Abstract
Context:
Hepatitis C virus (HCV) infection is a major cause of liver-morbidity and mortality among patients with thalassemia. Peginterferon plus ribavirin is currently the recommended therapy for hepatitis C infection in patients do not have thalassemia, but using ribavirin in patients with thalassemia is restricted due to its hemolytic effect. To evaluate the efficacy and safety of adding ribavirin to peginterferon or interferon, authors performed a systematic review on the available literatures.Evidence Acquisition:
Trials were identified through electronic database, manual searches of journals and bibliographies and approaching authors of trials. Randomized trials that enrolled patients with a diagnosis of thalassemia and chronic hepatitis C infection treated with interferon or peginterferon with or without ribavirin were included. Two investigators independently evaluated the trials for inclusion criteria, risk of bias and data extraction. The primary outcomes were sustained virological response (SVR), liver-related morbidity, mortality and adverse events. The odds ratios from each trial were calculated individually and in the subgroup analysis of trials. Data were analyzed with fixed-effect model.Results:
Three randomized clinical trials with 92 patients were included. All three trials had unclear risk of bias. Compared with peginterferon monotherapy, adding ribavirin to peginterferon had significant beneficial effect on sustained virological response (OR = 3.44, 95% CI: 1.18 - 10.06). There was no significant difference between combination therapy and monotherapy in the end of treatment achievement response. Other than about 30% increase in blood transfusion due to anemia that returned to normal level 2 - 3 months after treatment, there was no significant increase in side effects followed by adding ribavirin to pegylated interferon (Peg-IFN). Data were insufficient to determine the impact of genotype, viral load and age on the response to treatment.Conclusions:
Compared with monotherapy, adding ribavirin to treatment is more effective in removing hepatitis C virus from the bloodstream in patients with thalassemia, it is also more effective in reducing the relapse rate after treatment. Except the increase in blood transfusion, there was no significant increase in side effects followed by adding ribavirin.Keywords
Hepatitis C Virus Thalassemia Interferon Pegylated Interferon Ribavirin Treatment Response
1. Context
Hepatitis C virus (HCV) has infected about 170 million people worldwide (1). Most of acute hepatitis C cases are asymptomatic, nearly 80% of the infected patients develop chronic infection, 10 – 20% of chronic cases progress to cirrhosis and about 5% of chronic patients with cirrhosis will develop hepatocellular carcinoma over 20 – 25 years (2, 3). Transfusion of blood and its products and using intravenous drugs are the most common reasons of hepatitis C transmission (3, 4). Routine blood transfusion (red blood cell), which is the choice management of thalassemia, puts the patients at increased risk of HCV infection. Although screening programs of blood donors for HCV started in 1990, it reduced the rate of new infected patients. Some studies have demonstrated that the worldwide prevalence of HCV in transfusion dependent thalassemia was 21 – 45% (5, 6). Pegylated interferon (Peg-IFN) monotherapy regime is currently approved by clinicians as the first choice of treatment for HCV infection in patients with thalassemia (7). In patients with hepatitis C/without thalassemia, there are many clinical guidelines about the choice of accurate drugs, treatment timing, associated treatments and side effects. The best outcome obtained through the combination of pegylated interferon and ribavirin for a specific period of time which depends on the genotype of virus, extending from 24 weeks for genotypes 2 - 3 to 48 weeks for genotype 1. Responses to treatment are often characterized by the results of HCV RNA testing. Infection is assumed eradicated when there is a sustained virological response (SVR), defined as the lack of HCV RNA in serum by a sensitive test 24 weeks after completion of antiviral therapy (8). Addition of the polyethylene glycol to interferon molecules produces peginterferon. This process increases half-life of molecule, decreases renal clearance and changes metabolism of the molecule (9). Ribavirin is a guanine analog with antiviral activity that decreases infectivity of hepatitis C virus in a dose-dependent pattern, ribavirin is a well-tolerated drug but hemolysis due to oxidant damage is an important complication with side effects. Ribavirin dose reduction due to anemia happens in about 15 - 20% of patients. In patients with present anemia such as major thalassemia, this event is worse. Hemolysis and anemia are almost reversible after ribavirin discontinuation or dose reduction (10). First trials with peginterferon and ribavirin in HCV treatment in patients with major thalassemia resulted in a 30 – 40% increase of blood requirement and an associated increase of iron chelation therapy but enhanced SVR rate of patients was reported (11, 12).This review aimed to assess the SVR rate, compliance, benefits and harmful effects of combination therapy of ribavirin plus peginterferon, interferon versus monotherapy of peginterferon or interferon for treatment of hepatitis C in patients with thalassemia.
2. Evidence Acquisition
2.1. Literature Search
First, a systematic electronic literature search was performed on chronic hepatitis C treatment in patients with thalassemia in MEDLINE, EMBASE, SCOPUS, ISI, Cochrane Central Registry of Clinical Trials and Google Scholar without temporal limits using different combinations of the following keywords: thalassemia, chronic hepatitis C, HCV, hepatitis C virus, ribavirin, interferon, INF, pegylated interferon and Peg-IFN (Box 1). In addition, the references from all retrieved articles and relevant reviews were evaluated to find the studies not captured by the search. The registered unpublished and ongoing clinical trials were searched on http://clinicaltrials.gov, http://controlledtrials.gov and http://centerwatch.com. Google Scholar and Google search engine were searched to find gray literature and conference abstracts. Searches were performed in February 2014.
Search Strategy of Electronic Database
| Search Strategies |
|---|
| 1. Exp Ribavirin/ |
| 2. Ribavirin or riba or copegus or rebetol or ribasphere or vilona or virazole |
| 3. 1 or 2 |
| 4. Exp Interferons/ |
| 5. interferon or IFN or pegylated Interferon or peg-IFN or roferon* or intron*or multiferon* or rebif* or avonex or cinnovex or betaferon or pegasys or repiferon or pegatron or rebetron or alferon or infergen or actimmune |
| 6. 4 or 5 |
| 7. Exp Hepatitis C, Chronic/ |
| 8. (Chronic and (‘hepatitis C’ or ‘hep C’ or HCV)) or CHC |
| 9. 8 or 7 |
| 10. Exp thalassemia/ |
| 11. Beta thalassemia or beta thalassemia major or inherited anemia or inherited hemolytic anemia or congenital hemolytic anemia |
| 12. 10 or 11 |
| 13. 3 and 6 and 9 and 12 |
2.2. Inclusion Criteria
Randomized trial studies regardless of language, type, publication place and blinding were included. For inclusion, studies had to enroll patients with a confirmed diagnosis of thalassemia and chronic hepatitis C infection (positive HCV RNA in PCR) treated with interferon or peginterferon with or without ribavirin regardless of type of drug producing factory, dose and length of study (ribavirin with optimal dose included). Sustained virological response (SVR), defined as absence of HCV RNA six months after the end of treatment as outcome, morbidity, mortality, side effects and adverse events, should be reported. Studies with co-infection of hepatitis B or HIV and history of bone marrow transplantation and immunosuppressive therapy were excluded.
2.3. Data Extraction and Collection
An investigator inspected references identified by searches and another investigator rechecked it, in cases of disagreement between these two a third investigator was consulted. Also, extraction of data from studies was performed by one investigator and rechecked by another one and in case of disagreement the third one extracted the data. After study selection and data extraction authors documented the data and where necessary, contacted authors of trials via e-mail for clarification. The following data were extracted and documented: 1) Trials’ characteristics: location, date, publication condition, setting of trial, follow-up duration and sponsor; 2) Participants’ characteristics: number of patients (subjects) in every group, gender, age, race, virus genotype, viral load at the beginning and end of the treatment, degree of liver fibrosis before the treatment; 3) Interventions’ characteristics: dose and type of interferon or peginterferon, ribavirin dose, method of administration; 4) Characteristics of outcomes: number of patients with absent HCV RNA at the end of treatment (ETR) and SVR, blood transfusion amount to achieve the level of hemoglobin, dose and amount of iron chelators and side effects of drugs administration.
2.4. Quality Assessment of Randomized Controlled Trials
All of the included randomized controlled trials were assessed for risk of bias by two authors based on ‘Cochrane risk of bias tools’ including six items: allocation concealment, random sequence generation, blinding of outcome assessment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting and other sources of bias (13). Disagreements in assessing risk of bias were resolved by discussion.
2.5. Statistical Analysis
The study assessed the heterogeneity by chi-squared test and quantified the degree of heterogeneity by the Q statistics or I2 index as inconsistency measure. Significant heterogeneity was defined as P-value of chi-squared test less than 0.1 or I2 index greater than 50% (14). Analysis performed with the statistical software review manager (version 5.2; The Nordic Cochrane center, Copenhagen, Denmark). Outcomes were summarized as odd ratios (OR) and weighted mean difference (WMD) with 95% confidence interval (CI). The fix-effect method of Mantel and Haenszel was applied to analyze data (15) and in the presence of heterogeneity, DerSimonian and Laird random-effect models were used to analyze and to compute pooled OR (16). Rare events (mortality and morbidity) were estimated by Peto odds ratio (17).
3. Results
The study identified 1483 references through the electronic searches on MEDLINE, CENTRAL, EMBASE, SCOPUS, ISI, Google Scholar, clinicaltrials.gov, controlledtrials.gov, centerwatch.com and manual searches; after reading the abstracts and titles 1427 clearly irrelevant to topic and duplicate references were excluded (Figure 1). From 56 potentially relevant studies after reading full texts, eight case-reports and case series were excluded (18-25), one study was excluded because it was conducted on patients after bone marrow transplantation (26), another study was excluded for co-infection of participants with hepatitis B (27), two reviews were excluded (28, 29), seven studies were excluded because of low sample size 2 - 8 patients (30-36), one study was excluded due to lack of methodological report (37), a study was excluded because it reported just EVR (38), four studies were excluded because they were duplicate publications of the same patients data as seminar proceedings (39-42), 25 studies were excluded since they were prospective, without a control group (7, 12, 43-65), three non-randomized controlled trials were also excluded (66-68) and finally three randomized controlled trials were included in the meta-analysis (11, 69, 70).
Systematic Review Process Flowchart
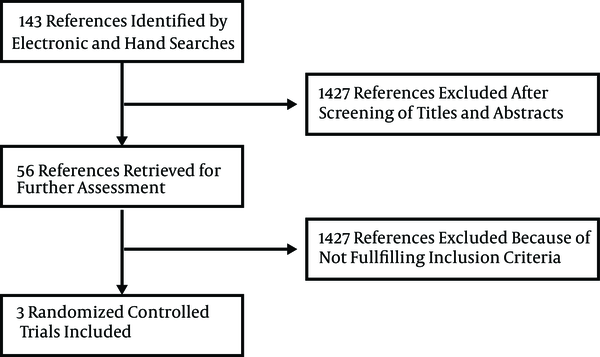
3.1. Qualitative Review
Tables 1 and 2, report the studies in details with design, patients’ characteristics, treatment protocol and response to treatment. The three included studies were randomized controlled trials on patients with thalassemia and chronic hepatitis C infection. Evaluation of bias risk according to Cochrane risk of bias tools was summarized in Table 3. Generation of random sequence was adequate in all studies, but allocation concealment was only adequate in one study and it was unclear in the other two studies. Also blinding was reported only in one of the studies as double blind and in other studies was unclear. All of studies assessed the patients with naive hepatitis C and no relapsed or non-responder patients were included. Overall, 92 patients (50 patients with interferon or peginterferon monotherapy and 42 patients with combination therapy with ribavirin) were included in the meta-analysis. Sixty-two percent of the patients were male and 40% were infected with genotype 1 hepatitis C virus; the difference in proportion of each genotype in both groups was insignificant.
Studies and Patients Characteristics
| RCT | Country | Sample Size | Gender, % Male | Mean Age of Treatment, y | Genotype 1, % | Serum Ferritin, µg/L | Liver Iron Content | HCV Viral Load, copies/mL | ALT, IU/mL |
|---|---|---|---|---|---|---|---|---|---|
| Inati et al. (11) | Lebanon | ||||||||
| 12 monotherapy | 83 | 21.6 | 67 | 1763 ± 1029 | 8 ± 3.9 | 371439 | 86.4 ± 44.5 | ||
| 8 combination therapy | 75 | 16.5 | 87 | 2757 ± 1007 | 8.2 ± 4 | 480835 | 102.6 ± 58 | ||
| Sood et al. (69) | India | ||||||||
| 20 monotherapy | 80 | 10 | 10 | ND | ND | 289333 | 125.8 ± 74.8 | ||
| 20 combination therapy | 60 | 9 | 20 | ND | ND | 372221 | 177.3 ± 140.2 | ||
| Kalantar and Rad (70) | Iran | ||||||||
| 18 monotherapy | 33 | 20.5 | 44 | ND | ND | ND | ND | ||
| 14 combination therapy | 50 | 21.5 | 57 | ND | ND | ND | ND |
| Study/Protocol | Interferon Dose | Ribavirin Doses | Duration, w | Interferon Total Dose in Each Patient | Sustained Virological Response, % | End of Treatment Response, % |
|---|---|---|---|---|---|---|
| Inati et al. | 48 | 8640 µg | ||||
| Peg-IFN α-2a+placebo | 180 µg/wk | - | 33 | 42 | ||
| Peg-IFN α-2a+ribavirin | 180 µg/wk | 10.6 mg/kg/d | 62.5 | 75 | ||
| Sood et al. | 24 or 48 | 2160 - 4320 µg | ||||
| Peg-IFN α-2b | 1.5 µg/kg/wk | - | 40 | 85 | ||
| Peg-IFN α-2b+ribavirin | 1.5 µg/kg/wk | 15 mg/kg/d | 70 | 85 | ||
| Kalantar and Rad | 24 or 48 | 216 – 432 million units | ||||
| INF α-2b+placebo | 9 million units/wk | - | 55 | 61 | ||
| INF α-2b+ribavirin | 9 million units/wk | 800 - 1200 mg/d | 28.5 | 64 |
Quality Assessment of Randomized Controlled Trials
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting |
|---|---|---|---|---|---|---|
| Inati et al. | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk |
| Sood et al. | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Kalantari and Rad | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear risk |
3.2. Effects of the Compared Interventions
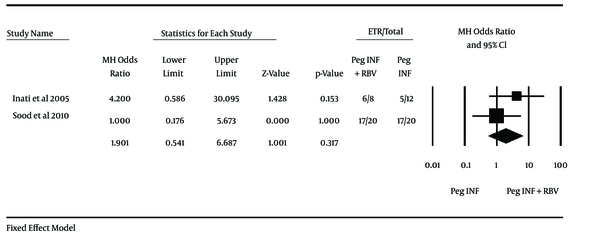
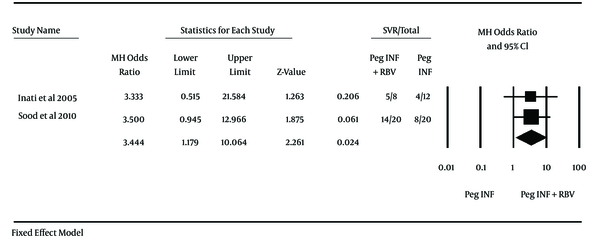
Two studies used Peg-IFN and one study used conventional interferon; therefore, because of the heterogeneity it was not possible to perform the meta-analysis but in subgroup analysis meta-analysis was performed in two studies that used Peg-IFN. Inati et al. (11) used Peg-IFN in a study on 20 patients (eight patients in combination therapy group and twelve patients in monotherapy group) and achieved OR of 3.33 (95% CI 0.51 - 21.58) for SVR and Sood et al. (69) experimented on 40 patients (20 patients each group) and showed OR of 3.50 (95% CI: 0.94 - 12.97) for SVR. End of treatment response (ETR) for these studies were 4.20 (0.59-30.10) for Inati et al. and 1.00 (0.18-5.67) for Sood et al. Odd ratio in both studies contains 1 and were not significant, but after merging these two studies data were analyzed and it was found that combination therapy with peginterferon and ribavirin vs. monotherapy with peginterferon had no significant differences in ETR through the analyzing with fixed effect method (OR = 1.90, 95% CI: 0.54 - 6.69; I2 = 13%) and proportion of patients who achieved SVR in combination therapy with peginterferon and ribavirin vs. monotherapy with peginterferon assessed with fixed effect method and there was significant difference between the groups (OR = 3.44, 95% CI: 1.18 - 10.06) (Figures 2 and 3). another study by Kalantari and Rad (70), that used conventional interferon, showed no significant differences in ETR and SVR between both groups (OR for ETR = 1.15, 95% CI: 0.27 - 4.87 and OR for SVR = 0.32, 0.07 - 1.41). The effect of other variables (e g. virus genotype, viral load, type of peginterferon, age at infection, age at treatment and gender) on response of patients to treatment was not identically addressed between studies and could not be analyzed. Just one study reported the response to treatment in different virus genotype groups and proportion of SVR in genotype 1 was 50% and 25% in monotherapy and combination therapy, respectively. Also Inati et al. (11) reported that patients younger than 18 have better chance of SVR.
Odds Ratios and 95% Confidence Interval for the End of Treatment Response Outcome in Combination Therapy Group Compared With Those of the Monotherapy Group
Odds Ratios (OR) and 95% Confidence Interval for the Sustained Virological Response Outcome in Combination Therapy Group Compared With Those of the Monotherapy Group
3.3. Safety
3.3.1. Increase in Blood Transfusion
Inati et al. (11) reported that blood transfusion increased 55% and 21% in patients with combination therapy and monotherapy, respectively. Sood et al. (69) reported that 20% of patients in combination therapy group and 5% of patients in monotherapy group needed one extra unit of blood. Kalanteri et al. (70) did not mentioned blood transfusion changes. All of the patients returned to normal two or three months after completion of treatment.
3.3.2. Ribavirin Dose Reduction
Four patients (two patients in each group) required ribavirin dose reduction (Sood et al. (69)).
3.3.3. Peginterferon Dose Reduction
Just one patient had 50% modification in peginterferon dose due to leukopenia and neutropenia, after four weeks and full dose therapy leukopenia did not occur. One patient had proteinuria (4.5 g/day) and peginterferon discontinued. Flu-like symptoms, bodyache and arthralgias were observed in 90% and 95% of patients of monotherapy and combination therapy groups respectively (Sood et al. (69)). The most expected adverse event was hemolytic anemia that needed an increase in transfusion requirements, which two months after antiviral treatment completion returned to their previous baseline before the treatment (Inati et al. (11)).
3.3.4. Mortality
One patient in monotherapy group died because of bacterial meningitis following periodontal infection and abscess about two months after completion of antiviral treatment; there was not any sign of cytopenia in this patient (Sood et al. (69)) and it did not seem to be caused by treatment.
The dropout rate in all three studies was 0. ALT, AST (aspartate aminotransferase) and liver fibrosis grade were reported only before the treatment; therefore, the effect of treatment on liver condition could not be evaluated.
4. Conclusions
Patients with congenital hemolytic anemia such as thalassemia are at high risk for HCV infection, which has become an important cause of liver related morbidity and mortality (3, 4). Combination of pegylated interferon and ribavirin, which is the standard treatment for patients with hepatitis C/without thalassemia, is limited in patients with hemolytic anemia such as major thalassemia because of the ribavirin associated hemolysis (5, 6).
Initial trials showed that adding ribavirin to Peg-IFN slightly increases the incidence of adverse events such as blood requirement and chelation therapy in patients with thalassemia but had improvement in the rate of patients with SVR (11, 12). The current review aimed to quantify the effect of adding ribavirin to Peg-IFN to treat HCV infection in patients with thalassemia. The current systematic review of three randomized trials included 92 patients. In the subgroup analysis, achievement of ETR had no significant difference in both groups (OR for ETR = 1.90, 95% CI: 0.54 - 6.69). The current review subgroup analysis showed that adding ribavirin to peginterferon had significant increase in SVR achievement (OR for SVR = 3.44, 95% CI: 1.18 - 10.06) that means 3.4-fold increase in SVR rate by adding ribavirin. Tabatabaei et al. (67) in a non-randomized controlled trial on 280 patients (199 subjects in combination therapy vs. 81 subjects in monotherapy group) reported that adding low dose ribavirin to monotherapy treatment increased the rate of SVR 2.2-fold (95% CI: 1.24 - 3.91) that was not against the current review findings, also the pegylated interferon plus ribavirin are used to treat hepatitis C in patients who do not have thalassemia (8, 71, 72) and despite the anemic condition of the patients with thalassemia, they benefit from adding ribavirin to peginterferon according to the current review.
When combined with peginterferon, ribavirin improves sustained virological response (SVR) rates by approximately 25 – 30%. Prevention of relapse after completion of treatment is an important role of ribavirin in SVR rate improvement. In patients with response to the antiviral effect of pegylated INF it is shown that ribavirin prevents relapse by more decrease in the slope of virus RNA (73, 74). However, after years of clinical research and broad use of ribavirin , the exact mechanism of ribavirin action is not well known, some of the perceived mechanisms of action for ribavirin against hepatitis C virus include: a direct effect on the hepatitis C virus-RNA dependent RNA polymerase (75), fatal mutagenesis of virus by induction of disjunction of nucleotides (76), evacuation of intracellular cytoplasm through inhibition of inosine-5’-monophosphate-dehydrogenase, change in the balance of cytokines between a Th2-type cytokines (anti-inflammatory) to a Th1-type cytokines (proinflammatory) and improving the effect of peginterferon through up-regulation of genes participating in peginterferon signaling (77-80). Authors found that adding ribavirin to peginterferon reduces the rate of relapse after treatment (significant increase in SVR rate in combination therapy regiment while there was no difference in end of the treatment response in both methods).
As it is known, thalassemia is quite prevalent and difficult to manage in Iran when co-occurred with hepatitis C. Although, there are several established treatment protocols for HCV in patients who do not have thalassemia, there is no standard therapy regime for this condition in patients with hemolytic anemia such as thalassemia. The present review assessed current literatures on the importance of addition of anti-nucleoside anti-viral drugs particularly ribavirin to interferon based regime to manage HCV in patients with thalassemia.
Other than the slight increase in blood transfusion about 30% due to anemia that returned to normal level two to three months after treatment (11, 12), there was no significant increase in side effects followed by adding ribavirin to Peg-IFN. Evaluation of different genotype subgroups was not possible due to lack of report, also assessment of effect of type of Peg-IFN was not performed due to the low number of studies. Biochemical responses were not mentioned in the studies. In general, adverse events except blood transfusion were insufficiently reported.
The current review had some limitations; only three randomized controlled trials with low sample sizes of totally 92 patients were included. Randomized controlled trials with such low sample size do not have strong and reliable evidences to be used clinically. Studies with more sample size, better design in randomization and blinding are needed to obtain reliable evidences. Two out of three studies were funded by pharmacy companies (11, 69).
The current review evaluated the current literature and included randomized controlled trials and found that combination therapy with peginterferon plus ribavirin versus monotherapy with peginterferon had significant difference in response to treatment and achievement of SVR in HCV infection in patients with thalassemia about more than three folds. Adverse events except slight increase in blood transfusion in combination therapy regiment did not differ in the groups, nonetheless on the current literature and trials authors could tell that adding ribavirin to pegylated interferon can enhance treatment response of hepatitis C infection in patients with thalassemia and can decrease the rate of relapse after treatment that lead to higher rate of sustained virological response.
Acknowledgements
References
-
1.
Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558-67. [PubMed ID: 16122679]. https://doi.org/10.1016/s1473-3099(05)70216-4.
-
2.
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-42. [PubMed ID: 23172780]. https://doi.org/10.1002/hep.26141.
-
3.
Heintges T, Wands JR. Hepatitis C virus: epidemiology and transmission. Hepatology. 1997;26(3):521-6. [PubMed ID: 9303478]. https://doi.org/10.1002/hep.510260338.
-
4.
Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553-62. [PubMed ID: 23817321]. https://doi.org/10.1038/nrgastro.2013.107.
-
5.
Mirmomen S, Alavian SM, Hajarizadeh B, Kafaee J, Yektaparast B, Zahedi MJ, et al. Epidemiology of hepatitis B, hepatitis C, and human immunodeficiency virus infecions in patients with beta-thalassemia in Iran: a multicenter study. Arch Iran Med. 2006;9(4):319-23. [PubMed ID: 17061602].
-
6.
Wasley A, Grytdal S, Gallagher K, Centers for Disease C. Surveillance for acute viral hepatitis--United States, 2006. MMWR Surveill Summ. 2008;57(2):1-24. [PubMed ID: 18354374].
-
7.
Angelucci E, Pilo F. Treatment of hepatitis C in patients with thalassemia. Haematologica. 2008;93(8):1121-3. [PubMed ID: 18669974]. https://doi.org/10.3324/haematol.13500.
-
8.
Strader DB, Wright T, Thomas DL, Seeff LB, American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147-71. [PubMed ID: 15057920]. https://doi.org/10.1002/hep.20119.
-
9.
Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33(2):433-8. [PubMed ID: 11172346]. https://doi.org/10.1053/jhep.2001.21747.
-
10.
Bodenheimer HC Jr, Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: a multicenter trial. Hepatology. 1997;26(2):473-7. [PubMed ID: 9252161]. https://doi.org/10.1002/hep.510260231.
-
11.
Inati A, Taher A, Ghorra S, Koussa S, Taha M, Aoun E, et al. Efficacy and tolerability of peginterferon alpha-2a with or without ribavirin in thalassaemia major patients with chronic hepatitis C virus infection. Br J Haematol. 2005;130(4):644-6. [PubMed ID: 16098081]. https://doi.org/10.1111/j.1365-2141.2005.05645.x.
-
12.
Li CK, Chan PK, Ling SC, Ha SY. Interferon and ribavirin as frontline treatment for chronic hepatitis C infection in thalassaemia major. Br J Haematol. 2002;117(3):755-8. [PubMed ID: 12028054].
-
13.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [PubMed ID: 22008217]. https://doi.org/10.1136/bmj.d5928.
-
14.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-58. [PubMed ID: 12111919]. https://doi.org/10.1002/sim.1186.
-
15.
Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6(3):341-50. [PubMed ID: 3616287].
-
16.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-88. [PubMed ID: 3802833].
-
17.
Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1):53-77. [PubMed ID: 16596572]. https://doi.org/10.1002/sim.2528.
-
18.
Behnava B, Keshvari M, Miri SM, Elizee PK, Alavian SM. Reactivation of brucellosis during pegylated interferon-alpha therapy in a thalassemic patient with chronic hepatitis C. Exp Clin Hepatol. 2011;7(1–2):CR57-9.
-
19.
Sherker AH, Senosier M, Kermack D. Treatment of transfusion-dependent thalassemic patients infected with hepatitis C virus with interferon alpha-2b and ribavirin. Hepatology. 2003;37(1):223. [PubMed ID: 12500209]. https://doi.org/10.1053/jhep.2003.50037.
-
20.
Hamidah A, Thambidorai CR, Jamal R. Peginterferon alfa-2b and ribavirin in thalassaemia/chronic hepatitis C virus-co-infected non-responder to standard interferon-based. Med J Malaysia. 2005;60(4):517-9. [PubMed ID: 16570722].
-
21.
Sharara AI, Aoun E, Koussa S, Inati A, Taher A. Treatment of acute hepatitis C in a child with thalassemia major using weight-based peginterferon alpha-2b. J Gastroenterol Hepatol. 2006;21(7):1221. [PubMed ID: 16824084]. https://doi.org/10.1111/j.1440-1746.2006.04174.x.
-
22.
Pagliano P, Costantini S, Gradoni L, Faella FS, Spasiano A, Mascarella G, et al. Distinguishing visceral leishmaniasis from intolerance to pegylated interferon-alpha in a thalassemic splenectomized patient treated for chronic hepatitis C. Am J Trop Med Hyg. 2008;79(1):9-11. [PubMed ID: 18606757].
-
23.
Mobini N. Severe muscle weakness during treatment with pegylated interferon alfa for chronic hepatitis C virus infection; A rare complication. Caspian J Intern Med. 2010;1(3):111-4.
-
24.
Hamidah A, Yong JF, Zulkifli HI, Jamal R. Treatment of chronic hepatitis C virus infection with interferon alfa and ribavirin: sustained response in two patients with transfusion dependent thalassaemia. Med J Malaysia. 2002;57(3):353-6. [PubMed ID: 12440276].
-
25.
Uslu N, Baysoy G, Demir H, Temizel IN, Yuce A. Safety of ribavirin in adolescent thalassemic patients with chronic hepatitis C virus infection. J Clin Virol. 2010;48(1):66-8. [PubMed ID: 20347610]. https://doi.org/10.1016/j.jcv.2010.02.011.
-
26.
Giardini C, Galimberti M, Lucarelli G, Polchi P, Angelucci E, Baronciani D, et al. Alpha-interferon treatment of chronic hepatitis C after bone marrow transplantation for homozygous beta-thalassemia. Bone Marrow Transplant. 1997;20(9):767-72. [PubMed ID: 9384479]. https://doi.org/10.1038/sj.bmt.1700968.
-
27.
Guptan RC, Thakur V, Raina V, Sarin SK. Alpha-interferon therapy in chronic hepatitis due to active dual infection with hepatitis B and C viruses. J Gastroenterol Hepatol. 1999;14(9):893-8. [PubMed ID: 10535471].
-
28.
Alavian SM, Tabatabaei SV. Treatment of chronic hepatitis C in polytransfused thalassaemic patients: a meta-analysis. J Viral Hepat. 2010;17(4):236-44. [PubMed ID: 19638104]. https://doi.org/10.1111/j.1365-2893.2009.01170.x.
-
29.
Bourliere M, Halfon P, Portal I. [Treatment of chronic hepatitis C in special groups]. Gastroenterol Clin Biol. 2002;26 Spec No 2:B238-47. [PubMed ID: 12180296].
-
30.
Uysal Z, Cin S, Arcasoy A, Akar N. Interferon treatment of hepatitis B and C in beta-thalassemia. Pediatr Hematol Oncol. 1995;12(1):87-9. [PubMed ID: 7703048].
-
31.
Russo-Mancuso G, Di Gregorio F, Passero E, Sciotto A, Mazzarino MC, Malaponte G, et al. Efficacy of an analysis of lymphocyte subsets in predicting the clinical response to alpha-interferon therapy in thalassaemia patients with chronic infection by hepatitis C virus: a pilot study. Br J Haematol. 1995;89(2):291-8. [PubMed ID: 7873379].
-
32.
Marcellini M, Kondili LA, Comparcola D, Spada E, Sartorelli MR, Palumbo M, et al. High dosage alpha-interferon for treatment of children and young adults with chronic hepatitis C disease. Pediatr Infect Dis J. 1997;16(11):1049-53. [PubMed ID: 9384338].
-
33.
Ancel D, Amiot X, Chaslin-Ferbus D, Hagege I, Garioud A, Girot R, et al. Treatment of chronic hepatitis C in sickle cell disease and thalassaemic patients with interferon and ribavirin. Eur J Gastroenterol Hepatol. 2009;21(7):726-9. [PubMed ID: 19404206]. https://doi.org/10.1097/MEG.0b013e3283097699.
-
34.
Paschos P, Vlachaki E, Pasvanti C, Sinakos E, Kalpaka A, Klonizakis P, et al. Safety and efficacy of combination therapy with pegylated interferon alpha-2a and ribavirin in treating patients with chronic hepatitis C and beta-thalassaemia major: a Greek single-center experience. Acta Haematol. 2011;126(4):231-3. [PubMed ID: 21934299]. https://doi.org/10.1159/000330516.
-
35.
Leonardi S, Avola E, Delfa WL, Di Gregorio F, Musumeci S. Interferon-α Therapy in Thalassemic Patients with Chronic Non-A, Non-B Hepatitis. Ann NY Acad Sci. 1990;612(1):553-5. https://doi.org/10.1111/j.1749-6632.1990.tb24364.x.
-
36.
Harmatz P, Butensky E, Foote D, Vichinsky E, editors. Report of the first patients with thalassemia in the United States treated with combination therapy for hepatitis C virus. Blood. 2003; Washington DC, USA. Amer Soc Hematology; 38B p.
-
37.
Eleftheriou A, Christou S, Skordos G, Pavlides N. Factors predicting progression and response to treatment of HCV infection in thalassaemia major patients. Blood. 1999;94(10):31B.
-
38.
Harmatz P, Olivieri NF, Vichinsky E, Kwiatkowski J, Giardina P, Shah F, et al. Early Hepatitis C Viral Response (EVR) to Peginterferon Alfa 2a and Ribavirin in Patients with β Thalassemia. ASH Ann Meet Abstr. 2004;104(11):3624.
-
39.
Harmatz P, Jonas MM, Kwiatkowski JL, Wright EC, Fischer R, Vichinsky E, et al. Safety and efficacy of pegylated interferon alpha-2a and ribavirin for the treatment of hepatitis C in patients with thalassemia. Haematologica. 2008;93(8):1247-51. [PubMed ID: 18556414]. https://doi.org/10.3324/haematol.12352.
-
40.
Inati A, Sharara AI, Ghorra S, Aoun E, Taha M, Koussa S, et al. Study on hepatitis C treatment using ribavirin and pegylated interferon in thalassemia patients (SCRIPT): One year report. ASH Ann Meet Abstr. 2004;104(11):3768.
-
41.
Di Marco V, Lo Iacono O, Capra M, Grutta S, Ciaccio C, Gerardi C, et al. Alpha interferon treatment of chronic hepatitis C in beta-thalassaemia. Gut. 1993;34(2 Suppl):S142-3. [PubMed ID: 8314484].
-
42.
Donohue SM, Wonke B, Hoffbrand AV, Reittie J, Ganeshaguru K, Scheuer PJ, et al. Alpha interferon in the treatment of chronic hepatitis C infection in thalassaemia major. Br J Haematol. 1993;83(3):491-7. [PubMed ID: 8387324].
-
43.
Di Marco V, Lo Iacono O, Capra M, Grutta S, Ciaccio C, Gerardi C, et al. alpha-Interferon treatment of chronic hepatitis C in young patients with homozygous beta-thalassemia. Haematologica. 1992;77(6):502-6. [PubMed ID: 1289187].
-
44.
Wonke B, Donohue SM, Hoffbrand AV, Scheuer PJ, Brown D, Dusheiko G. Recombinant alpha 2B interferon (IFN) in the treatment of chronic hepatitis C disease in thalassaemia major (TM). Bone Marrow Transplant. 1993;12 Suppl 1:24-5. [PubMed ID: 8397033].
-
45.
Malaponte G, Passero E, Leonardi S, Monte V, Lauria C, Mazzarino C, et al. Effect of alpha-interferon on natural killer cell activity and lymphocyte subsets in thalassemia patients with chronic hepatitis C. Acta Haematol. 1997;98(2):83-8. [PubMed ID: 9286304].
-
46.
Di Marco V, Lo Iacono O, Almasio P, Ciaccio C, Capra M, Rizzo M, et al. Long-term efficacy of alpha-interferon in beta-thalassemics with chronic hepatitis C. Blood. 1997;90(6):2207-12. [PubMed ID: 9310471].
-
47.
Telfer PT, Garson JA, Whitby K, Grant PR, Yardumian A, Hoffbrand AV, et al. Combination therapy with interferon alpha and ribavirin for chronic hepatitis C virus infection in thalassaemic patients. Br J Haematol. 1997;98(4):850-5. [PubMed ID: 9326177].
-
48.
Spiliopoulou I, Repanti M, Katinakis S, Karana-Ginopoulou A, Papanastasiou DA. Response to interferon alfa-2b therapy in mutitransfused children with beta-thalassemia and chronic hepatitis C. Eur J Clin Microbiol Infect Dis. 1999;18(10):709-15. [PubMed ID: 10584897].
-
49.
Sievert W, Pianko S, Warner S, Bowden S, Simpson I, Bowden D, et al. Hepatic iron overload does not prevent a sustained virological response to interferon-alpha therapy: a long term follow-up study in hepatitis C-infected patients with beta thalassemia major. Am J Gastroenterol. 2002;97(4):982-7. [PubMed ID: 12003436]. https://doi.org/10.1111/j.1572-0241.2002.05550.x.
-
50.
Syriopoulou V, Daikos GL, Kostaridou SL, Manolaki N, Nakopoulou L, Kattamis A, et al. Sustained response to interferon alpha-2a in thalassemic patients with chronic hepatitis C. A prospective 8-year follow-up study. Haematologica. 2005;90(1):129-31. [PubMed ID: 15642681].
-
51.
Artan R, Akcam M, Yilmaz A, Kocacik D. Interferon alpha monotherapy for chronic hepatitis C viral infection in thalassemics and hemodialysis patients. J Chemother. 2005;17(6):651-5. [PubMed ID: 16433196]. https://doi.org/10.1179/joc.2005.17.6.651.
-
52.
Chen AC, Peng CT, Wu SF, Wu KH, Chiang IP, Tsai CH. Effect of deferiprone on liver iron overload and fibrosis in hepatitis-C-virus-infected thalassemia. Hemoglobin. 2006;30(2):209-14. [PubMed ID: 16798645]. https://doi.org/10.1080/03630260600642518.
-
53.
Saffar MJ, Saffar H, Khalilian AR, Naqshvar F. Combination therapy with interferon and ribavirin for chronic hepatitis C infection in beta-thalassaemia major. East Mediterr Health J. 2009;15(4):785-91. [PubMed ID: 20187529].
-
54.
Ricchi P, Ammirabile M, Costantini S, Cinque P, Lanza AG, Spasiano A, et al. The impact of previous or concomitant IFN therapy on deferiprone-induced agranulocytosis and neutropenia: a retrospective study. Expert Opin Drug Saf. 2010;9(6):875-81. [PubMed ID: 20945995]. https://doi.org/10.1517/14740338.2010.510831.
-
55.
Ricchi P, Lanza AG, Ammirabile M, Costantini S, Cinque P, Spasiano A, et al. Hepatitis C virus distribution and clearance following interferon-monotherapy among thalassaemia major and intermedia patients. Br J Haematol. 2011;155(4):524-7. [PubMed ID: 21564076]. https://doi.org/10.1111/j.1365-2141.2011.08717.x.
-
56.
Jafroodi M, Asadi R, Heydarzadeh A, Besharati S. Effect of hepatic iron concentration and viral factors in chronic hepatitis C-infected patients with thalassemia major, treated with interferon and ribavirin. Int J Gen Med. 2011;4:529-33. [PubMed ID: 21845061]. https://doi.org/10.2147/IJGM.S19643.
-
57.
Di Marco V, Bronte F, Calvaruso V, Capra M, Borsellino Z, Maggio A, et al. IL28B polymorphisms influence stage of fibrosis and spontaneous or interferon-induced viral clearance in thalassemia patients with hepatitis C virus infection. Haematologica. 2012;97(5):679-86. [PubMed ID: 22180419]. https://doi.org/10.3324/haematol.2011.050351.
-
58.
Triantos C, Kourakli A, Kalafateli M, Giannakopoulou D, Koukias N, Thomopoulos K, et al. Hepatitis C in patients with beta-thalassemia major. A single-centre experience. Ann Hematol. 2013;92(6):739-46. [PubMed ID: 23412560]. https://doi.org/10.1007/s00277-013-1692-6.
-
59.
Lai ME, Origa R, Danjou F, Leoni GB, Vacquer S, Anni F, et al. Natural history of hepatitis C in thalassemia major: a long-term prospective study. Eur J Haematol. 2013;90(6):501-7. [PubMed ID: 23414443]. https://doi.org/10.1111/ejh.12086.
-
60.
Vafiadis I, Trilianos P, Vlachogiannakos J, Karagiorga M, Hatziliami A, Voskaridou E, et al. Efficacy and safety of interferon-based therapy in the treatment of adult thalassemic patients with chronic hepatitis C: a 12 years audit. Ann Hepatol. 2013;12(4):532-8. [PubMed ID: 23813130].
-
61.
Wonke B, Telfer P, Garson JA, Whitby K, Grant P, Rowntree C, et al. Alpha-interferon alone and in combination with ribavirin for hepatitis C infection in multiply tranfused patients with thalassemia major-the UK experience. Bone Marrow Transplant-Basingstoke-. 1997;19:163-5.
-
62.
Diamantis I, Vafiadis I, Voskaridou E, Dellatetsima J, Jaggi N, Gyr K, et al. Genotype distribution of hepatitis C virus infection in Greece: correlation with different risk factors and response to interferon therapy. Eur J Gastroenterol Hepatol. 1998;10(1):75-9. [PubMed ID: 9512957].
-
63.
Labropoulou-Karatza C, Goritsas C, Fragopanagou H, Matsouka P, Spiliopoulou II, Barbatis C. Adult beta-thalassemic patients with chronic hepatitis C: long-term efficacy of alpha-IFN treatment. Eur J Intern Med. 2000;11(3):161-4. [PubMed ID: 10854823].
-
64.
Mirmomen S, Ebrahimi Daryani N, Malekzadeh R, Zali MR, Haghpanah B, Poursamimi P. The efficacy and safety of peginterferon alpha-2a (PEGASYS) monotherapy in the treatment of chronic hepatitis C infected subjects with transfusion dependent thalassemia. Hepat Mon. 2004;4(7):65-70.
-
65.
Mirmomen S, Ghofrani H, Forootan Pishbuary H, Ebrahimi Daryani N, Jafar Farahvash M, Sharifian RA, et al. Safety and efficacy of interferon alfa for the treatment of chronic hepatitis C infected subjects with transfusion dependent thalassemia in Iran. Med J Islamic Repub Iran (MJIRI). 2003;17(2):87-95.
-
66.
Pizzarelli G, Di Gregorio F, Romeo MA, Carboni F, Gallisai D, Solinas A, et al. Interferon-alpha therapy in Sicilian and Sardinian polytransfused thalassaemic patients with chronic hepatitis C. BioDrugs. 1999;12(1):55-63. [PubMed ID: 18031162].
-
67.
Tabatabaei SV, Alavian SM, Keshvari M, Behnava B, Miri SM, Karimi Elizee P, et al. Low dose ribavirin for treatment of hepatitis C virus infected thalassemia major patients; new indications for combination therapy. Hepat Mon. 2012;12(6):372-81. [PubMed ID: 22879826]. https://doi.org/10.5812/hepatmon.6592.
-
68.
Clemente MG, Congia M, Lai ME, Lilliu F, Lampis R, Frau F, et al. Effect of iron overload on the response to recombinant interferon-alfa treatment in transfusion-dependent patients with thalassemia major and chronic hepatitis C. J Pediatr. 1994;125(1):123-8. [PubMed ID: 8021761].
-
69.
Sood A, Sobti P, Midha V, Singla D, Kaur A, Kaushal S, et al. Efficacy and safety of pegylated IFN alfa 2b alone or in combination with ribavirin in thalassemia major with chronic hepatitis C. Indian J Gastroenterol. 2010;29(2):62-5. [PubMed ID: 20443101]. https://doi.org/10.1007/s12664-010-0014-3.
-
70.
Kalantari H, Rad N. Efficacy of interferon alpha-2b with or without ribavirin in thalassemia major patients with chronic hepatitis C virus infection: A randomized, double blind, controlled, parallel group trial. J Res Med Sci. 2010;15(6):310-6. [PubMed ID: 21526103].
-
71.
Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-82. [PubMed ID: 12324553]. https://doi.org/10.1056/NEJMoa020047.
-
72.
Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346-55. [PubMed ID: 14996676].
-
73.
Bronowicki JP, Ouzan D, Asselah T, Desmorat H, Zarski JP, Foucher J, et al. Effect of ribavirin in genotype 1 patients with hepatitis C responding to pegylated interferon alfa-2a plus ribavirin. Gastroenterology. 2006;131(4):1040-8. [PubMed ID: 17030174]. https://doi.org/10.1053/j.gastro.2006.07.022.
-
74.
Dusheiko G, Nelson D, Reddy KR. Ribavirin considerations in treatment optimization. Antivir Ther. 2008;13 Suppl 1:23-30. [PubMed ID: 18432160].
-
75.
Hofmann WP, Polta A, Herrmann E, Mihm U, Kronenberger B, Sonntag T, et al. Mutagenic effect of ribavirin on hepatitis C nonstructural 5B quasispecies in vitro and during antiviral therapy. Gastroenterology. 2007;132(3):921-30. [PubMed ID: 17383421]. https://doi.org/10.1053/j.gastro.2006.12.005.
-
76.
Chevaliez S, Brillet R, Lazaro E, Hezode C, Pawlotsky JM. Analysis of ribavirin mutagenicity in human hepatitis C virus infection. J Virol. 2007;81(14):7732-41. [PubMed ID: 17494069]. https://doi.org/10.1128/JVI.00382-07.
-
77.
Chung RT, Gale M Jr, Polyak SJ, Lemon SM, Liang TJ, Hoofnagle JH. Mechanisms of action of interferon and ribavirin in chronic hepatitis C: Summary of a workshop. Hepatology. 2008;47(1):306-20. [PubMed ID: 18161743]. https://doi.org/10.1002/hep.22070.
-
78.
Asselah T, Bieche I, Sabbagh A, Bedossa P, Moreau R, Valla D, et al. Gene expression and hepatitis C virus infection. Gut. 2009;58(6):846-58. [PubMed ID: 19074178]. https://doi.org/10.1136/gut.2008.166348.
-
79.
Feld JJ, Lutchman GA, Heller T, Hara K, Pfeiffer JK, Leff RD, et al. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology. 2010;139(1):154-620000. [PubMed ID: 20303352]. https://doi.org/10.1053/j.gastro.2010.03.037.
-
80.
Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011;53(1):32-41. [PubMed ID: 21254160]. https://doi.org/10.1002/hep.23985.