Revised: June 27, 2014
Accepted: July 25, 2014
Published online: August 6, 2014
In 2012, about 16487 people received kidney transplants in the United States, whereas 95022 candidates were on the waiting list by the end of the year. Despite advances in renal transplant immunology, approximately 40% of recipients will die or lose graft within 10 years. The limitations of current therapies for renal failure have led researchers to explore the development of modalities that could improve, restore, or replace the renal function. The aim of this paper is to describe a reasonable approach for kidney regeneration and review the current literature regarding cell sources and mechanisms to develop a bioengineering kidney. Due to kidneys peculiar anatomy, extracellular matrix based scaffolds are rational starting point for their regeneration. The perfusion of detergents through the kidney vasculature is an efficient method for delivering decellularizing agents to cells and for removing of cellular material from the tissue. Many efforts have focused on the search of a reliable cell source to provide enrichment for achieving stable renal cell systems. For an efficient bioengineered kidney, these cells must be attached to the organ and then maturated into the bioractors, which simulates the human body environment. A functional bioengineered kidney is still a big challenge for scientists. In the last ten years we have got many improvements on the field of solid organ regeneration; however, we are still far away from the main target. Currently, regenerative centers worldwide have been striving to find feasible strategies to develop bioengineered kidneys. Cell-scaffold technology gives hope to end-stage renal disease patients who struggle with morbidity and mortality due to extended periods on dialysis or immunosupression. The potential of bioengineered organ is to provide a reliable source of organs, which can be refunctionalized and transplanted.
Core tip: In 2012, about 16487 people received kidney transplants in the United States, whereas 95022 candidates were on the waiting list by the end of the year. Despite advances in renal transplant immunology, 20% of recipients will experience an episode of acute rejection within 5 years of transplantation, and approximately 40% of recipients will die or lose graft function within 10 years. The aim of this paper is to describe a reasonable approach for kidney regeneration and review the current literature regarding possible cell sources and mechanisms to develop a bioengineering kidney.
- Citation: Zambon JP, Magalhaes RS, Ko I, Ross CL, Orlando G, Peloso A, Atala A, Yoo JJ. Kidney regeneration: Where we are and future perspectives. World J Nephrol 2014; 3(3): 24-30
- URL: https://www.wjgnet.com/2220-6124/full/v3/i3/24.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i3.24
In the United States, approximately 1 million patients live with end-stage renal disease (ESRD), with over 100000 new diagnoses every year. Although hemodialysis has increased the survival of patients with ESRD, kidney transplantation remains the only potential curative treatment. In 2012, about 16487 people received kidney transplants in the United States, whereas 95022 candidates were on the waiting list by the end of the year. Despite advances in renal transplant immunology, 20% of recipients will experience an episode of acute rejection within 5 years of transplantation, and approximately 40% of recipients will die or lose graft function within 10 years[1-4].
The limitations of current therapies for renal failure have led researchers to explore the development of alternative modalities that could improve, restore, or replace either partial or total renal function. Tissue engineering and regenerative medicine represents one of the newest innovations in modern-day science. It represents a broad spectrum of methodologies and techniques aiming to repair, augment, and regenerate damaged organs and tissues. The basis of tissue engineering is that cells can be expanded in vitro, placed on a tissue scaffold made of suitable biomaterial, and then implanted into the host[5-7].
Within the field of organ bioengineering, the methodology of seeding cells on supporting scaffolding material has shown great promise for generating viable organs. A simple structure such as vessels, bladders, upper airways, and urethras have been implanted into patients with acceptable results in the short and midterms. The structural simplicity of these organs enables them to meet the oxygen and nutrient requirements via diffusion from adjacent host tissues while angiogenesis has time to occur. Unfortunately, complex organs such as the kidney cannot be viably incorporated without the reconnection of new structure to the host vasculature, a task that has presented insurmountable challenges experimentally let alone clinically[8-12].
The aim of this paper is to describe a reasonable approach for kidney regeneration and review the current literature regarding possible cell sources and mechanisms to develop a bioengineering kidney.
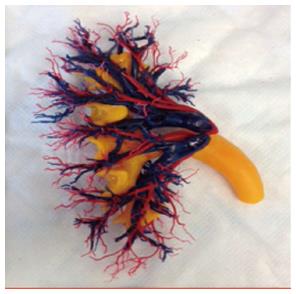
The kidneys are the primary organs for maintaining fluid, electrolyte, and acid-base balance. They produce hormones such as rennin, erythropoietin, and convert a precursor of vitamin D, 1,25-dihydroxyvitamin D, to active metabolite. Each kidney has more than thirty different cell types, approximately 2 million glomeruli, numerous arterioles, capillaries, and tubules that interconnect in a three dimensional pattern to filter the blood and excrete waste through the collecting system[13]. Figure 1 represents an example of a kidney’s vascular cast.
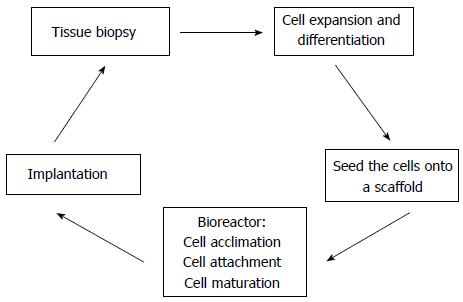
Ideally, a bioengineered kidney must be biocompatible, non-immunogenic and support cell growth. The basic idea for solid organ regeneration is to harvest a tissue biopsy from a donor and expand these cells in culture. Subsequently, they are seeded into a scaffold, and placed into a bioreactor in order to promote cell acclimation, attachment, and maturation. Once this is achieved, the cell-seeded scaffold can be implanted on the host[14,15]. Figure 2 represents a potential methodology for solid organ regeneration.
Extracellular matrix (ECM) is the naturally occurring scaffold material secreted and manufactured by the resident cells of each tissue and organ. The complex 3D organization of the ECM and its components are dictated by the tissue from which ECM is derived. The structural and functional molecules of the ECM are in a state of dynamic equilibrium within the surrounding tissues, and also provide the means by which cells communicate with each other and the external environment. The ECM contains growth factors and others bioinductive factors, which facilitates the remodeling process, cell attachment and tissue integration[16,17].
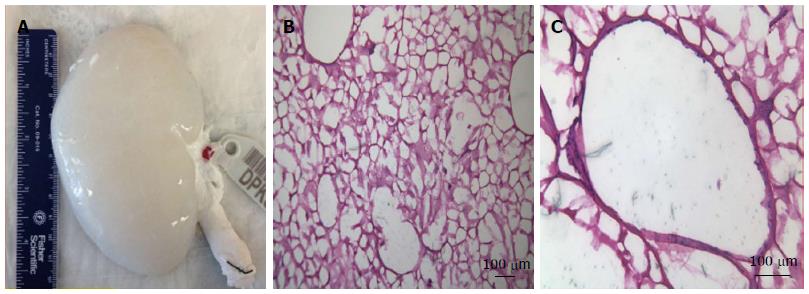
Due to their peculiar anatomy and physiology, ECM based scaffolds are a rational starting point for kidney regeneration. For this purpose, several protocols have been described for whole kidney decellularization. The perfusion of detergents and enzymes (e.g., DNase) through the kidney vasculature is an efficient method for delivering decellularizing agents to cells and for removing of cellular material from the tissue. However, in spite of being effective decellularization agents, the effects of these agents on kidney microvasculature have not been established, and further studies are necessary to elucidate them[18-20]. Figure 3 represents a decellularized pig kidney scaffold and its extra cellular matrix after decellularuzation.
The kidney has approximately 30 different specialized cell types. For an efficient scaffold, all cells must be characterized for repopulation, which represents a challenging task. Many efforts have focused on the search of a reliable cell source and optimal growth conditions to provide adequate enrichment for achieving stable renal cell expansion systems[4].
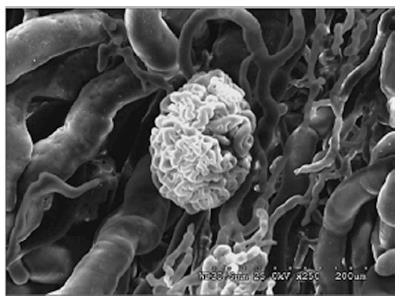
Regarding vascular anatomy, the main renal artery splits into segmental, interlobar, interlobular, and arcuate arteries. The venous system is formed by a complex net of veins, which drains to the main renal vein. Each kidney has approximately 2 million glomeruli, which are responsible for renal filtration process and an extensive net of capillaries[13]. Figure 4 represents a pig kidney’s glomerulus.
The regenerative capacity of a tissue is determined in part by whether it contains endogenous stem cells. These stem cell populations are housed in a niche, which regulates stem cell survival, self-renewal, and differentiation. In a normal environment, stem cells remain quiescent in the niche for long periods until they are activated by the requirement of new cells to maintain the tissue or because of the tissue damage. With regards to renal adult stem cells, a subset has been found in the Bowman’s capsule, glomeruli, pericytes, proximal tubules, and renal papilla. These cells expressed stem cell markers such as CD24, CD133, CD146 and Pax-2[21-25].
Regenerative mechanisms after acute renal failure have not been well established, but appear that tubular cells, growth factors and cytokines are involved in this process as well demonstrated by Humphreys et al[26]. They reported via a genetic maping technique, that tubular epithelial cells were the predominant source of regeneration after kidney ischemic injury, and distal tubular cells were more involved with growth factor production such as epidermal, IGF-1, and hepatocyte growth factor[26,27].
Harari-Steinberg et al[28] identified in human kidneys nephron progenitor cells (hNPCs), which were capable of generation of kidney structures and functional repair of chronic renal disease. These cells expressed NCAM1+ and had a high clonogenic potential. Moreover, when grafted in aggregates into a chorioallantoic membrane of the chick embryo they generate renal structures. Ultimately, hNPCs were injected directly into the kidney’s parenchyma of mice with chronic kidney disease. They reported that treatment with hNPCs halted disease progression and increased creatinine clearance throughout the 12-wk study period[28].
Buzhor et al[29] also demonstrated that human adult kidney epithelial cells (hKEpCs) positive for NCAM1+ overexpressed nephron progenitor markers, acquired a mesenchymal fate and produce epithelial renal tissue on single-cell grafting in chick chorioallantoic membrane and mouse.
Rinkevich et al[30] demonstrated an in vivo clonal analysis of progenitor cells found in mammalian kidneys. They used a long-term in vivo genetic lineage tracing and clonal analysis of individual cells isolated from kidneys and demonstrated that tissue and lineage-restricted precursors cells from tubules and non tubules structures such as glomerulus are directly involved in the kidney recovery after injury. As a future direction, the isolation and characterization of kidney precursor cells offer a therapeutic target to increase or restore the regenerative capacity of the mamallian kidney[30].
The bone marrow contains two major populations of stem cells, hematopoietic stem cells (HSCs), and mesenchymal stromal cells (MSC), which provide stromal support for HSCs. These stem cell therapies derived from bone marrow have been used to repair a variety of organs in experimental models. A possible explanation for apparent plasticity of these cells is a mechanism of transdifferentiation, dedifferentiation or cell fusion[31].
Despite studies have shown promising results with bone marrow derived stem cells, the biological relevance and clinical importance have not been well demonstrated for kidney regeneration. Initially, bone-marrow stem cells have been considered as a source of “replacement” cells that could be used for the treatment of different diseases. However, studies in experimental transplantation models or direct injection of these cells into tissue have shown that the contribution of bone-marrow cells to nonhematopoietic cell fates is uncommon. Therefore, the potential of these cells for whole organ regeneration is far from being considered as a treatment option[32,33].
Mesenchymal stem cells (MSC) are stromal cells that can be found and isolated from different tissues. Because of their multilineage differentiation potential and their ability to migrate to the site of injury, they have been studied in the last 10 years as a therapeutic agent in kidney injury. It has been demonstrated that MSC are involved in immune response through the activation of T and B-lymphocytes. In addition, they stimulate interleukins, β-TGF, hepatocyte, fibroblast, and vascular endothelial growth factors[34,35].
Adipocyte derived stem cells (ADSC) are a type of mesenchymal stem cell that in the last decade have been explored as an attractive source of cells with regenerative properties. These cells are abundant, easily harvested with low morbidity, and seem to stimulate angiogenesis. Regarding kidney regeneration, de Almeida et al[36] demonstrated in an experimental model of acute renal failure that the injection of ADSC in mice reduced renal fibrosis at six weeks; however, they did not find the injected cells in the kidney. Chen et al[37] also demonstrated in rats that an intra-renal injection of ADSC attenuated the deterioration of renal function at 14 d, improved angiogenesis, and preserved the architecture integrity[36,37].
Despite being an attractive source of stem cells for kidney regeneration, the exact mechanism of action of MSCs has not been established. Most of the experimental studies have been performed with rodents and have short follow up. Further studies with a longer follow up and using large animal models should be performed before translating to clinical trials.
Amniotic fluid, due to its contact with the fetus, has been considered an interesting source for undifferentiated or partially differentiated cells. The molecular composition of amniotic fluid and the presence of nutritive substances play a key role in the proliferation and differentiation of different cell types.
Human amniotic stem cells (HASC) express surface markers and transcription factors distinctive of embryonic stem cells (ESCs). These include octamer-binding transcription factor 4 (OCT-4) and stage specific embryonic antigen (SSEA-4)[38]. HASCs have high replicative self-renewal potential and multilineage differentiation capacity. Perin et al[39,40] showed that HASC integrated into metanephric structures after being injected into embryonic kidneys, which improved repair/recovery of kidneys with acute tubular necrosis[39-41].
In the field of cell therapy and regenerative medicine, many studies have been done to establish reliable animal models for different types of disease targeting the feasibility and benefits of human amniotic stem cell therapy.
ESC are pluripotent cells derived from blastocysts. These cells propagate readily and remain undifferentiated when cultured with leukemia inhibitory factor (LIF). When LIF is withdrawn, ESCs form aggregates called embryoid bodies that generate a variety of specialized cell types. However, the extraction of these cells involves the destruction of embryos, therefore their use is associated with controversial ethical dilemmas[42].
In spite of the self-renewing potential and the capability of differentiation into tissues derived from the three germ layers, ESCs are associated with uncontrolled growth and teratoma formation. Kidney regeneration studies have demonstrated that renal progenitor cells derived from ESCs differentiated into glomerular-like structures and integrated into renal proximal tubules when implanted in vivo[42,43].
This approach for generating kidney tissue can be achieved by the use of undifferentiated or partially developed kidney precursor cells derived from early embryos and fetal tissue. Dekel et al[44] transplanted in immunodeficient mice human or pig kidney precursors, which were obtained from 7 to 8 wk human fetus or 3.5 to 4 wk pig gestation. The rudimentar kidneys survive, grow and complete nephrogenesis, forming a functional organ able to produce urine. The successful organogenesis was achieved only when early progenitors with mesenchymal cells and ureteric bud branches were transplanted. Nevertheless, as well as embryonic cells, this approach also involves the destruction of embryos and is associated with controversial ethical dilemmas[44].
The first renal tissue created via therapeutic cloning techniques has been described by Lanza et al[45] in a bovine model. In this study, a skin fibroblast nucleus was microinjected into an enucleated oocyte that was transplanted in vitero for 12 wk. Cloned renal cells were then seeded into a biodegradable scaffold and transplanted in vivo to follow the growth of the construct. The authors reported that the kidney-like organ was capable of secreting urinary fluid, confirming that the implant contained cells capable of filtration, reabsorption and secretion. However, like ESCs, this technique also is associated with controversial ethical dilemmas[45,46].
Induced pluripotent stem cells (iPSC) were first described by Takahashi and Yamanaka in 2006 when they reprogrammed human fibroblasts to become pluripotent stem cells by the addition of four different genes: Oct3/4, Sox2, c-Myc, and Klf4. Despite being a good source of cells, not all adult stem cells can be reprogrammed using the same method, which means that each cell type may have critical factors. Unlike ESCs, iPS cells have no ethical issues and no immune rejection. On the other hand, these cells are reprogrammed through the addition of oncogenes, which increase the risk of uncontrolled growth[47-49].
The surrogate application of iPSC as representative of kidney disease is increasingly becoming reality given recent advances involving the production of iPSC from both mesangial and epithelial cells derived from urine[45]. In addition to that, iPSCs have been generated from proximal tubular cells and podocytes[46]. Despite promising results, some issues should be highlighted before clinical application. First of all, there are no established differentiation protocols for moving from pluripotent state to functional kidney cell. Second, there are no optimal culture conditions for targeting cells. Third, a stepwise differentiation depends on specific factors, which must be identified[50,51].
For whole organ regeneration, cells need to be attached to the organ and then maturated. The bioreactor simulates the human body environment; however, each organ depends on different conditions such as perfusion rate, temperature, CO2 concentration, growth factors, and nutrients, etc. Due to kidney complexity, the recellularization of a functional kidney is still a big challenge for scientists. In 2013, Song et al[52] published the first experimental orthotopic transplantation of a bioengineered kidney in rats. They repopulated acellular rat kidneys with endothelial and epithelial cells through the renal artery and ureter respectively. Engrafted epithelial cells were found to reestablish polarity and organize in tubular structures expressing Na/K-ATPase and aquaporin similar to native proximal tubular epithelium. Also, epithelial cells formed structures resembling native distal tubular epithelium and lined the renal pelvis similar to native transitional epithelium. Transmission and scanning electron microscopy of regenerated kidneys showed perfused glomerular capillaries with engrafted podocytes and formation of foot processes. However, as they suggested, before translating this technology, it will require optimization of cell seeding protocols, upscaling of biomimetic organ culture, as well as isolation, differentiation, and expansion of the required cell types[52].
In the field of regenerative medicine and whole organ regeneration many efforts have been carried out to translate this technology for clinical practice. The ultimate goal is to provide a feasible and reliable therapy for different types of diseases. In this context, although there are many improvements on kidney regeneration, there are still many hurdles to be overcome, and as our knowledge improves, more complex questions remain unclear.
A functional bioengineered kidney is still a big challenge for scientists. In the last ten years we have got many improvements on the field of solid organ regeneration; however, we are still faraway from the main target. Currently, regenerative centers worldwide have been striving to find feasible strategies to develop bioengineered kidneys. Cell-scaffold technology gives hope to end-stage renal disease patients who struggle with morbidity and mortality due to extended periods on dialysis or immunosupression. The potential of bioengineered organ is to provide a reliable source of organs, which can be refunctionalized and transplanted.
P- Reviewer: Olowu WA S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3684] [Cited by in F6Publishing: 3624] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 2. | Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedwald JJ, Hays R, Howard A, Jones E, Leichtman AB. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes QualityInitiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3:471–480. [DOI] [Cited in This Article: ] [Cited by in Crossref: 404] [Cited by in F6Publishing: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Organ Procurement and Transplantation Network: United Network for Organ Sharing 2012 Annual Report: Health Resources and Services Administration. Richmond, US Department of Health and Human Services, 2012. Available from: http://www.unos.org/docs/AnnualReport2012.pdf. [Cited in This Article: ] |
| 4. | Katari R, Peloso A, Zambon JP, Soker S, Stratta RJ, Atala A, Orlando G. Renal bioengineering with scaffolds generated from human kidneys. Nephron Exp Nephrol. 2014;126:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617-12621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1151] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 6. | Atala A. Regenerative medicine and urology. BJU Int. 2003;92 Suppl 1:58-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Atala A. Future perspectives in reconstructive surgery using tissue engineering. Urol Clin North Am. 1999;26:157-165, ix-x. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Orlando G, Wood KJ, De Coppi P, Baptista PM, Binder KW, Bitar KN, Breuer C, Burnett L, Christ G, Farney A. Regenerative medicine as applied to general surgery. Ann Surg. 2012;255:867-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Orlando G, Soker S, Stratta RJ. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Ann Surg. 2013;258:221-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 785] [Cited by in F6Publishing: 671] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 11. | Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol. 2009;20:2338-2347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, AbouShwareb T, De Coppi P, Wood KJ, Stratta RJ. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012;256:363-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Guimaraes-Souza N, Soler R, Yoo JJ. Regenerative medicine of the kidney. 1st ed. FL: CRC Press LLC 2009; 502-517. [DOI] [Cited in This Article: ] |
| 14. | Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A. Engineering complex tissues. Tissue Eng. 2006;12:3307-3339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 440] [Cited by in F6Publishing: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 15. | Basu J, Ludlow JW. Developmental engineering the kidney: leveraging principles of morphogenesis for renal regeneration. Birth Defects Res C Embryo Today. 2012;96:30-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608-616. [DOI] [Cited in This Article: ] [Cited by in Crossref: 705] [Cited by in F6Publishing: 688] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 18. | Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233-3243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2088] [Cited by in F6Publishing: 2148] [Article Influence: 165.2] [Reference Citation Analysis (0)] |
| 19. | Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, Zhang L, D’Amore A, Badylak SF. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014;10:183-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, Yoo JJ. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756-7764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 21. | Al-Awqati Q, Oliver JA. The kidney papilla is a stem cells niche. Stem Cell Rev. 2006;2:181-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Franquesa M, Flaquer M, Cruzado JM, Grinyó JM. Kidney regeneration and repair after transplantation. Curr Opin Organ Transplant. 2013;18:191-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443-2456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 497] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 24. | Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 25. | Bruno S, Camussi G. Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Methods Mol Biol. 2012;879:367-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Humphreys BD. Kidney structures differentiated from stem cells. Nat Cell Biol. 2014;16:19-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Kusaba T, Humphreys BD. Controversies on the origin of proliferating epithelial cells after kidney injury. Pediatr Nephrol. 2014;29:673-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Harari-Steinberg O, Metsuyanim S, Omer D, Gnatek Y, Gershon R, Pri-Chen S, Ozdemir DD, Lerenthal Y, Noiman T, Ben-Hur H. Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med. 2013;5:1556-1568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Buzhor E, Omer D, Harari-Steinberg O, Dotan Z, Vax E, Pri-Chen S, Metsuyanim S, Pleniceanu O, Goldstein RS, Dekel B. Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am J Pathol. 2013;183:1621-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, Lim X, Van-Amerongen R, Bowman A, Januszyk M. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep. 2014;7:1270-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 31. | Chou YH, Pan SY, Yang CH, Lin SL. Stem cells and kidney regeneration. J Formos Med Assoc. 2014;113:201-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Iwasaki M, Adachi Y, Minamino K, Suzuki Y, Zhang Y, Okigaki M, Nakano K, Koike Y, Wang J, Mukaide H. Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. J Am Soc Nephrol. 2005;16:658-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Nishida M, Fujimoto S, Toiyama K, Sato H, Hamaoka K. Effect of hematopoietic cytokines on renal function in cisplatin-induced ARF in mice. Biochem Biophys Res Commun. 2004;324:341-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 314] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 35. | Evren S, Loai Y, Antoon R, Islam S, Yeger H, Moore K, Wong K, Gorczynski R, Farhat WA. Urinary bladder tissue engineering using natural scaffolds in a porcine model: role of Toll-like receptors and impact of biomimetic molecules. Cells Tissues Organs. 2010;192:250-261. [PubMed] [Cited in This Article: ] |
| 36. | de Almeida DC, Donizetti-Oliveira C, Barbosa-Costa P, Origassa CS, Câmara NO. In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. Clin Biochem Rev. 2013;34:131-144. [PubMed] [Cited in This Article: ] |
| 37. | Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9-51. [Cited in This Article: ] |
| 38. | De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1406] [Cited by in F6Publishing: 1205] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 39. | Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, Warburton D, Atala A, De Filippo RE. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, Lemley KV, Rosol M, Wu S, Atala A. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One. 2010;5:e9357. [PubMed] [Cited in This Article: ] |
| 41. | Da Sacco S, De Filippo RE, Perin L. Amniotic fluid as a source of pluripotent and multipotent stem cells for organ regeneration. Curr Opin Organ Transplant. 2011;16:101-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Kramer J, Steinhoff J, Klinger M, Fricke L, Rohwedel J. Cells differentiated from mouse embryonic stem cells via embryoid bodies express renal marker molecules. Differentiation. 2006;74:91-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, Fehling HJ, Keller G, Burrow C, Wilson P. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18:1709-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH. Human and porcine early kidney precursors as a new source for transplantation. Nat Med. 2003;9:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 45. | Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT. Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol. 2002;20:689-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 314] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 46. | Colman A, Kind A. Therapeutic cloning: concepts and practicalities. Trends Biotechnol. 2000;18:192-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Vogelstein B, Alberts B, Shine K. Genetics. Please don’t call it cloning! Science. 2002;295:1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17989] [Cited by in F6Publishing: 17055] [Article Influence: 947.5] [Reference Citation Analysis (0)] |
| 49. | Zhou T, Benda C, Dunzinger S, Huang Y, Ho JC, Yang J, Wang Y, Zhang Y, Zhuang Q, Li Y. Generation of human induced pluripotent stem cells from urine samples. Nat Protoc. 2012;7:2080-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 50. | Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 51. | O’Neill AC, Ricardo SD. Human kidney cell reprogramming: applications for disease modeling and personalized medicine. J Am Soc Nephrol. 2013;24:1347-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 595] [Article Influence: 54.1] [Reference Citation Analysis (0)] |