Articles
Article Tools
Stats or Metrics
Article
Original Article
Exp Neurobiol 2018; 27(5): 377-386
Published online October 31, 2018
https://doi.org/10.5607/en.2018.27.5.377
© The Korean Society for Brain and Neural Sciences
Ubiquitin C-terminal Hydrolase L1 Regulates Lipid Raft-dependent Endocytosis
Seo-Jun Kang1,2,3,†, Jin Soo Kim1,2,†, and Sang Myun Park1,2,3*
1Department of Pharmacology, Ajou University School of Medicine, Suwon 16499, Korea.
2Chronic Inflammatory Disease Research Center, Ajou University School of Medicine, Suwon 16499, Korea.
3BK21 plus program, Department of Biological Sciences, Ajou University School of Medicine, Suwon 16499, Korea.
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-31-219-5063, FAX: 82-31-219-5069
e-mail: sangmyun@ajou.ac.kr
†These authors contributed equally to this work.
Abstract
Ubiquitin C-terminal hydrolase L1 (UCH-L1) is a deubiquitinating enzyme that is highly expressed in neurons, and gathering evidence indicates that UCH-L1 may play pathogenic roles in many neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease (PD). Additionally, lipid rafts have attracted interest in neurodegeneration as playing a common role in many neurodegenerative diseases. In the present study, we demonstrated that UCH-L1 associates with lipid rafts as with other PD-associated gene products. In addition, UCH-L1 regulates lipid raft-dependent endocytosis and it is not dependent on the expression and degradation of caveolin-1 or flotillin-1. Finally, UCH-L1 regulates cell-to-cell transmission of α-synuclein. This study provides evidence that many PD-associated gene products share common signaling pathways to explain the pathogenesis of PD.
Graphical Abstract
Keywords: alpha-synuclein, UCH-L1, Parkinson's disease, prion disease
INTRODUCTION
Ubiquitin C-terminal hydrolase L1 (UCH-L1) is a deubiquitinating enzyme that is highly expressed in neurons, comprising 1~2% of total neuronal proteins [1]. Accumulating evidence indicates that UCH-L1 may be involved in the pathogenesis of many neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD). UCH-L1 is detected in cortical Lewy bodies and neurofibrillary tangles in patients with diffuse Lewy body disease and AD, respectively [2]. Down-regulation and extensive oxidative modification of UCH-L1 have been observed in the brains of AD and PD patients [3]. A missense mutation, I93M, in UCH-L1 has been identified in patients with autosomal dominant familial PD [4], and an S18Y mutation in UCH-L1 has been reported to exert a neuroprotective effect against PD [5], although these genetic studies are controversial [6,7].
Recently, lipid rafts have attracted interest in neurodegeneration. Lipid rafts are specialized membrane microdomains that are enriched in cholesterol and glycosphingolipids. They serve as platforms for the assembly of signaling molecules and regulate receptor-mediated signal transduction and endocytosis [8,9]. Changes in lipid rafts content are present in many neurodegenerative diseases such as AD, PD, Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), and prion disease [10]. Also, significant alteration in numerous proteins in lipid rafts has been reported in AD and ALS mouse models [11,12]. Aβ, α-synuclein, mutant huntingtin and prion, which are key players of AD, PD, HD and prion disease, respectively, have been found in lipid rafts [13,14,15,16] and the main proteins responsible for Aβ generation such as amyloid precursor protein, β-secretase [17,18], and presenilin-1 [19] are present in lipid rafts. Many PD-associated gene products such as parkin, PINK1, DJ-1, and LRRK2 [20,21,22,23] also associates with lipid rafts, implying that lipid rafts may play a common role in many neurodegenerative diseases.
Additionally, recent evidence indicates that regional and intercellular spreading of tau and α-synuclein are significant in the pathogenesis of AD and PD, respectively. It has attracted much attention because intercellular spreading may explain unveiled pathogenesis and help with developing novel therapeutic interventions such as blocking secretion and reuptake [24,25]. Previously, we demonstrated that α-synuclein is internalized into microglia in a lipid raft-dependent manner [26]. In addition, parkin, which has also been known to associate with lipid rafts, regulates caveolin-1 expression, which alters lipid rafts and the cell-to-cell transmission of α-synuclein [27], implying that lipid raft components may be involved in cell-to-cell transmission of α-synuclein.
Although UCH-L1 is mainly cytosolic protein, 20~50% of UCH-L1 is membrane-associated [28,29,30]. However, association of UCH-L1 with lipid rafts has not been explored. In the present study, we explored whether UCH-L1 associates with lipid rafts and whether altered UCH-L1 expression is involved in lipid raft function. Additionally, we explored the role of UCH-L1 in cell-to-cell transmission of α-synuclein.
MATERIALS AND METHODS
Antibodies against flotillin-1 and flotillin-2 were purchased from BD Bioscience (Fraklin Lakes, NJ, USA). Antibodies against GAPDH, CD71 (transferrin receptor) and UCH-L1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Methyl-β-cyclodextrin and LDN-57444 were purchased from Sigma-Aldrich (St Louis, MO, USA). Rhodamine-conjugated transferrin and BOIPY® FL C5-Lactosylceramide were purchased from Molecular Probes (Leiden, the Netherlands).
SH-SY5Y, Hela, and human alpha-synuclein overexpressing SH-SY5Y cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Primary cortical neurons were cultured from Sprague-Dawley rat embryos at embryonic day 18 and maintained in Neurobasal medium (Invitrogen, Carlsbad, CA, USA) with L-glutamine and B-27 supplement (Invitrogen). Hela cells were transfected using lipofectamine 2000 (Invitorgen) according to the manufacturer's instruction. After 24 hr of transfection, the cells were used for further experiments. The plasmid for UCH-L1-myc was kindly provided by Prof. K. J. Lee at Ewha Womans University, Korea. UCH-L1 knockdown SH-SY5Y cells were prepared using lentiviral constructs that expressed shRNA for UCH-L1 (Sigma-Aldrich) as described previously [31] and selected using puromycin.
Cells were lysed in ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% sodium deoxycholate, 1% Triton X-100, 0.1% SDS and protease inhibitor mixture (GenDEPOT, Barker, TX)) for 20 min on ice after sonication for 3 s. The lysates were cleared by centrifugation at 13,000×g for 30 min at 4℃. The supernatants were collected and mixed with sample buffer, resolved by SDS-PAGE, transferred to a nitrocellulose membrane and immunoblotted with the indicated antibodies. They were then detected using an enhanced chemiluminescence system (Thermo Fisher Scientific, Waltham, MA, USA).
Cell were washed three times with ice-cold phosphate-buffered saline (PBS) and lysed in ice-cold PBS containing 1% Triton X-100 and protease inhibitor mixture. After the lysates were incubated for 10 min at 4℃, they were centrifuged at 13,000×g for 15 min at 4℃. Supernatants were used as the soluble fraction. The pellets were washed with ice-cold PBS, solubilized with 1 x sample buffer and used as the insoluble fraction. These individual fractions were analyzed by SDS-PAGE and Western blot. For sucrose density gradient centrifugation fractionation analysis, cells were harvested in lysis buffer (25 mM MES, pH 6.5, 50 mM NaCl, 1 mM Na3VO4 and 1% Triton X-100) with protease inhibitor mixture and phosphatase inhibitor added, and incubated for 30 min at 4℃ with Dounce homogenization every 10 min. The lysates were adjusted to 42.5% sucrose, overlaid with 35 and 5% sucrose in lysis buffer without Triton X-100. The mixed lysates were centrifuged at 275,000×g for 20 hr at 4℃. From the top of the gradient, eleven 1-ml fractions were collected, and some volumes of each fraction were analyzed by Western blot.
Endocytosis assay was performed as described previously [22]. SH-SY5Y cells were incubated with 50 nM BOIPY® FL C5-Lactosylceramide and 2.5 µg/ml rhodamine-conjugated transferrin for indicated times. The cells were then fixed and observed by confocal microscopy. The intensity was analyzed by ImageJ (http://imagej.nih.gov/ij/).
Cell-to-cell transmission assay was performed as described previously [27,32]. α-Synuclein-overexpressing SH-SY5Y cells [26] as donor cells were differentiated by treatment with 50 µM retinoic acid for 5 days. Then, SH-SY5Y cells cultured on coverslips on 12-well plates as recipient cells were cocultured with differentiated α-synuclein-overexpressing SH-SY5Y cells cultured on the insert for 12 h. The recipient cells were prepared for staining with the anti-α-syn antibody (BD Bioscience, Franklin Lakes, NJ, USA).
Cells cultured on coverslips were washed twice with PBS and fixed in 4% paraformaldehyde for 10 min at room temperature; the fixed cells were then washed with PBS and incubated with PBS containing 0.1% Triton X-100 for 10 min at room temperature. After they were washed with PBS, the cells were blocked with PBS containing 1% bovine serum albumin (GenDEPOT, Barker, TX, USA) for 1 hr at room temperature, and then incubated overnight with primary antibodies at 4℃. Preparations were then stained with fluorescence-conjugated secondary antibody (Jackson Immunoresearch, West Grove, PA, USA) for 2 h, mounted, and observed using a model LSM510 or LSM710 confocal microscope (Carl Zeiss, Jena, Germany).
All values are expressed as the mean±SEM. Statistical significance was evaluated using Graphpad Prism 5 (San Diego, CA, USA).
RESULTS
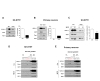
To explore whether UCH-L1 associated with lipid rafts in the same way as other PD-associated proteins, we first isolated lipid rafts of SH-SY5Y cells based on their solubility in 1% Triton X-100 on ice [33] and performed Western blot. As shown in Fig. 1A, we observed about ~20% of total UCH-L1 in the cold Triton X-100 insoluble fraction, and we obtained similar results in the primary neurons (Fig. 1B). When SH-SY5Y cells were treated with methyl-β-cyclodextrin (MβCD), a cholesterol-depleting agent, to exclude the possibility that UCH-L1 was isolated as a contaminant of lipid raft preparations [34], UCH-L1 was translocated into the Triton X-100 soluble fraction (Fig. 1C). To further confirm these observations, we isolated lipid rafts using sucrose density gradient centrifugation fractionation [22], and we also observed a small portion of UCH-L1 in the low-density lipid raft fractions (fractions #4~#6) of SH-SY5Y cells and primary neurons. Additionally, treatment with MβCD induced the translocation of UCH-L1 into non-lipid rafts fractions such as flotillin-1 (flot-1), a lipid raft marker (Fig 1D and 1E), suggesting that UCH-L1 associates with lipid rafts as a lipid raft protein and not as a contaminant.
In our previous report, DJ-1, a PD-associated genes, associate with lipid rafts and regulates lipid raft-dependent endocytosis in astrocytes [22] by regulating flot-1 and caveolin-1 (cav-1) expression [35]. Parkin, which has been known to associate with lipid rafts [20], also regulates lipid raft-dependent endocytosis in MEF cells through regulating cav-1 expression [27]. To explore whether altered UCH-L1 expression was involved in lipid raft function, we generated stable cell lines with downregulated UCH-L1 expression. UCH-L1 expression was efficiently downregulated in both fractions (Fig. 2A, B), and then performed general endocytosis assay using these cell lines. We used lactosylceramide as a marker for lipid raft-dependent endocytosis [36,37] and transferrin as a marker for clathrin-dependent endocytosis [38,39]. As shown in Fig. 2C, downregulation of UCH-L1 in SH-SY5Y cells enhanced lipid raft-dependent endocytosis, but not clathrin-dependent endocytosis. The treatment with LDN-57444, a UCH-L1 inhibitor that inhibits UCH-L1 hydrolase activity [40,41], also enhanced lipid raft-dependent, but not clathrin-dependent endocytosis, suggesting that UCH-L1 regulates lipid raft-dependent endocytosis (Fig. 2D). Next, to explore whether UCH-L1 regulated lipid raft-dependent endocytosis by regulating the expression of flot-1 or cav-1 in the same way as DJ-1 or parkin, we performed Western blot analysis. We observed no changes in flot-1 or -2 expression in UCH-L1 knock-down (KD) cell lines (Fig. 3A) or with LDN-57444 treatment (Fig. 3B). Overexpression of UCH-L1 did not alter the expression of flot-1 and cav-1 or -2 (Fig. 3C), suggesting that lipid raft-dependent endocytosis changes with UCH-L1 do not depend on the expression of flotillins and caveolins.
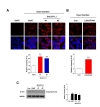
Previously, we demonstrated that extracellular α-synuclein is internalized into cells in a lipid raft-dependent manner [26], and also, signaling for lipid raft-dependent endocytosis is involved in cell-to-cell transmission of α-synuclein [32]. Accordingly, we explored whether UCH-L1 regulates cell-to-cell transmission of α-synuclein. As shown in Fig. 4A, cell-to-cell transmission of α-synuclein was greater in UCH-L1 KD cell lines than in the controls. In addition, UCH-L1 inhibition by LDN-57444 treatment also enhanced cell-to-cell transmission of α-synuclein (Fig. 4B). Endogenous α-synuclein expression did not altered by downregulation of UCH-L1 (Fig. 4C). These data suggest that UCH-L1 regulates cell-to-cell transmission of α-synuclein via lipid raft-dependent endocytosis.
DISCUSSION
The accumulating evidence indicates that the molecular pathways of neurodegeneration triggered by each mutation may be shared by several genetic forms of PD and may also play a role in the common sporadic disease [42,43,44,45,46]. Considering that the sublocalization of proteins is very important for protein function and that many PD-associated proteins, including a-synuclein, parkin, PINK1 and LRRK2, associate with lipid rafts, it is very informative to know whether other PD-associated proteins also associate with lipid rafts to elucidate the common molecular pathways involved in lipid rafts.
In the present study, we demonstrated that UCH-L1 also associates with lipid rafts. Lipid modifications of proteins such as palmitoylation and farnesylation facilitate the association of soluble proteins with cell membranes. Palmitoylation has been known to play important roles in trafficking into lipid rafts [47]; for instance, DJ-1 has been known to associate with lipid rafts via palmitoylation [22]. On the contrary, farnesylation drives membrane proteins to non-rafts domains [48], and UCH-L1 has been known to associate with membranes via farnesylation [30]. Nevertheless, UCH-L1 also associates with lipid rafts; FTI-277, a farnesylation inhibitor [49], and 2-bromopalmitate, a palmitoylation inhibitor [22] did not affect the association of UCH-L1 into lipid rafts (data not shown), suggesting that UCH-L1 associates with lipid rafts in palmitoylation- and farnesylation- independent manners, which is also supported by the finding that UCH-L1 membrane association is neuron specific and not dependent on farnesylation [50]. UCH-L1 also lacks obvious lipid interaction domains, but many deubiquitinases operate as part of larger protein complexes, which may induce the association of UCH-L1 with lipid rafts [1]. Farnesylated H-ras also dynamically associates with lipid rafts by bound nucleotides, regulating downstream signals [51]. More studies will be needed to explore how UCH-L1 associates with lipid rafts.
We also demonstrated that UCH-L1 regulates lipid raft-dependent endocytosis, but it is not dependent on the expression and degradation of caveolin-1 and flotillin-1, unlike DJ-1 and parkin. UCH-L1 is intimately involved in regulating protein ubiquitination, which has emerged as a common element in the internalization of plasma membrane proteins. Cell surface turnover of GLT-1 is mediated by ubiquitination [41], and ubiquitinated EGFR has been reported to be endocytosed through a lipid raft-dependent route [52]. Although we could not identify the exact substrates of UCH-L1 for regulating lipid raft-dependent endocytosis, UCH-L1 may participate in lipid raft-dependent endocytosis by regulating the ubiquitination or deubiquitination of lipid raft proteins.
Interestingly, loss of UCH-L1 enhanced lipid raft-dependent endocytosis and then, cell-to-cell transmission of α-synuclein. Prion-like propagation of protein inclusions such as α-synuclein, tau, and mutant huntingtin in many neurodegenerative diseases has received a great deal of attention. Although the detailed mechanism of intercellular propagation of protein inclusions has not been well understood, this propagation has been anticipated to explain the unveiled pathogenesis of many neurodegenerative diseases [25]. Given that the molecular pathways of neurodegeneration triggered by each mutation may be shared by several genetic forms of PD, it is unsurprising that many PD-related genes have been shown to affect one or more of these processes. Loss of parkin has been reported to be involved in lipid rafts-dependent endocytosis and cell-to-cell transmission of α-synuclein [27]. Glucocerebrosidase depletion, which is strongly associated with PD, promotes propagation of α-synuclein aggregates [53]. The knock-down of some orthologs of some PD-related genes such as catp-6 (
Decreased UCH-L1 expression and oxidative modification of UCH-L1 have been observed in the brains of AD and PD patients [3]. The overexpression of UCH-L1 reduces the number of amyloid beta plaques and improves memory deficits in AD mice [55], suggesting that UCH-L1 has a protective effect in AD models. Accordingly, enhancing UCH-L1 activity may become a useful target against AD and PD progression.
In summary, we demonstrated that UCH-L1 associates with lipid rafts in the same way as other PD-associated gene products, and also that UCH-L1 regulates lipid raft-dependent endocytosis and cell-to-cell transmission of alpha-synuclein. This study provides more evidence that many PD-associated gene products share common signaling pathways to explain the pathogenesis of PD and evidence of UCH-L1 activity as a useful target against the progression of PD.
Figures
References
- Bishop P, Rocca D, Henley JM. Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction. Biochem J 2016;473:2453-2462.
- Lowe J, McDermott H, Landon M, Mayer RJ, Wilkinson KD. Ubiquitin carboxyl-terminal hydrolase (PGP 9.5) is selectively present in ubiquitinated inclusion bodies characteristic of human neurodegenerative diseases. J Pathol 1990;161:153-160.
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem 2004;279:13256-13264.
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson's disease. Nature 1998;395:451-452.
- Maraganore DM, Farrer MJ, Hardy JA, Lincoln SJ, McDonnell SK, Rocca WA. Case-control study of the ubiquitin carboxy-terminal hydrolase L1 gene in Parkinson's disease. Neurology 1999;53:1858-1860.
- Lincoln S, Vaughan J, Wood N, Baker M, Adamson J, Gwinn-Hardy K, Lynch T, Hardy J, Farrer M. Low frequency of pathogenic mutations in the ubiquitin carboxy-terminal hydrolase gene in familial Parkinson's disease. Neuroreport 1999;10:427-429.
- Zhu R, Zhu Y, Liu X, He Z. UCH-L1 S18Y variant and risk of Parkinson's disease in Asian populations: an updated meta-analysis. Neurodegener Dis 2014;14:194-203.
- Korade Z, Kenworthy AK. Lipid rafts, cholesterol, and the brain. Neuropharmacology 2008;55:1265-1273.
- Marin R, Fabelo N, Fernández-Echevarría C, Canerina-Amaro A, Rodríguez-Barreto D, Quinto-Alemany D, Mesa-Herrera F, Díaz M. Lipid raft alterations in aged-associated neuropathologies. Curr Alzheimer Res 2016;13:973-984.
- Schengrund CL. Lipid rafts: keys to neurodegeneration. Brain Res Bull 2010;82:7-17.
- Zhai J, Ström AL, Kilty R, Venkatakrishnan P, White J, Everson WV, Smart EJ, Zhu H. Proteomic characterization of lipid raft proteins in amyotrophic lateral sclerosis mouse spinal cord. FEBS J 2009;276:3308-3323.
- Chadwick W, Brenneman R, Martin B, Maudsley S. Complex and multidimensional lipid raft alterations in a murine model of Alzheimer's disease. Int J Alzheimers Dis 2010;2010:604792.
- Rushworth JV, Hooper NM. Lipid rafts: linking Alzheimer's amyloid-β production, aggregation, and toxicity at neuronal membranes. Int J Alzheimers Dis 2010;2011:603052.
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci 2004;24:6715-6723.
- Valencia A, Reeves PB, Sapp E, Li X, Alexander J, Kegel KB, Chase K, Aronin N, DiFiglia M. Mutant huntingtin and glycogen synthase kinase 3-beta accumulate in neuronal lipid rafts of a presymptomatic knock-in mouse model of Huntington's disease. J Neurosci Res 2010;88:179-190.
- Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem 1997;272:6324-6331.
- Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem 2005;280:36815-36823.
- Hur JY, Teranishi Y, Kihara T, Yamamoto NG, Inoue M, Hosia W, Hashimoto M, Winblad B, Frykman S, Tjernberg LO. Identification of novel γ-secretase-associated proteins in detergent-resistant membranes from brain. J Biol Chem 2012;287:11991-12005.
- Wada S, Morishima-Kawashima M, Qi Y, Misono H, Shimada Y, Ohno-Iwashita Y, Ihara Y. Gamma-secretase activity is present in rafts but is not cholesterol-dependent. Biochemistry 2003;42:13977-13986.
- Fallon L, Moreau F, Croft BG, Labib N, Gu WJ, Fon EA. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem 2002;277:486-491.
- Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet 2005;14:3477-3492.
- Kim KS, Kim JS, Park JY, Suh YH, Jou I, Joe EH, Park SM. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet 2013;22:4805-4817.
- Hatano T, Kubo S, Imai S, Maeda M, Ishikawa K, Mizuno Y, Hattori N. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum Mol Genet 2007;16:678-690.
- Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol 2010;6:702-706.
- Stopschinski BE, Diamond MI. The prion model for progression and diversity of neurodegenerative diseases. Lancet Neurol 2017;16:323-332.
- Park JY, Kim KS, Lee SB, Ryu JS, Chung KC, Choo YK, Jou I, Kim J, Park SM. On the mechanism of internalization of alpha-synuclein into microglia: roles of ganglioside GM1 and lipid raft. J Neurochem 2009;110:400-411.
- Cha SH, Choi YR, Heo CH, Kang SJ, Joe EH, Jou I, Kim HM, Park SM. Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: implications for Parkinson's disease. Mol Neurodegener 2015;10:63.
- Chen J, Huang RY, Turko IV. Mass spectrometry assessment of ubiquitin carboxyl-terminal hydrolase L1 partitioning between soluble and particulate brain homogenate fractions. Anal Chem 2013;85:6011-6017.
- Nagamine S, Kabuta T, Furuta A, Yamamoto K, Takahashi A, Wada K. Deficiency of ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1) leads to vulnerability to lipid peroxidation. Neurochem Int 2010;57:102-110.
- Liu Z, Meray RK, Grammatopoulos TN, Fredenburg RA, Cookson MR, Liu Y, Logan T, Lansbury PT. Membrane-associated farnesylated UCH-L1 promotes alpha-synuclein neurotoxicity and is a therapeutic target for Parkinson's disease. Proc Natl Acad Sci U S A 2009;106:4635-4640.
- Choi YR, Kang SJ, Kim JM, Lee SJ, Jou I, Joe EH, Park SM. FcγRIIB mediates the inhibitory effect of aggregated α-synuclein on microglial phagocytosis. Neurobiol Dis 2015;83:90-99.
- Choi YR, Cha SH, Kang SJ, Kim JB, Jou I, Park SM. Prion-like propagation of α-synuclein is regulated by the FcγRIIB-SHP-1/2 signaling pathway in neurons. Cell Reports 2018;22:136-148.
- Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci 1997;110:673-679.
- Zheng YZ, Berg KB, Foster LJ. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J Lipid Res 2009;50:988-998.
- Kim JM, Cha SH, Choi YR, Jou I, Joe EH, Park SM. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci Rep 2016;6:28823.
- Marks DL, Singh RD, Choudhury A, Wheatley CL, Pagano RE. Use of fluorescent sphingolipid analogs to study lipid transport along the endocytic pathway. Methods 2005;36:186-195.
- Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell 2003;14:3254-3265.
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 2004;5:121-132.
- Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev 2003;55:1439-1466.
- Tan YY, Zhou HY, Wang ZQ, Chen SD. Endoplasmic reticulum stress contributes to the cell death induced by UCH-L1 inhibitor. Mol Cell Biochem 2008;318:109-115.
- Martínez-Villarreal J, García Tardón N, Ibáñez I, Giménez C, Zafra F. Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia 2012;60:1356-1365.
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006;441:1162-1166.
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006;441:1157-1161.
- Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet 2011;20:40-50.
- Greggio E, Bisaglia M, Civiero L, Bubacco L. Leucinerich repeat kinase 2 and alpha-synuclein: intersecting pathways in the pathogenesis of Parkinson's disease?. Mol Neurodegener 2011;6:6.
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 2010;29:3571-3589.
- Levental I, Lingwood D, Grzybek M, Coskun U, Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A 2010;107:22050-22054.
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 1996;65:241-269.
- Lerner EC, Qian Y, Blaskovich MA, Fossum RD, Vogt A, Sun J, Cox AD, Der CJ, Hamilton AD, Sebti SM. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J Biol Chem 1995;270:26802-26806.
- Bishop P, Rubin P, Thomson AR, Rocca D, Henley JM. The ubiquitin C-terminal hydrolase L1 (UCH-L1) C terminus plays a key role in protein stability, but its farnesylation is not required for membrane association in primary neurons. J Biol Chem 2014;289:36140-36149.
- Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol 2001;3:368-375.
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A 2005;102:2760-2765.
- Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, Lee HJ, Kim S, Masliah E, Sardi SP, Lee SJ. Glucocerebrosidase depletion enhances cell-to-cell transmission of α-synuclein. Nat Commun 2014;5:4755.
- Tyson T, Senchuk M, Cooper JF, George S, Van Raamsdonk JM, Brundin P. Novel animal model defines genetic contributions for neuron-to-neuron transfer of α-synuclein. Sci Rep 2017;7:7506.
- Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 2006;126:775-788.



