonging to the Acanthaceae family. This plant is known as the “King of Bitters.” In Indonesia, A. paniculata is known as “Sambiloto,” one of the medicinal plants extensively studied because of some beneficial health effects (Akowuah et al., 2009). In traditional medicine, especially in Asian countries, Sambiloto is widely used to treat fever, cold, laryngitis, and infections. The extracts and fractions of Sambiloto containing ANDR have been evaluated for the biological activities including antioxidant (Akowuah et al., 2008), hepatoprotector from cell death induced by hydrogen peroxide (Mittal et al., 2016), carbon tetrachloride (Chen et al., 2014), inducer of glutathione S-transferase pi class (Lu et al., 2011), inhibitor of inflammatory responses in lipopolysaccaride-stimulated macrophages (Kim et al., 2019), and to have antidiabetic activities (Xu et al., 2012). These activities are correlated with phytochemical contents present in A. paniculata, mainly ANDR; therefore, analytical methods capable of a fast and reliable technique for the determination of ANDR are continuously developed, validated, and applied in any type of sample matrices.
 | Figure 1. The chemical structure of andrographolide (ANDR). [Click here to view] |
Several methods have been applied for quantitative analysis of ANDR, and the most reported ones are chromatographic-based methods. High-performance liquid chromatography (HPLC) using detector UV 224 nm has been used for purity analysis of ANDR and ANDR quantification in bulk materials (Indrati et al., 2018) and HPLC using detector UV 210 nm has been used for the analysis of ANDR in methanol extract of A. paniculata. Liquid chromatography-mass spectrometry has also been widely used for the analysis of ANDR in extracts and biological fluids (Gu et al., 2007; Sajeeb et al., 2015; Xu et al., 2009; Zhang and Fan, 2012). The other methods used for ANDR quantification are thin layer chromatography (Akowuah et al., 2006), electrokinetic chromatographic (MEEKC) method (Yanfang et al., 2006), and proton NMR-spectroscopy (Yang et al., 2012). These methods involve sophisticated instruments, complex sample preparation, and skillful analyst; therefore, a reliable method offering accurate and precise results based on Fourier transform infrared (FTIR) spectra could be developed as an alternative method for the determination of ANDR.
FTIR spectra were reported for characterization of vibrational properties of ANDR extracted from A. paniculata (Singh et al., 2006) and for confirmation and qualitative analysis of ANDR. FTIR spectroscopy has been successfully used for the analysis of total lactones in dried and powdered A. paniculata (Shivali et al., 2012). To the best of our knowledge, FTIR spectra in conjunction with chemometrics of multivariate analysis for quantitative analysis of ANDR have not been reported as yet (Indrati et al., 2018). Therefore, in the present research, FTIR spectra assisted with Partial least square regression (PLSR) was used for the prediction of ANDR. The levels of ANDR quantified by HPLC were used as actual values to be correlated with predicted values obtained from FTIR spectra with the aid of multivariate calibration.
MATERIALS AND METHODS
Materials
The samples of A. paniculata herbs (15 samples) were obtained from several regions in Daerah Istimewa Yogyakarta, West Java, and Central Java (Bantul, Sleman, Kulon Progo, Semarang, Boyolali, and Bogor), Indonesia. The plant identification was carried out in the Laboratory of Pharmacognosy and Phytochemistry, Faculty of Pharmacy, UGM, Yogyakarta. The reference standards of ANDR and methanol HPLC grade were purchased from Merck (Darmstadt, Germany). Water for injection was bought from Ikapharmindo (Indonesia). The chemicals used for the analysis were of a pro analytical grade.
Samples’ extraction
The herbs of A. paniculata were cleaned and chopped into pieces. The chopped herbs were dried in a conventional oven for 24 hours. The dried herbs were grounded into a powder. The extraction method used was maceration by weighing 5 g of powdered samples from each region using an analytical balance with a sensitivity of 0.1 mg (Mettler Toledo). The powders were macerated with 50 ml of absolute ethanol pro analytical grade for 24 hours. Filtration was carried out to obtain a liquid extract and then ethanol was added to a volumetric flask of 50.0 ml. This sample solution was analyzed using HPLC.
Analysis of ANDR using HPLC
The reference standards of ANDR were dissolved in methanol HPLC grade to get stock solution with a concentration of 1,000 μg/ml. A series of working solutions in certain concentration ranges (25, 50, 75, 100, and 125 μg/ml) were also prepared from the stock solution. Sample solutions were prepared by transferring 1 ml sample extract into a volumetric flask of 10 ml, filled until 10 mL with ethanol and filtered with 0.45 μm filter before being subjected for injection into LC chromatograph. HPLC method for the analysis of reference standards and sample solutions was carried out according to Syukri et al. (2016). HPLC analysis of ANDR was carried out using chromatograph Shimadzu LC-20AD (Kyoto, Japan) equipped with binary gradient pump using injection valve of Rheodyne 7725i with 20 μl loop. HPLC separation was carried out on Cosmosil C18 column (250 × 4.6 mm, 5 μm) using a mobile phase of methanol and water (6 : 4 v/v) and delivered isocratically at a flow rate of 0.8 ml/minutes. The injection volume and wavelength of the wavelength detector were 20 μl and 229 nm.
FTIR spectra measurement
The measurement of FTIR spectra was carried out according to Irnawati et al. (2020b). The powdered A. paniculata samples were placed on a Smart iTR™ attenuated total reflectance at a mid-infrared region of 4,000–650 cm−1, recorded for 32 scans at a resolution of 8 cm−1.
Data analysis
The multivariate calibrations were carried out through chemometric software of TQ Analyst® software version 9 (Thermo Fisher Scientific, Inc., Waltham, MA). The multivariate calibrations used were PLSR and principal component regression (PCR). HPLC data were used as the actual values and FTIR data were used as a predicted value. The selection of wavenumber regions is based on its capability of giving high coefficient of determination (R2) and low values of errors, either Root mean square error of calibration (RMSEC) or Root mean square error of prediction (RMSEP).
RESULTS AND DISCUSSION
In the present research, FTIR spectra combined with multivariate calibrations were used for the quantification of ANDR in herbs of A. paniculata. As its property as fingerprint technique, FTIR spectra could be used for selecting specific peaks corresponding to target analytes (ANDR). But, FTIR spectra used as tools for the analysis of ANDR in herbs are nonstandard methods; therefore, the actual values of analytes must be determined using a reference method, namely, HPLC. Quantification of ANDR using HPLC was carried out using external calibration by preparing the linearity curve correlating between concentrations of ANDR (x-axis) and peak area or area under curve (y-axis). The linearity was obtained from five concentrations of standard solutions (25, 50, 75, 100, and 125 μg/ml). From the calibration plot in Figure 2, the (R2) value for ANDR was 0.99998, indicating a good linearity with the following equation: y = 50,513.92 × −16,180.4.
HPLC, a reference method for analysis of analyte of interest, was utilized for the quantitative estimation of ANDR. Standard and samples showed similar retention time values. Figure 3 shows the HPLC chromatogram, either in ANDR obtained from Sigma-Aldrich (at a concentration of 75 μg/ml) with retention time 7.140 minutes or in ethanolic extract of A. paniculata (AP3) with the retention time of 7.121 minutes (data were not shown). An analyte can be characterized by its retention time, which is not affected by the quantity of injected samples. Table 1 shows the concentrations of analyte (ANDR) in some samples of ethanolic extracts of the A. paniculata herb in some regions. ANDR contents in the analyzed extracts were diverse, mainly due to the differences in season of cultivation, region, age, and time of harvesting (Hossain et al., 2014). The concentrations of ANDR were used as actual values to be correlated with ANDR contents predicted by the FTIR method facilitated with two multivariate calibrations of PCR and PLSR.
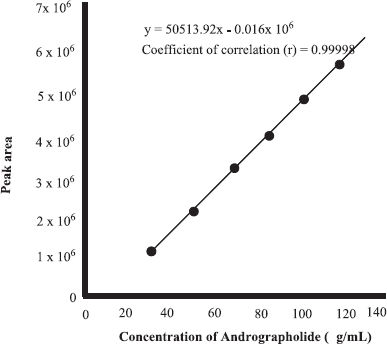 | Figure 2. Linear regression curve for correlation between the concentration of andrographolide and area under the curve, as analyzed using HPLC. [Click here to view] |
Figure 4 shows the FTIR spectra of dried powder of A. paniculata herb from different regions. The main component present in A. paniculata is ANDR. The peak and shoulders shown have originated from the functional groups’' absorption present in the evaluated samples. From the analysis, the FTIR spectra showed similar peaks, which can be interpreted as a similar profile in chemical components. The differences in peak intensities caused by different levels of chemical contents could be seen in the dried powders. The peak at (a) 3,286 cm−1 may be due to the presence of stretching vibration of the O-H bond. The peaks at (b) 2,919 and (c) 2,851 cm−1 originated from stretching vibrations of C-H. The group of C=O was observed at (d) 1,731 cm−1 with stretching vibration mode, while the peaks at (e) 1,605 and (f) 1,416 cm−1 were coming from C=C alkenes and bending vibration of CH2, respectively. The peaks at (g) 1,320 and (h) 1,239 cm−1 were originating from C–O in stretching vibration mode. The peak at (i) 1,030 cm−1 may be due to the presence of amine C–N stretching vibration (Lestari et al., 2017).
 | Figure 3. HPLC chromatogram of andrographolide at a concentration of 75 μg/ml. HPLC condition, column: Cosmosil C18 column (250 mm × 4.6 mm, 5 μm); mobile phase: methanol : water (60 : 40); flow rate: 0.8 ml/minutes; injection volume: 20 μl; detector: ultraviolet 229 nm. [Click here to view] |
 | Table 1. Levels of andrographolide in herb of A. paniculata from several regions. [Click here to view] |
 | Figure 4. FTIR spectra of dried powder of A. paniculata herb from different regions scanned at midwavenumbers of 4,000–650 cm−1. Inset: the chemical structure of andrographolide. AP1–AP3 = A. paniculata herb from Bantul, AP4–AP6 and AP15 = A. paniculata herb from Sleman, AP7–AP9 = A. paniculata herb from Kulon Progo, AP10 and AP12 = A. paniculata herb from Semarang, AP11 and AP13 = A. paniculata herb from Boyolali, and AP14 = A. paniculata herb from Bogor. [Click here to view] |
Quantitative analysis of ANDR in herbs of A. paniculata can be difficult in FTIR spectroscopy due to the overlapping spectra of the molecules in the sample. FTIR spectra combined with multivariate calibrations of PCR and PLSR are useful for the quantitative analysis of analytes in complex mixtures (Rohman, 2014). In PLSR and PCR, the variables used during modeling was absorbance values at specific wavenumbers. The absorbance values were then combined to obtain principal components (PCs) and regressed toward actual values obtained by HPLC analysis
The wavenumbers used were selected based on some variations that existed, especially in peak intensities. The FTIR spectra in normal and derivatization modes were compared for modeling. The derivatization of FTIR spectra could make the overlapping peaks be more resolved, but the sensitivity was decreased (Irnawati et al., 2020a). To obtain the best prediction models, the optimizations in terms of the selection of wavenumber regions and the modes of FTIR spectra either in normal or in the first and the second derivatives were optimized (Rohman et al., 2015). The selection of optimization parameters was relied on its capability of giving high R2 and low values of errors, either in calibration models (RMSEC) or in prediction models called RMSEP. The lower errors indicated a more precise model, while the higher R2 value exhibited the more accurate developed models (Siregar et al., 2018).
Table 2 showed the optimization results of FTIR spectra combined with PLSR and PCR for quantitative analysis of ANDR using normal and derivative spectra at specific wavenumbers. These results were expressed by R2 and RMSEC and RMSEP values. After optimization, PLSR using variables of absorbance values at 3,700–665 cm−1 was finally chosen for the prediction of ANDR because this condition could give the highest R2 values of 0.9997 in the calibration model and 0.9765 in the validation model. The values of RMSEC and RMSEP were relatively low, that isi.e., 0.005% and 0.055%, respectively. These results exhibited that PLSR models offered good accuracy and precision (Miller and Miller, 2010).
 | Table 2. The performance of principal PCR and PLSR for quantitative analysis of A. paniculata herb. [Click here to view] |
 | Figure 5. The relationship between actual values (x-axis) of andrographolide and the predicted values of andrographolide (y-axis) in powdered samples of A. paniculata using FTIR spectroscopy (A) along with residual analysis (B.). [Click here to view] |
Figure 5(A) revealed the scatter plot which explains the correlation between actual (x-axis) and predicted values (y-axis) of ANDR in powder herbs as determined by HPLC and FTIR spectra with the aid of PLSR using the second derivative FTIR spectra at 3,700–665 cm−1. Figure 5(B) showed a residual analysis of the model, and it indicates the difference between the actual values and predicted values to see the error patterns; therefore, the error that occurred during modeling is negligible because all point differences between actual and predicted value falls above and below zero value. The model developed is reliable to predict ANDR content.
CONCLUSION
The combination of FTIR spectra and PLSR was successfully used for quantification of ANDR using derivative-2 FTIR spectra at 3,700–665 cm−1, with R2 for the correlation of actual values and FTIR predicted values of 0.9997 (calibration) and 0.9765 (validation), respectively, with RMSEC (0.005%) and RMSEP (0.055%). As a reference, the HPLC method is useful to determine the contents of ANDR in A. paniculata herb. The levels of ANDR quantified with HPLC were used as actual values during prediction with FTIR spectroscopy.
ACKNOWLEDGMENTS
The authors acknowledge the Ministry of Research, Technology and Higher Education, Republic of Indonesia, for financial support during this study through the World Class University Program of Universitas Gadjah Mada for financial support of this research by Riset Kolaborasi Indonesia Grant 2019 awarded to Prof. Dr. Abdul Rohman.
AUTHORS’ CONTRIBUTIONS
Hanifah Luthfianasari and Irnawati carried out the research activities, data acquisition, and analyzed data. Abdul Rohman, Sugeng Riyanto, Mohamad Rafi, Bambang Prajogo, Muhammad Bachri Amran designed the research, drafted the manuscript, and made critical thinking on the manuscript.
CONFLICT OF INTEREST
Authors declare that there are no conflicts of interest.
FUNDING
None.
REFERENCES
Akowuah GA, Zhari I, Mariam A. Analysis of urinary andrographolides and antioxidant status after oral administration of Andrographis paniculata leaf extract in rats. Food Chem Toxicol, 2008; 46(12):3616–20. CrossRef
Akowuah GA, Zhari I, Mariam A, Yam MF. Absorption of andrographolides from Andrographis paniculata and its effect on CCl4-induced oxidative stress in rats. Food Chem Toxicol, 2009; 47(9):2321–6. CrossRef
Akowuah GA, Zhari I, Norhayati I, Mariam A. HPLC and HPTLC densitometric determination of andrographolides and antioxidant potential of Andrographis paniculata. J Food Compost Anal, 2006; 19(2–3):118–26. CrossRef
Chen HW, Huang CS, Li CC, Lin AH, Huang YJ, Wang TS. Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol Appl Pharmacol, 2014; 280(1):1–9. CrossRef
Gu Y, Ma J, Liu Y, Chen B, Yao S. Determination of andrographolide in human plasma by high-performance liquid chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2007; 854(1):328–31. CrossRef
Hossain MS, Urbi Z, Sule A, Rahman KMH. Andrographis paniculata (Burm. f.) Wall. ex Nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J, 2014; 2014:1–8. CrossRef
Indrati O, Martien R, Rohman A, Nugroho AK. Employment of ATR-FTIR and HPLC-UV method for detection and quantification of andrographolide. Int J Appl Pharm, 2018; 10(6):135–8. CrossRef
Irnawati, Riyanto S, Martono S, Rohman A. Determination of sesame oil, rice bran oil and pumpkin seed oil in ternary mixtures using FTIR spectroscopy and multivariate calibrations. Food Res, 2020a; 4(1):135–42. CrossRef
Irnawati, Riyanto S, Martono S, Rohman A. The employment of FTIR spectroscopy and chemometrics for authentication of pumpkin seed oil from sesame oil. Food Res, 2020b; 4(1):42–8. CrossRef
Kim N, Lertnimitphun P, Jiang Y, Tan H, Zhou H, Lu Y. Andrographolide inhibits inflammatory responses in LPS-stimulated macrophages and murine acute colitis through activating AMPK. Biochem Pharmacol, 2019; 170:1136-46. CrossRef
Lestari HP, Martono S, Wulandari R, Rohman A. Simultaneous analysis of curcumin and demethoxycurcumin in Curcuma xanthorriza using FTIR spectroscopy and chemometrics. Int Food Res J, 2017; 24(5):2097–101.
Lu CY, Li CC, Lii CK, Yao HT, Liu KL, Tsai CW. Andrographolide-induced pi class of glutathione S-transferase gene expression via PI3K/Akt pathway in rat primary hepatocytes. Food Chem Toxicol, 2011; 49(1):281–9. CrossRef
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. Pearson Education, North York, ON, 2010.
Mittal SPK, Khole S, Jagadish N, Ghosh D, Gadgil V, Sinkar V. Andrographolide protects liver cells from H2O2 induced cell death by upregulation of Nrf-2/HO-1 mediated via adenosine A2a receptor signalling. Biochim Biophys Acta, 2016; 1860(11):2377–90. CrossRef
Rohman A. Spektroskopi inframerah dan kemometrika untuk analisis farmasi [Infrared spectroscopy and chemometrics for pharmaceutical analysis]. Pustaka Pelajar, Yogyakarta, Indonesia, 2014.
Rohman A, Sudjadi, Devi, Ramadhani D, Nugroho A. Analysis of curcumin in Curcuma longa and Curcuma xanthorriza using FTIR spectroscopy and chemometrics. Res J Med Plant, 2015; 9(4):179–86. CrossRef
Sajeeb BK, Kumar U, Halder S, Bachar SC. Identification and quantification of andrographolide from Andrographis paniculata (Burm. f.) Wall. ex Nees by RP-HPLC method and standardization of its market preparations. Dhaka Univ J Pharm Sci, 2015; 14(1):71–8. CrossRef
Shivali G, Praful L, Vijay G. A validated Fourier transform infrared spectroscopy method for quantification of total lactones in Inula racemosa and Andrographis paniculata. Phytochem Anal, 2012; 23(2):171–6. CrossRef
Singh PK, Hasan T, Prasad O, Sinha L, Raj K, Misra N. FT-IR spectra and vibrational spectroscopy of andrographolide. Spectroscopy, 2006; 20(5):275–83. CrossRef
Siregar C, Martono S, Rohman A. Application of Fourier transform infrared (FTIR) spectroscopy coupled with multivariate calibration for quantitative analysis of curcuminoid in tablet dosage form. J Appl Pharm Sci, 2018; 8(8):151–6. CrossRef
Syukri Y, Martien R, Lukitaningsih E, Nugroho AE. Quantification of andrographolide isolated from Andrographis paniculata nees obtained from traditional market in Yogyakarta using validated HPLC. Indones J Chem, 2016; 16(2):190–7. CrossRef
Xu J, Li Z, Cao M, Zhang H, Sun J, Zhao J. Synergetic effect of Andrographis paniculata polysaccharide on diabetic nephropathy with andrographolide. Int J Biol Macromol, 2012; 51(5):738–42. CrossRef
Xu L, Xiao DW, Lou S, Zou JJ, Zhu YB, Fan HW. A simple and sensitive HPLC-ESI-MS/MS method for the determination of andrographolide in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci, 2009; 877(5):502–6. CrossRef
Yanfang Z, Xingping L, Zongde Z, Liren C, Yongmin L. Simultaneous determination of andrographolide and dehydroandrographolide in Andrographis paniculata and Chinese medicinal preparations by microemulsion electrokinetic chromatography. J Pharm Biomed Anal, 2006; 40(1):157–61. CrossRef
Yang M, Wang J, Kong L. Quantitative analysis of four major diterpenoids in Andrographis paniculata by 1H NMR and its application for quality control of commercial preparations. J Pharm Biomed Anal, 2012; 70(11):87–93. CrossRef
Zhang SQ, Fan YM. Determination of andrograpolide sodium bisulphite in beagle dog plasma by LC-MS/MS and its application to pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci, 2012; 907(10):173–7.