Published online Dec 19, 2021. doi: 10.5498/wjp.v11.i12.1206
Peer-review started: March 26, 2021
First decision: June 17, 2021
Revised: June 20, 2021
Accepted: October 25, 2021
Article in press: October 25, 2021
Published online: December 19, 2021
Attention deficit hyperactivity disorder (ADHD) is a common and impairing behavioral health disorder, impacting over 5% of children worldwide. There are multiple evidence-based pharmacological and psychosocial treatments for ADHD, and greater service utilization is associated with improved acute and long-term outcomes. However, long-term outcomes are suboptimal as multimodal treatments are often not accessed and most care ends prematurely. This narrative review discusses barriers to engagement for children and adolescents with ADHD and their families as well as interventions to overcome these barriers. Families face a variety of structural and attitudinal barriers, ranging from cost and access to stigma and low self-efficacy to successfully implement change. There are multiple interventions that may enhance engagement with ADHD care including psychoeducation, integration of behavioral services in general medical settings, telehealth as well as specific adaptations to existing ADHD treatments, such as the use of motivational interviewing or shared decision making. Integration of behavioral health into general medical settings and telehealth have been found in controlled studies to increase access by reducing both structural and attitudinal barriers. Adding motivational interviewing, shared decision making and other engagement interventions to evidence-based ADHD treatments has been found to reduce attitudinal barriers that translates into improved participation and satisfaction while enhancing outcomes. However, little is known about how to promote extended engagement with ADHD services even though a chronic care model for ADHD is recommended.
Core Tip: Assessment of families’ motivation for care at treatment initiation and recurrently over the course of treatment, especially during times of increasing stress and declining functioning, is essential to promote sustained engagement. Aspects of motivation that predict sustained engagement include desire and readiness for care, treatment preferences, self-efficacy to access and implement the selected treatment and perceived barriers to the treatment. Integrating services into trusted medical settings to reduce stigma, telehealth to reduce the burden of care, shared decision making to promote autonomy, psychoeducation about treatment options and how attention deficit hyperactivity disorder impacts current functioning and motivational interviewing can be employed to promote engagement.
- Citation: Baweja R, Soutullo CA, Waxmonsky JG. Review of barriers and interventions to promote treatment engagement for pediatric attention deficit hyperactivity disorder care. World J Psychiatr 2021; 11(12): 1206-1227
- URL: https://www.wjgnet.com/2220-3206/full/v11/i12/1206.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i12.1206
Attention deficit hyperactivity disorder (ADHD) is a common and impairing behavioral health (BH) disorder, impacting over 5% of children worldwide[1]. Impairment often persists into adulthood leading to recommendation to treat ADHD with a chronic care model[2,3]. Multiple evidence-based pharmacological and psychosocial interventions exist for ADHD[3-5]. In the United States, most children diagnosed with ADHD receive some treatment for it with 5% of school-aged children prescribed ADHD medication. In other countries rates of ADHD medication use in children are often below the reported prevalence rates, varying from 3% (France, Japan) to 5% (Iceland)[6]. Across countries, multimodal treatment and rates of any counseling services rates under 50%, even in countries where treatment guidelines prioritize initial treatment with behavioral interventions[7-11]. In the United States, most care starts in primary care, with primary care clinicians (PCCs) being the most common provider of ADHD services[12]. However, a number of specialty medical providers treat ADHD and schools offer a variety of therapeutic supports[7,12].
Most countries in the European Union and the United Kingdom have universal access, National Health Systems, funded by the State via taxpayer deduction on the worker´s paychecks. These systems usually also cover the rest of the family, including children, even if the parents are not working at the time. There are different levels of coverage within the European Union especially for medication and counseling services leading to appreciable variations in ADHD care across national boundaries. Access to specialists varies widely across countries, as do the administrative requirements to get to specialty care. Initial assessment and diagnosis can start in general or specialty settings[13,14]. For example, in the United Kingdom, patients with possible ADHD are referred to a Child Psychiatry and Psychology Evaluation Unit that performs a comprehensive medical, psychiatric, psychological, school and social evaluation that may take several months to be completed. In Italy, there are less than 10 Pediatric Neuropsychiatry Outpatients Units that are allowed to prescribe methylphenidate, creating substantial waits for a country with a population of 60 million. In Spain, Child and Adolescent Psychiatry is not recognized as a specialty yet. Pediatricians can start medication, and can refer to either Pediatric Neurology (usually younger children and those with medical comorbidity as epilepsy or neurodevelopmental delays) or Child Psychiatry Units usually staffed by general psychiatrists with variable levels of expertise.
Despite well-established assessments and evidence-based and available treatments, long-term outcomes are suboptimal across countries[2,15,16]. One major challenge to achieving optimal long-term outcome is treatment engagement. Initiating care can be a challenge as many referred families fail to access any care[17]. When initiated, care is often interrupted or quickly discontinued. In both primary care and specialty care settings, utilization rapidly declines over the first year of treatment[18-20]. In the United States, care for nearly 60% of publically insured youth does not meet federal guidelines for frequency of reassessment[21]. Similarly, low rates have been found in Australia, with only 28% children diagnosed with ADHD receiving any services in the past 6 mo[22] and sizable percentage of children with ADHD in United Kingdom are not receiving treatment[23]. When stopping care, most patients still exhibit appreciable impairment[21] and many who experience interrupted care never return[24].
Numerous studies have shown rapid declines in ADHD medication usage over time. In both Europe and the United States, over half of children stop medication within 6 mo[25,26]. Rates of treatment utilization for CNS stimulants continue to decline after year one[27]. By the end of adolescence, only 10% of youth with ADHD are using medication even half of the time[2]. Even short-term medication adherence is challenging as over one month, only about 40% of children take every dose of medication prescribed[28].
Engagement with counseling services face similar if not greater challenges to those for medication treatments. In the United States, many families never access counseling, as these services are less likely to be available in general medical settings than medication treatments[29,30]. Up to half of referrals for counseling services for ADHD fail to translate to any treatment[31] as families often stop care when referred to external BH providers[18]. For those who do connect, care often ends prematurely[20,32]. In a review of insurance claims data in the United States from 2008-2014, under half of families with a child diagnosed with ADHD accessed any billable psychosocial services. Among families accessing any treatment, only half attended 4+ sessions over any 12-month period[10]. Other reports from the United States[33], other western Countries and across the globe[34,35] show similarly low rates. Multimodal treatment is recommended for children[3,5], most desired by caretakers[36] and is most likely to optimize functioning especially for low-income youth[4,37,38]. However, combined treatment is particularly challenging to establish and maintain, with only 20%-33% youth accessing both counseling and medication in the same year[10,39].
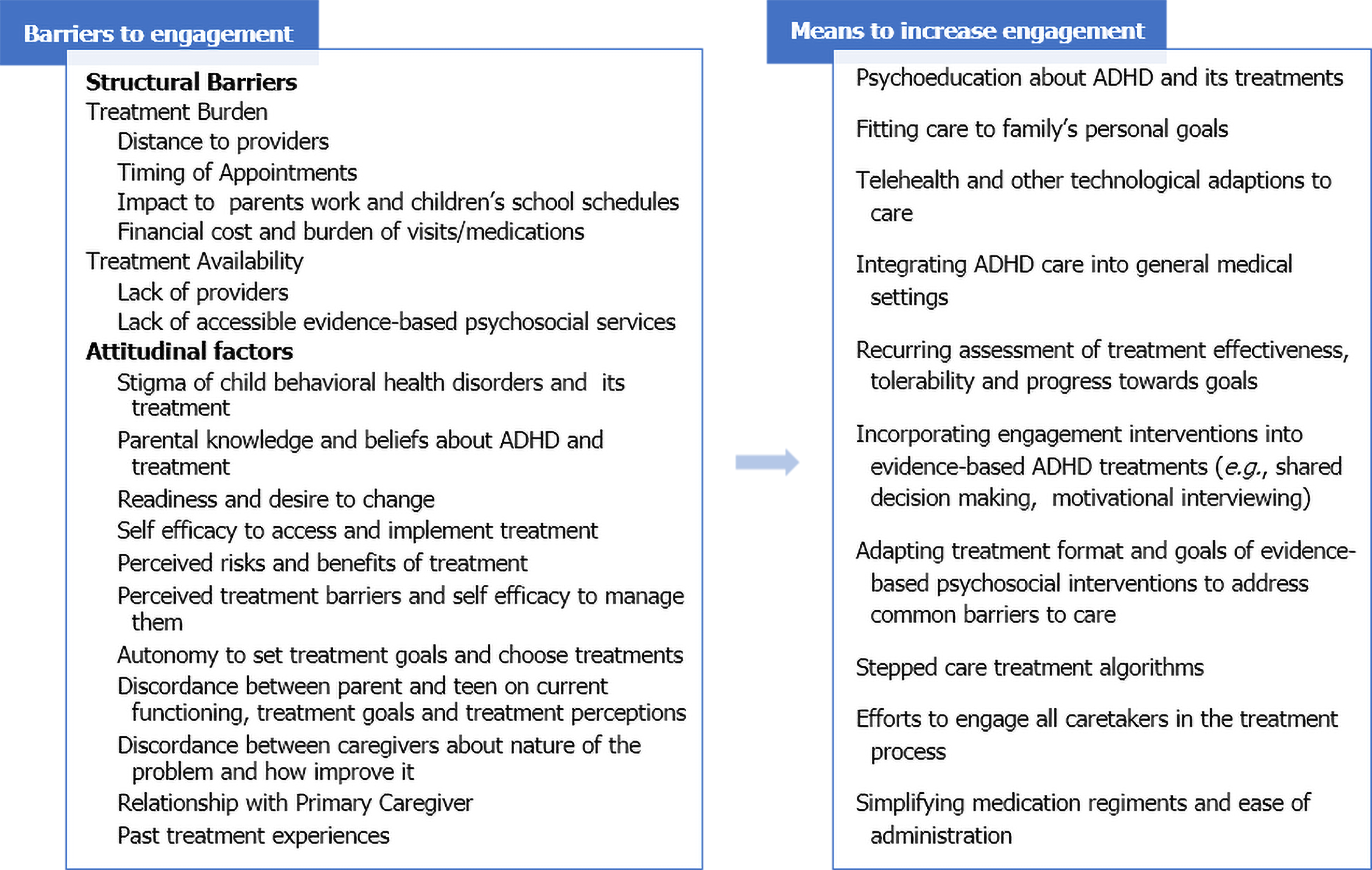
While challenging to achieve, greater service utilization is associated with improved acute and long-term outcomes[16,20,40], especially in primary care where treatment usage rates are particularly low[31,41,42]. Therefore, efforts to promote sustained utilization may be one means to enhance long-term outcome. Current ADHD guidelines[3,5] recommend engagement efforts that begin when care starts. They advise measuring progress towards personal goals along with symptoms using scheduled assessments, while promoting families to be informed advocates in their child’s care. This review will discuss identified barriers to engagement with pediatric ADHD care and interventions to overcome these barriers (Figure 1).
Existing literature was ascertained in the English language, published until March 2021, using searches of MEDLINE and PsycInfo for the following categories: ADHD, children, adolescence, engagement, adherence, methylphenidate, amphetamine, pharmacotherapy, drugs, CNS stimulants, nonstimulants, medication, psychotherapy, psychosocial intervention, counseling, parent training, behavioral therapy, and multimodal treatment. References from identified articles were reviewed to ensure that all relevant papers were included.
Numerous barriers have been identified to sustained engagement with pediatric mental health services. These can be classified as structural such as long waiting lists, limited hours of operation or cost and attitudinal, which encompass a variety of perceptions about mental health and its treatment. These include beliefs that mental health disorders do not exist or cannot be improved, to stigma about receiving a diagnosis or treatment for it.
A variety of physical, geographical, financial and access barriers have all been found to impede ADHD care in over half of families of a child with the disorder[19,43]. Geographical barriers such as distance to a provider have been found to be particularly relevant to BH care, especially in rural areas where there are few specialty treatment options[44,45]. These structural barriers exist to varying degrees in different healthcare models. Despite having a Universal National Health System in the European Union and the United Kingdom, there are still structural barriers to ADHD care. In a study from Taiwan, families who are able to access specialty care appear more likely to stay in care than those getting care in general medical settings[46]. Mostly problematic is the limited availability of Child Psychiatrist and Child Psychology providers. That lack of availability generates sizable travel times, long waiting lists and suboptimal reassessment schedules.
Financial cost is a commonly cited structural barrier to the treatment for ADHD[22,47,48]. In the United States, treatment coverage can vary widely by insurance plan, creating substantive financial barriers even for those with health insurance. For example, behavioral interventions for children are not covered as an essential benefit under the Affordable Care Act[49]. Bussing et al[50] reported that 38% families reported cost as a main reason for not to pursue treatment, while rates of medication usage are positively correlated with income[51]. Lowering costs has been found to improve treatment adherence for ADHD care[52]. In Europe, ADHD care is not universally covered by public insurance[53]. Even in countries with universal healthcare coverage that encompasses ADHD services, the long wait times have created a private healthcare market where cost of services can be an appreciable barrier. ADHD significantly increases a family’s medical cost, more than many other chronic medical conditions. ADHD in children can also reduce parents’ capacity to attend work. Parents of ADHD youth are more likely to have ADHD themselves[54], which is associated with reduced educational achievement and earning potential[55]. Parental psychopathology may also impact the ability to engage with and implement BH services for their children[56,57].
When socio-economic status (SES) has been formally examined as a treatment barrier, the data is mixed. Lower SES is associated with less likely to engage and adhere to ADHD treatment[20,50]. In the United States, public vs private insurance is associated with higher rates of counseling services[7,10,12]. In the Multimodal Treatment of ADHD study (MTA), SES moderated adherence to behavioral and combined treatment, but not medication[19]. Other analysis has observed that SES is inversely associated with medication adherence[58]. In the United States, differences in treatment utilization across race/ethnicity are diminished when sociodemographic variables are taken into consideration[59].
Taking ADHD medication several times per day can be an appreciable challenge[28,48,60,61]. Extended-release versions that only require once daily dosing are associated with improved adherence[25], but even youth using extended-release preparations struggle with adherence[40]. Furthermore, extended-release medications often come with higher costs forcing families and prescribers to balance between finances and convenience[62]. More than 40% stimulants medication prescription for ADHD require prior-authorization in countries where they have managed care[63] creating additional barriers to more convenient and palatable care. In other countries, office visits are required to adjust the dose and the long waits between appointments can create a sizable impediment to timely dose optimization.
Uptake of care can be low even when it is readily accessible[38,42,64,65]. Attitudinal barriers[66] more strongly predict BH utilization than treatment type or provider, especially for low-income families[38]. Parents are the main agent of change for a child’s BH, making parental attitudes, perceptions and preferences important variables to assess when trying to promote engagement[67]. Parental knowledge and beliefs regarding ADHD and its treatment affect decisions to both initiate and continue with care[68]. Parents who feel that they have little influence over their child’s behavior or that their child is choosing to misbehave are less likely to engage in counseling interventions for ADHD[69,70]. Parental attributions for a child’s behavioral problems appear to be particularly robust predictors for fathers who are more likely to engage in care when they feel they can influence their child’s behavior[71].
Parental views on ADHD medication vary widely, ranging from unacceptable to the preferred treatment[27,72]. Parents who view their child’s symptoms as a medical disorder are more likely to initiate medication[72]. In contrast parents who view ADHD as age normative fluctuations in behavior[48,73] are less likely to use medication. Parental knowledge about ADHD and medication treatment is predictive of medication acceptance and usage[19,34,74]. While having more knowledge may increase willingness of parents to initiate medication, it does not reliably predict medication adherence or long-term usage and may reduce interest in evidence-based psychosocial treatments[34,75]. The degree of symptom reduction also does not predict adherence. Rather parents appear to weigh the perceived risks of medication usage vs its perceived benefits while deciding how often and for how long to use medication[24]. Pretreatment preferences for treatment modality do not reliably predict uptake of counseling services as most adults rate psychosocial treatments as palatable[76]. Initial treatment decisions also impact future care. Past medication use is one the most robust predictors for future medication use for ADHD, while use of medication appears to lower motivation for future counseling services even when impairment persists, both in clinical trials offering free care[77] and in routine care[10].
There are meaningful differences in treatment utilization across racial/ethnic groups. Black children are less likely than White children to use medication, but more likely to utilize counseling. Hispanic families are less likely than White non-Hispanic families to utilize counseling and medication. However, rates of medication are increasing over time amongst families of different races and ethnicities, particularly for Hispanic families[78,79]. Parental health beliefs about ADHD also appear to be influenced by race and ethnicity. Non-Hispanic White families are more likely to report greater knowledge about ADHD, more likely to view it as a medical disorder and more likely to view medication as safe than Black or Hispanic families. Moreover, Black and Hispanic families are more likely to report side effect concerns regarding ADHD medication and expect less benefit from medication[80-84]. In contrast to White Non-Hispanic families, increasing parental knowledge about ADHD in Hispanic families is associated with increased odds to utilize counseling but not medication[75].
Parents’ perception about the impact of the child behaviors on parental and family functioning influences decision to pursue, accept and persist with treatment for ADHD[19,22]. For example, parents who perceive their child’s behavior to be negatively affecting their career, are more likely to seek treatment[19]. The child’s level of current functioning as well as the impact of treatment on their functioning appears to be particularly relevant for decisions regarding when to use medication for Hispanic families[75]. While increased parental stress may drive treatment seeking, persistently stressed parents are prone to disengage care[84]. Similarly, children with comorbid disorders, especially other externalizing behavioral disorders, may be more apt to present for treatment but experience higher rates of premature dropout[19].
Stigma related to child mental health and specifically to ADHD have been identified as sizable barriers to initiating treatment[81,85]. Unfortunately, there is continuous to be an appreciable stigma regarding having a child with ADHD and surrounding treatment for ADHD[74]. In the MTA study, stigma was a common reason for discontinuing medication[24]. However, failure to treat also increases the risk of being stigmatized as Singh et al[86] observed youth are more likely to experience stigma due to their symptomatic behaviors than to disclosing medication use. Parental perceptions of their relationship with their child’s healthcare provider influences decisions to initiate ADHD medication and can counteract stigma concerns, especially when the primary care provider is also managing ADHD[72]. However, providers do not always talk with families about their goals and preferences for the treatment for the ADHD[87]. Opinions of others also impact care decisions as parents are most likely to engage in a treatment when medical advice meshes with feedback from family and friends[88].
Attitudinal factors may also influence national healthcare policy. The acceptance of biological psychiatry may be a barrier to both diagnosis and treatment. For example, in France (population 65 million), where there is still a strong psychoanalytic tradition and the Diagnostic and Statistical Manual of Mental Disorders (DSM) or International Classification of Diseases nomenclatures are not widely accepted, there are only a few approved ADHD medications and very limited specialty treatment centers[89,90]. Spain (population 47 million), where Child and Adolescent Psychiatry is still not a recognized medical specialty, has just one child psychiatry fellowship program at the University of Navarra[91].
Patients and families value treatment choices and typically will access more preferred treatments. Treatment goals have also been found to predict which type of ADHD intervention is used. Parents prioritizing academic goals are more likely to use medication, while those with behavioral goals lean towards psychosocial treatments[65]. However, for ADHD, it does not appear that families access only preferred treatments. Most treatment seeking families rate psychosocial treatments as most palatable but medication is more commonly used in the United States, even in young children[7,10,12,76]. Using easily accessible but unpalatable treatments has been identified as one cause for poor sustained utilization of ADHD services[40]. However, even caretakers preferring psychosocial treatments often fail to persist with treatments[76] suggesting using preferred treatments is insufficient to produce sustained engagement.
Perceptions about the burden and safety of treatment also predict utilization. Many parents report hesitancy to use ADHD medications due to side effect concerns[92,93]. Side effects are also one of the most common reasons for discontinuing ADHD medication reported by parents and adolescents[24,93]. The most common side effect concerns related to changes in sleep, appetite, mood and perceived risk of addiction[94]. For counseling services, the perceived intensity and burden of care are relevant factors as parental motivation for care is one of the most robust predictors of counseling dropout and treatment response[20,95-97].
Self-efficacy is another variable impacting engagement with ADHD care, especially for counseling where parents are the primary agents of change for their child. Parents of children with ADHD report increased parental stress and reduced self-efficacy[64,98]. In fact, parental self-efficacy mediates the relationship between parental ADHD and increased parenting stress[99]. Low self-efficacy in mothers and fathers predict poor treatment outcome in children with ADHD. In the MTA study, parents with low self-efficacy had difficulties in implementing behavioral plans, consistently administering medications, and reported more stress[100].
Engaging all caretakers improves treatment utilization and durability[101]. Engaging fathers can be particularly challenging as ADHD is associated with higher rates of divorce, single parenting and separated households and fathers are less likely to present for office-based care[102,103]. Perceived criticism by the other parent is a major risk factor for dropout for fathers[104], so parental conflict can present a major barrier to sustained care. Single parent families also face unique challenges to accessing, persisting and implementing ADHD treatments including higher rates of parental psychopathology and smaller social support networks[19,102].
For adolescents to initiate and persist with a treatment, the patient and parent must recognize and agree on a problem, desire better functioning and believe that the treatment is an acceptable means to achieve a goal that they all feel capable of implementing and accessing. Adolescents prioritize autonomy to set their own goals and the freedom to pick treatments that match their goals[105,106]. All these aspects of motivation have been found to predict utilization of pharmacological and counseling services for ADHD[40,74,107]. Self-awareness about functioning is often poor in adolescents with ADHD and has been found to correlate with medication adherence[108,109]. Adolescents’ limited insight of their current impairments often leads to parents and teens disagreeing about the need for treatment, and the level of discordance predicts treatment persistence[24,107]. Higher trait antagonism in adolescents also predicts reduced treatment uptake[110]. Not surprisingly, treatment adherence, especially for medication, declines rapidly over adolescence. Across countries, medication use drops significantly during adolescents[2,111]. Typically, the decision to stop is driven by the adolescent with the most common reason for discontinuation being the adolescent felt medication was not needed anymore. However, parents and teachers continue to report high rates of impairment in adolescents stopping medication. Therefore, it appears that adolescents’ limited self-awareness is an appreciable barrier to both starting and continuing with ADHD care[24,107,108]. Side effect concerns, followed by stigma are the next most common reason reported by adolescents for stopping medication[24,110].
As parental knowledge and perceptions of ADHD influence decisions to start ADHD care, psychoeducation has been explored as a means to improve treatment uptake. For example, more extended ADHD assessments are associated with increased parental willingness to start medication[112]. The largest study assessing this issue was set in the United States and examined the efficacy of a neuro-educational intervention in 658 families of children with ADHD whose parents had either discontinued or declined medication for their children following their initial diagnosis of ADHD. At study entry, lack of direct testing of their child’s attention span and side effect concerns were the most commonly reported barriers to treatment. The study intervention consisted of a semi-structured diagnostic interview supplemented with two objective measures of attention and impulse control over 3 sessions with last session dedicated to a systematic review of findings with the family along with psychoeducation about ADHD and its treatment. This visit was run using a manualized format which included information about the causes of ADHD, rationale for medication use, strategies for reducing side effects, tips for improving sleep and diets, importance of school support and accommodation and practical strategies to address common behavioral, emotional and social problems during early phase of medication treatment. Following this brief diagnostic and educational intervention, over 70% of parents started medication for their children with treatment rates increasing to over 95% by month 6. At the 2-year follow-up, 95% were still taking medication for ADHD[113]. However, other studies have not observed associations between psychoeducation and persistent treatment engagement[34,75,114], and a systematic review found that patient education alone has limited impact on medication adherence in youth[115]. In a small study set in Spain, adding a brief nurse run psychoeducation intervention to medication treatment led to lower mean doses with no loss of efficacy vs medication alone[116]. Therefore, psychoeducation may also be a means to improve treatment tolerability.
Families report greater comfort in primary care settings vs BH settings[117,118]. However, specialty care is associated with increased contact and more frequent medication adjustments. Integration of BH services into primary care is one means to accomplish increased contact with patients that predicts greater service utilization[41], especially in countries where generalists provide much of the ADHD care[7,119]. Most models employ two main techniques: Embedding specialty BH providers in primary care to offer counseling services and training primary care providers to employ systematic medication pathways for ADHD supported by remote child psychiatrists.
One of the first randomized trials of an integrated care ADHD intervention was published by Kolko et al[120], where 163 children were randomly assigned to nurse-administered intervention or to enhanced usual care (diagnostic assessment, recommendations, and facilitated referral to a specialty mental health provider in the community). The nurses completed an extensive training period and received ongoing supervision from specialists for the study duration. The core components of the intervention were: in office application of a menu of evidence-based counseling interventions, school consultation, case coordination and crisis management. The intervention arm was more likely to receive and complete mental health services, reported fewer barriers for services, more satisfaction, and were more likely to meet personalized treatment goals even though symptoms levels did not differ between groups[120]. A second similarly sized randomized controlled trial (RCT) of the intervention across 4 primary care sites using masters level care managers produced similar findings as well as greater overall improvement on the Clinical Global Impressions Scale[121]. A more recent larger RCT of over 300 youth from 8 practices vs enhanced usual care (psychoeducation and care coordination services) found that the intervention led to greater rates of treatment initiation and completion, as well as larger changes in internalizing and externalizing symptoms, parental stress and satisfaction and primary care provider ADHD care competencies[122].
In a randomized comparative effectiveness trial, families of children being evaluated in primary care for ADHD (N: 156, ages 6-12), received care management with decision support using a collaborative care model and were randomized to enhanced care vs basic care. All treatment occurred in the primary care setting using care managers without a formal BH background, and families were recruited from low-income neighborhoods. Care managers in the enhanced care arm were trained in both motivational interviewing (MI) and parent management techniques to help parents identify and initiate ADHD care, address their own mental health concerns and improve their child’s behaviors. In the enhanced arm, half of the families attended the primary care-based parenting intervention and 72% initiated medication for ADHD in their children. For the entire sample, there was no difference between basic and enhanced arm on means changes in scores for inattention, hyperactivity/ impulsivity, oppositionality and social skills at both 6 mo and 12 mo of follow-up. However, after 12 mo, the enhanced arm experienced greater improvement among children with ADHD consistent presentations, with moderate to large effect sizes (0.57 for hyperactivity/impulsivity, 0.55 for oppositionality, and 0.69 for social skills)[42].
In a quasi-experimental design, Power et al[123] compared a multimodal primary care-based intervention to treatment as usual enhanced by a psychoeducation intervention. The Partnering to Achieve School Success (PASS) included family engagement strategies, behavioral therapy targeting the whole family, school consultation using principles of trauma-informed care. It was compared to treatment as usual supplemented with parent education and support groups. PASS was associated with greater changes in objectively measured negative parenting behaviors (ES = 30) and larger changes in child impairment (ES = 35) but not symptom scores[123].
Technology has also been used to enhance ADHD care through creation of specialized databases that prompt providers when and how to collect critical information such as parent and teacher ratings of ADHD symptoms and side effects[41]. Other technological advancements such as text message reminders from electronic medical records for when to take medication have increased adherence in adults with the disorder[124]. A recent review on telemedicine in the management of ADHD concluded that telemedicine is well accepted and valued by clinicians, caregivers, and educators. Use of telemedicine was also associated with improved outcomes although the results were limited by the small number of studies, most of which did not employ a control arm[125].
Telehealth services have also been used to reduce the structural barriers to integrating BH into pediatric primary care. The Children’s ADHD Telemental Health Treatment Study randomized families of children with ADHD to one of two different telehealth delivery models in a predominantly rural area[126]. In the direct care telehealth intervention arm, participating families received 6 medication sessions over 22 wk by child psychiatrists via telehealth and parent behavior training, provided in person by community therapists who were supervised remotely. The Control arm received treatment from PCCs augmented with a telepsychiatry consultation from study psychiatrists. Children in both service models improved but those in the direct care telehealth arm experienced greater reductions in symptom scores and impairment[127].
A European study examined the efficacy of a behavioral parent training administered via telehealth in children already treated with CNS stimulants. The RCT compared the telephone-assisted self-help (TASH) intervention to routine clinical care. TASH did not separate on the primary outcome of parent rated impairment but group differences were seen on externalizing symptoms and negative parenting behaviors. Parents also expressed high satisfaction with the program, but completion rates were higher for families with a greater educational level and fewer additional stressors[128].
Several evidence-based psychosocial interventions for ADHD have incorporated formal engagement interventions to promote treatment uptake. For pediatric BH, engagement interventions have greater impact than those only addressing structural barriers[129]. These interventions target multiple aspects of motivation, including desire and readiness for care, self-efficacy, treatment goals and preferences and planning for potential care barriers that all predict counseling and medication use and efficacy[65,74] and treatment persistence[20,24,95]. MI is the most commonly employed engagement intervention in pediatric ADHD treatments. It is a collaborative conversation designed to strengthen a patient’s desire to change[130,131]. MI has been applied in medical and BH settings with moderate effects for improving a range of outcomes[132,133]. The other common components of these engagement interventions are a family interview to identify goals, a strengths-based assessment to promote self-efficacy, identify areas of need and a feedback session emphasizing discrepancies between current and desired functioning. These programs are often tailored to a specific population prone to greater challenges engaging with ADHD care, such as adolescents, fathers or single mothers.
In a study among single mothers of children with ADHD, participants were randomly assigned to an enhanced behavioral parent training program-the Strategies to Enhance Positive Parenting (STEPP) program or a traditional behavioral parent training program. The STEPP program (nine 21/2 h session) focused on enhancements to the format, delivery, and content of traditional parent training programs including: (1) An enhanced intake procedure to addresses practical barriers for participation in treatment, define realistic expectations from treatment, and identify attributions related children’s behavior that promote parental efficacy; (2) Programming to improve social support for parents; and (3) A systematic problem-solving skill. Both arms led to improvements in child behavior but STEPP resulted in better attendance, participation and satisfaction[102]. COACHES (Coaching Our Acting-out Children: Heightening Essential Skills) is a behavioral parenting program designed for fathers that packages evidenced based techniques into a youth sporting event. During the first hours, fathers learn how to implement effective parenting strategies, while children practice soccer skills combined with a contingency management approach for appropriate behavior. During the second hour, the parent and child groups join for a soccer game, where fathers practice learned parenting strategies and get live feedback from therapists. COACHES was associated with better attendance and greater change in parenting behaviors and children’s problem behaviors than standard behavioral parent training[134].
The STAND (Supporting Teens’ Academic Needs Daily) program was designed to promote adolescents and parents in partnering to improve the teen’s academic and home functioning. The first two sessions employ MI to aide parents and teens in identifying mutually agreeable goals and therapeutic techniques they will use prior to starting any specific treatments. STAND has been found to significantly improve adolescent functioning under randomized conditions, with parents and adolescents reporting high satisfaction, credibility and therapeutic engagement[108]. STAND has also been administered via videoconferencing with acceptable therapeutic alliance, treatment fidelity and effects[135]. When applied by community therapists, STAND led to increased parent participation and satisfaction vs treatment as usual. No differences in adolescent attendance or satisfaction were seen but STAND was associated with increased rates of starting or restarting ADHD medication vs treatment as usual[136].
Hamrin et al[137] used MI to improve medication adherence in 48 adolescents with mood disorders. The trial was set in specialty offices with psychiatrists applying MI with good fidelity during medication management visits. There was significant improvement in objectively measured medication adherence over a 30-d period (d = 0.65). While this trial did not require ADHD for entry, half of the sample had comorbid ADHD, and there was no evidence of reduced efficacy in those with ADHD vs those without it.
In Europe, where treatment of ADHD largely occurs in specialty settings[14], standardized care pathways have been developed for these settings. One example is the Dundee ADHD Clinical Care Pathway in Scotland[138]. It has standardized protocols for four stages derived from the NICE guidelines[5]: Referral and pre-assessment, assessment, initiating treatment and continuing care. Patients are referred to the program with initial screening conducted by nurse specialists who gather structured parent and teacher ratings prior to direct assessment, which focuses on functional impairments in the child and the level of family functioning. The Schedule for Affective Disorders and Schizophrenia for School-Aged Children for DSM-IV--Present and Lifetime (KSADS-PL) is used for diagnostic determination. The assessment concludes with the family meeting with a senior clinician to review results. Initial treatment offerings are stratified by patient age with families of children under 6 referred to evidence-based behavioral parent training programs and those over 6 offered medication. A 4-wk dose optimization protocol is used for medication, preferencing methylphenidate products. It consists of 3 to 4 visits that can be remote or in person with structured rating scales from parents and teachers used to titrate to optimal effect. Standardized definitions of meaningful response are used to determine if the initial treatment choice should be maintained or switched. Once optimized, nurse visits occur every 6 mo that measure adherence, stigma, other care barriers and functioning at home, school and with peers. Behavioral interventions are offered targeting identified impairments. The creators of the model report high rates of initial medication use, large reductions in ADHD symptoms and maintenance of effects over time. However, there are no randomized trials of this care pathway to date.
One limitation of these programs is that they were set in either university-based or other specialty BH settings that families had to be referred to or discover on their own. In addition, they employ trained BH staff, which may not be available outside of specialty settings. While some of the same principles have been applied in general pediatric settings as part of an integrated care model, there has been limited formal examination of their capacity to promote ADHD treatment in these settings. Silverstein et al[42] addressed this limitation by comparing the efficacy of an enhanced ADHD care management system vs a basic collaborative care intervention in urban primary care pediatric practices predominantly serving low-income families. All families referred by their primary care providers for an ADHD assessment were eligible with 40% of participating children found to have presentations consistent with ADHD. Nonclinical care managers in the enhanced arm were trained in an evidence-based parenting intervention and used preset MI scripts that focused on the benefits of medication usage. The two arms did not differ in levels of observed improvement over 12 mo with both groups showing reduced symptoms and improved social functioning over time. When analyses were limited only to the 40% of the sample with ADHD consistent presentations, significant differences in symptoms of ADHD and ODD as well as social skills were observed. Enhanced care was also associated with increased medication usage but had little impact on externally referred ADHD services[42]. Moreover, the specific impact of the use of MI was not able to be assessed apart from the larger treatment package.
Brinkman et al[139] completed a smaller study set in pediatric primary care designed to assess the capacity of a brief shared decision-making intervention to improve treatment uptake. The intervention offers psychoeducation about ADHD and its treatments while eliciting parental goals and treatment preferences. It was added to the initial treatment discussion with pediatrician for their child with ADHD (age range 6-10 years). This shared decision-making intervention was found to be feasible and well received by parents without increasing the duration of the visits. It improved parental knowledge of ADHD and increased initial interest in ADHD treatment. However, it had no impact on office contacts/visits or medication adherence over the next 90 d.
There are multiple evidence-based pharmacological and psychosocial treatments for ADHD, and greater service utilization is associated with improved acute and long-term outcomes[16,20]. However, long-term outcomes are suboptimal across countries and a major identified challenge to achieving optimal long-term outcome is sustained engagement with care. There are both structural and attitudinal barriers to care that serve as appreciable impediments to sustained engagement for many families of children with ADHD. Over the past two decades there has been increasing research identifying these barriers and developing interventions to overcome them. Many structural barriers are hard to modify such as geography and SES[12,19,20]. However, frequency of contact whether in person or remote improves treatment persistence[41,140]. Modifications to how and where pediatric BH care is administered have been effectively employed to increase contact and overcome other structural barriers. These interventions also broach attitudinal barriers by embedding care in less stigmatizing settings than specialty BH clinics. Engagement strategies have also been developed that directly address attitudinal barriers that can be applied wherever ADHD care occurs. These interventions include psychoeducation, MI or shared decision making. Other treatments, such as stepped care models, hold promise but their impact on engagement has not been systematically examined.
Integrated care for pediatric behavior problems have shown to be feasible and effective across several studies. Integrating BH into primary care has enhanced outcomes, largely through increased service utilization of both medication and on-site counseling services[31,42,127]. Integration has been able to address both structural and attitudinal barriers by locating BH services in accessible primary care settings and building on families established relationships with their primary care providers[72]. Models employing full integration vs colocation of services have led to greater impact on the frequency of appointments for ADHD management[141]. Most models employ care managers who serve as the family’s primary point of contact and guide them through the multiple aspects of care to reduce the burden of managing a chronic disease. Nurses and other primary care staff with little formal BH experience can be remotely trained and supported to accomplish these roles. Integrated models have also proved feasible and effective in populations with increased barriers to care[142]. However, integrated models require sizable effort and financial investment and are only beginning to become common occurrences in the United States[143]. Child Psychiatry access programs where primary care providers can access child psychiatrists when an acute need arises have become increasingly popular in the United States as they allow a single specialist to impact a broad geographic area. Many of these programs also offer care coordination services to families to help them connect to care. These programs have been effective at improving access to care and promoting evidence-based BH treatments for ADHD and other BH disorders in primary care[29,144].
Even when integration occurs, it is often not feasible to embed a full range of BH services into primary care. Therefore, referrals to external BH providers remain a common occurrence, and is a point in the care pathway where dropout often occurs[145]. Integrated models do not address the challenges of accessing referred care, which have proved particularly challenging to overcome[146]. Moreover, a sizable subset of families fails to engage integrated ADHD therapy services[42] in part because some stressed families do not inform PCCs about their child’s struggles even when they view care as accessible[111]. Efforts to streamline and personalize the BH referral process that leverages the family’s established relationship with their PCCs have been found to improve access to externally referred care[147].
For ADHD, increasing visits may promote engagement[140], but adding visits is challenging in primary care as stressed families are unlikely to come more frequently and long waits often preclude more frequent office visits with specialists. Rating scales and remote contact with families have proved as effective as office visits for improving ADHD outcomes[41,140], with less burden to staff and families than sole reliance on face-to-face interactions[148]. Therefore, collection of rating scales, communication through a patient portal or other forms of asynchronous or synchronous remote contact can be used to build engagement, especially in areas with long travel distances to care.
Telehealth has been recommended as an effective means to increase access and improve engagement for pediatric BH services[149,150]. In children with ADHD, meaningful changes in functioning have been captured through telehealth interventions[125-127], and several evidence-based psychosocial interventions for pediatric behavioral problem have been adapted for telehealth application[151]. There is an emerging evidence base to support both direct patient care and peer-to-peer consultation models using telehealth[29,126,127]. These tele-methods are effective for underserved families and have found to be palatable across race and ethnicity[126,127]. The effectiveness of these techniques for the chronic care of ADHD has not been studied as published studies have not extended beyond 6 mo[152].
Tele methods have become increasingly common during the coronavirus pandemic[153-155] given the multiple barriers to face-to-face contact. Treatment guidelines for ADHD telecare during the pandemic are being developed[154,155] and rules requiring a face-to-face visit for prescription of controlled substances medications have been loosed during the pandemic in the United States. European ADHD Guidelines report it is reasonable to start ADHD medications during the pandemic for patients who did not have a baseline, face-to face cardiovascular assessment if they meet following 3 criteria: (1) No personal history of cardiac symptoms; (2) No family history of early (< 40 years) sudden death in a first degree relative; and (3) Patient must have baseline measurement of blood pressure and heart rate by a family member or another person remotely on three separate occasions. If the first two conditions are not satisfied, a referral to a pediatric cardiologist should be made before starting the medication for the ADHD[155].
Chronic care models assessing functioning and motivation starting when care begins are recommended[156]. Failure to synchronize treatment intensity for a chronic disorder like ADHD to the motivational state of the family can lead to feelings of defeat and disengagement[157]. Families of ADHD youth have higher rates of parental ADHD, depression, divorce and exposure to adverse childhood experiences, which all impair motivation[20,158]. Initial evaluations should assess for a wide range of psychosocial stressors impacting the patient and the family as well as assessing parent-child and family relationships[64]. Initially, stress may promote change as it can lead to treatment seeking efforts[19]. However, chronically worsening stress impedes change, as stressed families are more apt to decide the current state is unmodifiable, especially when there are financial barriers to care[38]. Waiting too long to assess motivation risks families dropping out of care[84]. The combination of waning motivation and reports of increasing impairment or family strain indicate increased risk for dropout.
The success of any initial treatment is always contingent on the family’s motivation to make a change in their child’s behavior, whether this is through giving medication or attending a counseling visit. According to the transtheoretical model, change is a process, progressing from recognizing an undesirable behavior, to contemplating change, to intending to act, to modifying the behavior and then to maintaining the desired state[130,159]. Goal achievement is most likely when progression is sequential and the motivational stage is used to inform treatment decisions. Therefore, efforts to improve motivation should be a fluid process tailored to the status of the patient and family at the current time. Current ADHD guidelines recommend repeatedly measuring motivation and using results to tailor an individualized care plan[3,5].
Cunningham has applied the transtheoretical model of change to ADHD care[160]. Contacting the PCCs is the most common first step in the ADHD care pathway[119]. Parents presenting to a care provider with concerns about their child’s ADHD are likely at the preparation stage[159] as they have achieved an awareness of their child’s struggles and have a goal to improve them. However, adolescents are often not at the same stage as their parents, and direct assessment of their motivation for care is critical. To move towards action, a palatable treatment option that parents and adolescents feel they can access and successfully implement to achieve their goal must be identified[161]. Psychoeducation may be help for more receptive families towards starting treatment[113] but simply providing information about treatment options is unlikely to move many parents and teens from preparation to action to maintenance of changes[34,113,114]. Some families presenting for assessment are not ready for active treatment even if their child is appreciably impaired. For example, external forces such as schools may drive people into the office who are only in the contemplation stage. For them, it may be critical to address motivation for care prior to directly promoting treatment.
MI is widely used to help patients down to age 12 to clarify goals, explore the benefits and risks of engaging in treatment[130,162,163], address stigma and other attitudinal barriers and promote self-efficacy to benefit from treatment seeking efforts[132]. Simple single item motivation rulers can be employed to measure each of these core aspects of motivation (readiness and desire to change, self-efficacy to implement change, treatment preferences and overcoming perceive barriers) as they have been found to predict health behaviors in adults[164] and adolescents[165].
Initial change behaviors can be nurtured by expressing an interest in family’s views about the sources of their child’s problems while rolling with their resistance to change. Minimizing their concerns or attempting to solve problems for them may reduce self-efficacy and threaten autonomy. Empathetically providing patients with objective feedback on differences between current vs desired functioning can be used to generate change talk to enhance readiness and desire to change. Recognizing areas where the child is doing well and instances where parents have successfully achieved past goals for their child promotes self-efficacy to move forward with identified changes. Once parents have identified a specific goal that they feel motivated to and capable of achieving, they may be most receptive to discussing specific treatments. Shared decision-making can then be used to identify a treatment that matches their goals, while also crafting realistic treatment expectations to increase the chances that families will initiate new services for ADHD. To engage adolescents, it is essential that treatment centers around their goals vs parental or provider goals to create engagement[139]. Clinicians may need to aide parents and teens in identifying a mutually acceptable treatment goal. Parental input on ways to incentivize the adolescent working towards their goal (making curfews or screen time contingent on making visible effort to achieve a goal) can be elicited. To foster sustained engagement, providers should empathically affirm treatment seeking behaviors by parents and adolescents, aide in finding supports and reinforcers to sustain ongoing change behavior and offer to help them plan to overcome identified barriers[159,166].
These techniques of MI have been integrated with high fidelity into several counseling interventions for ADHD[102,123,135]. They have also been used to increase medication adherence in general medical and specialty settings[42,136,137]. Modifying the format of existing counseling interventions to increase interest (e.g., Fabiano’s COACHES for fathers) or supplementing the content to address specific barriers for populations with multiple impediments to engagement (Chacko’s STEPP for single mothers or Sibley’s STAND for adolescents with academic struggles) have also been employed. Modifying content could potentially reduce efficacy for primary outcomes or increase dropout if new content is added; however, controlled results support that these adaptations increase attendance, participation and satisfaction with some producing better outcomes than the unmodified interventions[108,134].
Medication issues: Parents’ most frequently cited reasons for discontinuing ADHD medication is lack of efficacy or intolerable adverse effects. Health care providers should closely monitor patients during the dose optimization phase using structured measures of efficacy and tolerability. Once dose is optimized, monitoring should assess functioning, adherence, tolerability and ongoing need for medication. For adolescents, it is also important to directly assess their perceptions of the need for medication and ensure that medication addresses a goal that is relevant to them. Reducing the number of doses per day by switching to long-acting preparations has been found to increase compliance with medications for ADHD[25,26,28,167]. Formulation alterations, such as liquids, patches or chew tabs can also be an option, for children either who have difficulties in swallowing pills.
Involvement of all caregivers: Caretakers may have differing views on the severity and cause of the child’s behavioral struggles. They may also be at different stages of change in regards to treatment seeking efforts, especially when they reside apart[3,168]. Therefore, efforts should be made to contact all custodial caretakers to assess their personal views. It has been found identifying parental attributions for the child’s problem behaviors that emphasize the capacity of the parent to promote positive change has been found to increase engagement for fathers not residing full time with the child[71]. Strengthening support networks enhances service initiation and persistence with care, while fathers’ participation in care improves maintenance of treatment effects[169]. Therefore, engaging all caretakers can be a means to increase the amount and the impact of utilized services. Telehealth may be a particularly useful tool to engage fathers, as they are less likely to present for office-based care[20]. It can also be used to separately engage caretakers prone to disagree with each other. Reducing conflict between parents may be another means to engage fathers as perceived criticism from the mother is a major risk for their dropout from treatment[104].
Stepped care: The appreciable commitments required of parents for intensive counseling interventions may be one reason why stressed families receive less benefit from these programs[170]. Stepped care reduces burden without sacrificing impact, as low intensity treatments are initiated for all, followed by incrementally more intensive services if impairment persists. Combining stepped care with tailoring approaches to individualize subsequent care further minimizes burden and is recommended for treating childhood behavior problems[152].
There is evidence of efficacy of stepped care for ADHD in controlled settings, with the sequence of behavior therapy followed by medication leading to the greatest enhancements in functioning at a lower cost than starting with medication[62,77]. Stepped care models for ADHD have not been examined outside the confines of research studies. However, there is evidence that medication usage reduces parental motivation for psychosocial services both in controlled and naturalistic settings[10,77]. For multiply stressed families, lower intensity counseling programs have been found to be less costly and not inferior in efficacy to more intensive programs such as Parent Child Interaction[152]. They may be the preferred initial intervention especially for families with multiple barriers to high intensity care.
Most studies assessing barriers to care are drawn from Western Europe and the United States while the vast majority of the trials of interventions designed to promote engagement were run in the United States. Integrated models for pediatric BH care have not been well studied outside of the United States, possibly in part due to national policies regarding the payment and provision of BH care that are separate from the those for the rest of medicine. Cultural perceptions of ADHD and health care policy varies widely across the globe, limiting the generalizability of these findings. While behavioral parenting interventions are efficacious in countries beyond their origin[171], the impact of engagement barriers and interventions for them could variably appreciably given the impact of culture on perceptions of pediatric BH[43]. It was encouraging to see that several studies recruited racially and ethnically diverse families[42,123] with a focus on populations that are less likely to access BH care[102,134]. Furthermore, there are only a few RCT specifically designed to measure engagement and even less that track engagement for more than 6 mo. Additionally, most interventions were multi-pronged, so it is hard to determine the impact of any single engagement technique. The heterogeneity of the interventions and assessment methods used to measure and promote engagement further inhibited the ability to draw conclusions from the collective literature base. Only a limited number of studies have examined how to improve uptake of externally referred services, which remain a common form of treatment[146].
Despite a number of evidence-based treatments, long-term outcomes for youth with ADHD remain suboptimal. ADHD care often ends quickly even when high levels of impairment persist. Families face a variety of structural and attitudinal barriers to engagement, ranging from cost and access to stigma and low self-efficacy to successfully implement change. Integration of BH into general medical settings and telehealth have been found in controlled studies to increase access by reducing both structural and attitudinal barriers. Adding MI, shared decision-making and other engagement interventions to evidence-based ADHD treatments reduces altitudinal barriers that translates to improved participation and satisfaction with care while enhancing outcomes. However, improving uptake of externally referred BH services remains a challenge and little is known about how to enhance long-term engagement with ADHD services even though a chronic care model for ADHD is recommended.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Thummer RP S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942-948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2714] [Cited by in F6Publishing: 2826] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 2. | Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, Abikoff HB, Stehli A, Owens EB, Mitchell JT, Nichols Q, Howard A, Greenhill LL, Hoza B, Newcorn JH, Jensen PS, Vitiello B, Wigal T, Epstein JN, Tamm L, Lakes KD, Waxmonsky J, Lerner M, Etcovitch J, Murray DW, Muenke M, Acosta MT, Arcos-Burgos M, Pelham WE, Kraemer HC; MTA Cooperative Group. Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. J Child Psychol Psychiatry. 2017;58:663-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | Wolraich ML, Hagan JF Jr, Allan C, Chan E, Davison D, Earls M, Evans SW, Flinn SK, Froehlich T, Frost J, Holbrook JR, Lehmann CU, Lessin HR, Okechukwu K, Pierce KL, Winner JD, Zurhellen W; Subcommittee on Children and Adolescents with Attention-Deficit/Hyperactive Disorder. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 519] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 4. | Froehlich TE, Brinkman WB. Multimodal Treatment of the School-aged Child With Attention-Deficit/Hyperactivity Disorder. JAMA Pediatr. 2018;172:109-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 5. | National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: Diagnosis and management (NG87). [cited 26 March 2021]. Available from: https://www.nice.org.uk/guidance/ng87. [Cited in This Article: ] |
| 6. | Cortese S. Pharmacologic Treatment of Attention Deficit-Hyperactivity Disorder. N Engl J Med. 2020;383:1050-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 7. | Walls M, Allen CG, Cabral H, Kazis LE, Bair-Merritt M. Receipt of Medication and Behavioral Therapy Among a National Sample of School-Age Children Diagnosed With Attention-Deficit/Hyperactivity Disorder. Acad Pediatr. 2018;18:256-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Hodgkins P, Setyawan J, Mitra D, Davis K, Quintero J, Fridman M, Shaw M, Harpin V. Management of ADHD in children across Europe: patient demographics, physician characteristics and treatment patterns. Eur J Pediatr. 2013;172:895-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Quintero J, Ramos-Quiroga JA, Sebastián JS, Montañés F, Fernández-Jaén A, Martínez-Raga J, Giral MG, Graell M, Mardomingo MJ, Soutullo C, Eiris J, Téllez M, Pamias M, Correas J, Sabaté J, García-Orti L, Alda JA. Health care and societal costs of the management of children and adolescents with attention-deficit/hyperactivity disorder in Spain: a descriptive analysis. BMC Psychiatry. 2018;18:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Waxmonsky JG, Baweja R, Liu G, Waschbusch DA, Fogel B, Leslie D, Pelham WE Jr. A Commercial Insurance Claims Analysis of Correlates of Behavioral Therapy Use Among Children With ADHD. Psychiatr Serv. 2019;70:1116-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, de Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lépine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, Arbabzadeh-Bouchez S, Autonell J, Bernal M, Buist-Bouwman MA, Codony M, Domingo-Salvany A, Ferrer M, Joo SS, Martínez-Alonso M, Matschinger H, Mazzi F, Morgan Z, Morosini P, Palacín C, Romera B, Taub N, Vollebergh WA; ESEMeD/MHEDEA 2000 Investigators, European Study of the Epidemiology of Mental Disorders (ESEMeD) Project. Use of mental health services in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;47-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Danielson ML, Visser SN, Chronis-Tuscano A, DuPaul GJ. A National Description of Treatment among United States Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. J Pediatr. 2018;192:240-246.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Hinshaw SP, Scheffler RM, Fulton BD, Aase H, Banaschewski T, Cheng W, Mattos P, Holte A, Levy F, Sadeh A, Sergeant JA, Taylor E, Weiss MD. International variation in treatment procedures for ADHD: social context and recent trends. Psychiatr Serv. 2011;62:459-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5:175-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 15. | Setyawan J, Fridman M, Hodgkins P, Quintero J, Erder MH, Katic B, Harpin V. Physician-reported treatment outcomes for ADHD among children and adolescents in Europe. Neuropsychiatry. 2013;3:587-600. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Kollins SH. Moving Beyond Symptom Remission to Optimize Long-term Treatment of Attention-Deficit/Hyperactivity Disorder. JAMA Pediatr. 2018;172:901-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Hacker K, Arsenault L, Franco I, Shaligram D, Sidor M, Olfson M, Goldstein J. Referral and follow-up after mental health screening in commercially insured adolescents. J Adolesc Health. 2014;55:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Zima BT, Bussing R, Tang L, Zhang L, Ettner S, Belin TR, Wells KB. Quality of care for childhood attention-deficit/hyperactivity disorder in a managed care medicaid program. J Am Acad Child Adolesc Psychiatry. 2010;49:1225-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Corkum P, Bessey M, McGonnell M, Dorbeck A. Barriers to evidence-based treatment for children with attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2015;7:49-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Chacko A, Jensen SA, Lowry LS, Cornwell M, Chimklis A, Chan E, Lee D, Pulgarin B. Engagement in Behavioral Parent Training: Review of the Literature and Implications for Practice. Clin Child Fam Psychol Rev. 2016;19:204-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 178] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 21. | Chiedi JM. Many Medicaid-Enrolled Children Who Were Treated for ADHD Did Not Receive Recommended Followup Care. [cited 26 March 2021]. Available from: https://oig.hhs.gov/oei/reports/oei-07-17-00170.asp. [Cited in This Article: ] |
| 22. | Sawyer MG, Rey JM, Arney FM, Whitham JN, Clark JJ, Baghurst PA. Use of health and school-based services in Australia by young people with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1355-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Sayal K, Ford T, Goodman R. Trends in recognition of and service use for attention-deficit hyperactivity disorder in Britain, 1999-2004. Psychiatr Serv. 2010;61:803-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Brinkman WB, Simon JO, Epstein JN. Reasons Why Children and Adolescents With Attention-Deficit/Hyperactivity Disorder Stop and Restart Taking Medicine. Acad Pediatr. 2018;18:273-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Marcus SC, Wan GJ, Kemner JE, Olfson M. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159:572-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Raman SR, Marshall SW, Gaynes BN, Haynes K, Naftel AJ, Stürmer T. An observational study of pharmacological treatment in primary care of children with ADHD in the United kingdom. Psychiatr Serv. 2015;66:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8:1563-1571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Gau SS, Shen HY, Soong WT, Gau CS. An open-label, randomized, active-controlled equivalent trial of osmotic release oral system methylphenidate in children with attention-deficit/hyperactivity disorder in Taiwan. J Child Adolesc Psychopharmacol. 2006;16:441-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Aupont O, Doerfler L, Connor DF, Stille C, Tisminetzky M, McLaughlin TJ. A collaborative care model to improve access to pediatric mental health services. Adm Policy Ment Health. 2013;40:264-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Olfson M, Blanco C, Wang S, Laje G, Correll CU. National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiatry. 2014;71:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 31. | Kolko DJ, Campo J, Kilbourne AM, Hart J, Sakolsky D, Wisniewski S. Collaborative care outcomes for pediatric behavioral health problems: a cluster randomized trial. Pediatrics. 2014;133:e981-e992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Gellad WF, Stein BD, Ruder T, Henderson R, Frazee SG, Mehrotra A, Donohue JM. Geographic variation in receipt of psychotherapy in children receiving attention-deficit/hyperactivity disorder medications. JAMA Pediatr. 2014;168:1074-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Barkley RA, Shelton TL, Crosswait C, Moorehouse M, Fletcher K, Barrett S, Jenkins L, Metevia L. Multi-method psycho-educational intervention for preschool children with disruptive behavior: preliminary results at post-treatment. J Child Psychol Psychiatry. 2000;41:319-332. [PubMed] [Cited in This Article: ] |
| 34. | Corkum P, Rimer P, Schachar R. Parental knowledge of attention-deficit hyperactivity disorder and opinions of treatment options: impact on enrollment and adherence to a 12-month treatment trial. Can J Psychiatry. 1999;44:1043-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Huang HL, Chao CC, Tu CC, Yang PC. Behavioral parent training for Taiwanese parents of children with attention-deficit/hyperactivity disorder. Psychiatry Clin Neurosci. 2003;57:275-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Gage JD, Wilson LJ. Acceptability of Attention-Deficit/Hyperactivity Disorder interventions: A comparison of parents. J Atten Disord. 2000;4:174-182. [DOI] [Cited in This Article: ] |
| 37. | Jensen PS, Hinshaw SP, Swanson JM, Greenhill LL, Conners CK, Arnold LE, Abikoff HB, Elliott G, Hechtman L, Hoza B, March JS, Newcorn JH, Severe JB, Vitiello B, Wells K, Wigal T. Findings from the NIMH Multimodal Treatment Study of ADHD (MTA): implications and applications for primary care providers. J Dev Behav Pediatr. 2001;22:60-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 38. | Jones DJ, Anton M, Zachary C, Pittman S, Turner P, Forehand R, Khavjou O. A Review of the Key Considerations in Mental Health Services Research: A Focus on Low-Income Children and Families. Couple Family Psychol. 2016;5:240-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Hoagwood KE, Kelleher K, Zima BT, Perrin JM, Bilder S, Crystal S. Ten-Year Trends In Treatment Services For Children With Attention Deficit Hyperactivity Disorder Enrolled In Medicaid. Health Aff (Millwood). 2016;35:1266-1270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Chacko A, Newcorn JH, Feirsen N, Uderman JZ. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16:2416-2423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Epstein JN, Kelleher KJ, Baum R, Brinkman WB, Peugh J, Gardner W, Lichtenstein P, Langberg JM. Specific Components of Pediatricians' Medication-Related Care Predict Attention-Deficit/Hyperactivity Disorder Symptom Improvement. J Am Acad Child Adolesc Psychiatry. 2017;56:483-490.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Silverstein M, Hironaka LK, Walter HJ, Feinberg E, Sandler J, Pellicer M, Chen N, Cabral H. Collaborative care for children with ADHD symptoms: a randomized comparative effectiveness trial. Pediatrics. 2015;135:e858-e867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Weisenmuller C, Hilton D. Barriers to access, implementation, and utilization of parenting interventions: Considerations for research and clinical applications. Am Psychol. 2021;76:104-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Young AS, Rabiner D. Racial/ethnic differences in parent-reported barriers to accessing children's health services. Psychol Serv. 2015;12:267-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Janicke DM, Davis AM. Introduction to the special section: rural health issues in pediatric psychology. J Pediatr Psychol. 2011;36:647-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Chen CY, Yeh HH, Chen KH, Chang IS, Wu EC, Lin KM. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21:265-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Chan E, Zhan C, Homer CJ. Health care use and costs for children with attention-deficit/hyperactivity disorder: national estimates from the medical expenditure panel survey. Arch Pediatr Adolesc Med. 2002;156:504-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | Sitholey P, Agarwal V, Chamoli S. A preliminary study of factors affecting adherence to medication in clinic children with attention-deficit/hyperactivity disorder. Indian J Psychiatry. 2011;53:41-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | U.S. Centers for Medicare and Medicaid Services. Preventative care benefits for children. [cited 26 March 2021]. Available from: https://www.healthcare.gov/preventive-care-children. [Cited in This Article: ] |
| 50. | Bussing R, Zima BT, Gary FA, Garvan CW. Barriers to detection, help-seeking, and service use for children with ADHD symptoms. J Behav Health Serv Res. 2003;30:176-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Bloom B, Cohen RA, Freeman G. Summary health statistics for US children: National Health Interview Survey, 2007. [cited 26 March 2021]. Available from: https://europepmc.org/article/MED/19326838. [Cited in This Article: ] |
| 52. | Bokhari FAS, Heiland F, Levine P, Ray GT. Risk factors for discontinuing drug therapy among children with ADHD. Health Serv Outcomes Res Method. 2008;8:134-158. [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Hayden JC, Flood M, Gavin B, Maršanić VB, McNicholas F. Barriers to medication entitlements after diagnosis of ADHD. Lancet Psychiatry. 2018;5:18-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Starck M, Grünwald J, Schlarb AA. Occurrence of ADHD in parents of ADHD children in a clinical sample. Neuropsychiatr Dis Treat. 2016;12:581-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Pelham WE, Page TF, Altszuler AR, Gnagy EM, Molina BSG, Pelham WE. The long-term financial outcome of children diagnosed with ADHD. J Consult Clin Psychol. 2020;88:160-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 56. | Sayal K, Mills J, White K, Merrell C, Tymms P. Predictors of and barriers to service use for children at risk of ADHD: longitudinal study. Eur Child Adolesc Psychiatry. 2015;24:545-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Babinski DE, Waxmonsky JG, Pelham WE Jr. Treating parents with attention-deficit/hyperactivity disorder: the effects of behavioral parent training and acute stimulant medication treatment on parent-child interactions. J Abnorm Child Psychol. 2014;42:1129-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Brownell MD, Mayer T, Chateau D. The incidence of methylphenidate use by Canadian children: what is the impact of socioeconomic status and urban or rural residence? Can J Psychiatry. 2006;51:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Coker TR, Elliott MN, Kataoka S, Schwebel DC, Mrug S, Grunbaum JA, Cuccaro P, Peskin MF, Schuster MA. Racial/Ethnic disparities in the mental health care utilization of fifth grade children. Acad Pediatr. 2009;9:89-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Gau SS, Chen SJ, Chou WJ, Cheng H, Tang CS, Chang HL, Tzang RF, Wu YY, Huang YF, Chou MC, Liang HY, Hsu YC, Lu HH, Huang YS. National survey of adherence, efficacy, and side effects of methylphenidate in children with attention-deficit/hyperactivity disorder in Taiwan. J Clin Psychiatry. 2008;69:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27 Suppl 2:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Page TF, Pelham WE 3rd, Fabiano GA, Greiner AR, Gnagy EM, Hart KC, Coxe S, Waxmonsky JG, Foster EM, Pelham WE Jr. Comparative Cost Analysis of Sequential, Adaptive, Behavioral, Pharmacological, and Combined Treatments for Childhood ADHD. J Clin Child Adolesc Psychol. 2016;45:416-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Wilens TE, Wilens K, Woodworth KY, Chippari V, Firmin ES. Prior Authorizations: A Necessary Evil? J Am Acad Child Adolesc Psychiatry. 2020;59:1005-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Cunningham CE. A family-centered approach to planning and measuring the outcome of interventions for children with attention-deficit/hyperactivity disorder. J Pediatr Psychol. 2007;32:676-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Fiks AG, Mayne S, Debartolo E, Power TJ, Guevara JP. Parental preferences and goals regarding ADHD treatment. Pediatrics. 2013;132:692-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Gopalan G, Goldstein L, Klingenstein K, Sicher C, Blake C, McKay MM. Engaging families into child mental health treatment: updates and special considerations. J Can Acad Child Adolesc Psychiatry. 2010;19:182-196. [PubMed] [Cited in This Article: ] |
| 67. | Heath CL, Curtis DF, Fan W, McPherson R. The association between parenting stress, parenting self-efficacy, and the clinical significance of child ADHD symptom change following behavior therapy. Child Psychiatry Hum Dev. 2015;46:118-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Johnston OG, Burke JD. Parental Problem Recognition and Help-Seeking for Disruptive Behavior Disorders. J Behav Health Serv Res. 2020;47:146-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Johnston C, Mah JW, Regambal M. Parenting cognitions and treatment beliefs as predictors of experience using behavioral parenting strategies in families of children with attention-deficit/hyperactivity disorder. Behav Ther. 2010;41:491-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Sawrikar V, Dadds M. What Role for Parental Attributions in Parenting Interventions for Child Conduct Problems? Clin Child Fam Psychol Rev. 2018;21:41-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | DosReis S, Mychailyszyn MP, Evans-Lacko SE, Beltran A, Riley AW, Myers MA. The meaning of attention-deficit/hyperactivity disorder medication and parents' initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | Cormier E. How parents make decisions to use medication to treat their child's ADHD: a grounded theory study. J Am Psychiatr Nurses Assoc. 2012;18:345-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Bussing R, Koro-Ljungberg M, Noguchi K, Mason D, Mayerson G, Garvan CW. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74:92-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Breaux R, Waschbusch DA, Marshall R, Humphrey H, Pelham WE Jr, Waxmonsky JG. The Role of Parental Knowledge and Attitudes about ADHD and Perceptions of Treatment Response in the Treatment Utilization of Families of Children with ADHD. Evid Based Pract Child Adolesc Ment Health. 2020;5:102-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Brinkman WB, Epstein JN. Treatment planning for children with attention-deficit/hyperactivity disorder: treatment utilization and family preferences. Patient Prefer Adherence. 2011;5:45-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Pelham WE Jr, Fabiano GA, Waxmonsky JG, Greiner AR, Gnagy EM, Pelham WE 3rd, Coxe S, Verley J, Bhatia I, Hart K, Karch K, Konijnendijk E, Tresco K, Nahum-Shani I, Murphy SA. Treatment Sequencing for Childhood ADHD: A Multiple-Randomization Study of Adaptive Medication and Behavioral Interventions. J Clin Child Adolesc Psychol. 2016;45:396-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 78. | Collins KP, Cleary SD. Racial and ethnic disparities in parent-reported diagnosis of ADHD: National Survey of Children's Health (2003, 2007, and 2011). J Clin Psychiatry. 2016;77:52-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Getahun D, Jacobsen SJ, Fassett MJ, Chen W, Demissie K, Rhoads GG. Recent trends in childhood attention-deficit/hyperactivity disorder. JAMA Pediatr. 2013;167:282-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 80. | Bussing R, Gary FA, Mills TL, Garvan CW. Cultural variations in parental health beliefs, knowledge, and information sources related to attention-deficit/hyperactivity disorder. J Family Issues. 2007;28:291-318. [DOI] [Cited in This Article: ] |
| 81. | Bailey RK, Owens DL. Overcoming challenges in the diagnosis and treatment of attention-deficit/hyperactivity disorder in African Americans. J Natl Med Assoc. 2005;97:5S-10S. [PubMed] [Cited in This Article: ] |
| 82. | Dosreis S, Zito JM, Safer DJ, Soeken KL, Mitchell JW Jr, Ellwood LC. Parental perceptions and satisfaction with stimulant medication for attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2003;24:155-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 83. | Arcia E, Fernández MC, Jáquez M. Latina mothers' stances on stimulant medication: complexity, conflict, and compromise. J Dev Behav Pediatr. 2004;25:311-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Hooven JT, Fogel BN, Waxmonsky JG, Sekhar DL. Exploratory study of barriers to successful office contacts for attention deficit hyperactivity disorder. Atten Defic Hyperact Disord. 2018;10:237-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Bussing R, Mehta AS. Stigmatization and self-perception of youth with attention deficit/hyperactivity disorder. Patient Intelligence. 2013;5:15-27. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Singh I, Kendall T, Taylor C, Mears A, Hollis C, Batty M, Keenan S. Young People's Experience of ADHD and Stimulant Medication: A Qualitative Study for the NICE Guideline. Child Adolesc Ment Health. 2010;15:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Hart CN, Kelleher KJ, Drotar D, Scholle SH. Parent-provider communication and parental satisfaction with care of children with psychosocial problems. Patient Educ Couns. 2007;68:179-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Fiks AG, Hughes CC, Gafen A, Guevara JP, Barg FK. Contrasting parents' and pediatricians' perspectives on shared decision-making in ADHD. Pediatrics. 2011;127:e188-e196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Mises R, Quemada N, Botbol M, Burzsteijn C, Garrabe J, Golse B, Jeammet P, Plantade A, Portelli C, Thevenot JP. French classification for child and adolescent mental disorders. Psychopathology. 2002;35:176-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Weibel S, Menard O, Ionita A, Boumendjel M, Cabelguen C, Kraemer C, Micoulaud-Franchi JA, Bioulac S, Perroud N, Sauvaget A, Carton L, Gachet M, Lopez R. Practical considerations for the evaluation and management of Attention Deficit Hyperactivity Disorder (ADHD) in adults. Encephale. 2020;46:30-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Child and Adolescent Psychiatry. Cilinica Universidad De Navarra. [cited 26 March 2021]. Available from: https://www.cun.es. [Cited in This Article: ] |
| 92. | Schatz NK, Fabiano GA, Cunningham CE, dosReis S, Waschbusch DA, Jerome S, Lupas K, Morris KL. Systematic Review of Patients' and Parents' Preferences for ADHD Treatment Options and Processes of Care. Patient. 2015;8:483-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Frank E, Ozon C, Nair V, Othee K. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76:e1459-e1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Toomey SL, Sox CM, Rusinak D, Finkelstein JA. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Chacko A, Wymbs BT, Rajwan E, Wymbs E, N F. Characteristics of Parents and Children with ADHD who Never Attend, Drop Out and Complete Behavioral Parent Training. J Child Fam Stud. 2017;28:950-960. [DOI] [Cited in This Article: ] |
| 96. | He Y, Gewirtz AH, Lee S, August G. Do Parent Preferences for Child Conduct Problem Interventions Impact Parenting Outcomes? J Marital Fam Ther. 2018;44:716-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Gewirtz AH, Lee SS, August GJ, He Y. Does Giving Parents Their Choice of Interventions for Child Behavior Problems Improve Child Outcomes? Prev Sci. 2019;20:78-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Primack BA, Hendricks KM, Longacre MR, Adachi-Mejia AM, Weiss JE, Titus LJ, Beach ML, Dalton MA. Parental efficacy and child behavior in a community sample of children with and without attention-deficit hyperactivity disorder (ADHD). Atten Defic Hyperact Disord. 2012;4:189-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 99. | Williamson D, Johnston C. Maternal ADHD Symptoms and Parenting Stress: The Roles of Parenting Self-Efficacy Beliefs and Neuroticism. J Atten Disord. 2019;23:493-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 100. | Hoza B, Owens JS, Pelham WE, Swanson JM, Conners CK, Hinshaw SP, Arnold LE, Kraemer HC. Parent cognitions as predictors of child treatment response in attention-deficit/hyperactivity disorder. J Abnorm Child Psychol. 2000;28:569-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 101. | Niec LN, Barnett ML, Gering CL, Triemstra K, Solomon DT. Differences in Mothers' and fathers' readiness for Change in Parent Training. Child and Family Behavior Therapy 2015; 37: 224-235. [DOI] [Cited in This Article: ] |
| 102. | Chacko A, Wymbs BT, Wymbs FA, Pelham WE, Swanger-Gagne MS, Girio E, Pirvics L, Herbst L, Guzzo J, Phillips C, O'Connor B. Enhancing traditional behavioral parent training for single mothers of children with ADHD. J Clin Child Adolesc Psychol. 2009;38:206-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Wymbs BT, Pelham WE Jr, Molina BS, Gnagy EM, Wilson TK, Greenhouse JB. Rate and predictors of divorce among parents of youths with ADHD. J Consult Clin Psychol. 2008;76:735-744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Piotrowska PJ, Tully LA, Lenroot R, Kimonis E, Hawes D, Moul C, Frick PJ, Anderson V, Dadds MR. Mothers, Fathers, and Parental Systems: A Conceptual Model of Parental Engagement in Programmes for Child Mental Health-Connect, Attend, Participate, Enact (CAPE). Clin Child Fam Psychol Rev. 2017;20:146-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 105. | McCarthy S. Pharmacological interventions for ADHD: how do adolescent and adult patient beliefs and attitudes impact treatment adherence? Patient Prefer Adherence. 2014;8:1317-1327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Meaux JB, Hester C, Smith B, Shoptaw A. Stimulant medications: a trade-off? J Spec Pediatr Nurs. 2006;11:214-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Gajria K, Lu M, Sikirica V, Greven P, Zhong Y, Qin P, Xie J. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1543-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Sibley MH, Graziano PA, Kuriyan AB, Coxe S, Pelham WE, Rodriguez L, Sanchez F, Derefinko K, Helseth S, Ward A. Parent-teen behavior therapy + motivational interviewing for adolescents with ADHD. J Consult Clin Psychol. 2016;84:699-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 109. | Emilsson M, Gustafsson PA, Öhnström G, Marteinsdottir I. Beliefs regarding medication and side effects influence treatment adherence in adolescents with attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2017;26:559-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Emilsson M, Gustafsson P, Öhnström G, Marteinsdottir I. Impact of personality on adherence to and beliefs about ADHD medication, and perceptions of ADHD in adolescents. BMC Psychiatry. 2020;20:139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | McCarthy S, Asherson P, Coghill D, Hollis C, Murray M, Potts L, Sayal K, de Soysa R, Taylor E, Williams T, Wong IC. Attention-deficit hyperactivity disorder: treatment discontinuation in adolescents and young adults. Br J Psychiatry. 2009;194:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 112. | Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9:223-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 113. | Monastra VJ. Overcoming the barriers to effective treatment for attention-deficit/hyperactivity disorder: a neuro-educational approach. Int J Psychophysiol. 2005;58:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Johnston C, Hommersen P, Seipp C. Acceptability of behavioral and pharmacological treatments for attention-deficit/hyperactivity disorder: relations to child and parent characteristics. Behav Ther. 2008;39:22-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 115. | Hamrin V, McCarthy EM, Tyson V. Pediatric psychotropic medication initiation and adherence: a literature review based on social exchange theory. J Child Adolesc Psychiatr Nurs. 2010;23:151-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 116. | Morentin ADMd, Esperón CS, Tricas-Sauras S, Nuin MB, Sotos TP, Gracia KM. Effectiveness of a nurse led psychoeducation program in parents of children with attention-deficit hyperactivity disorder. Revista de psiquiatría infanto-juvenil. 2013;30:55-63. [Cited in This Article: ] |
| 117. | Davis DW, Jones VF, Logsdon MC, Ryan L, Wilkerson-McMahon M. Health promotion in pediatric primary care: importance of health literacy and communication practices. Clin Pediatr (Phila). 2013;52:1127-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | DeMaso D, Martini R, Sulik LR. A guide to building collaborative mental health care partnerships in pediatric primary care. J Am Acad Child Adolesc Psychiatry. 2010;. [Cited in This Article: ] |
| 119. | Visser SN, Zablotsky B, Holbrook JR, Danielson ML, Bitsko RH. Diagnostic Experiences of Children With Attention-Deficit/Hyperactivity Disorder. Natl Health Stat Report. 2015;1-7. [PubMed] [Cited in This Article: ] |
| 120. | Kolko DJ, Campo JV, Kelleher K, Cheng Y. Improving access to care and clinical outcome for pediatric behavioral problems: a randomized trial of a nurse-administered intervention in primary care. J Dev Behav Pediatr. 2010;31:393-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 121. | Kolko DJ, Campo JV, Kilbourne AM, Kelleher K. Doctor-office collaborative care for pediatric behavioral problems: a preliminary clinical trial. Arch Pediatr Adolesc Med. 2012;166:224-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Kolko DJ, Hart JA, Campo J, Sakolsky D, Rounds J, Wolraich ML, Wisniewski SR. Effects of Collaborative Care for Comorbid Attention Deficit Hyperactivity Disorder Among Children With Behavior Problems in Pediatric Primary Care. Clin Pediatr (Phila). 2020;59:787-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 123. | Power TJ, Mautone JA, Marshall SA, Jones HA, Cacia J, Tresco K, Cassano MC, Jawad AF, Guevara JP, Blum NJ. Feasibility and Potential Effectiveness of Integrated Services for Children With ADHD in Urban Primary Care Practices. Clin Pract Pediatr Psychol. 2014;2:412-426. [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 124. | Biederman J, Fried R, DiSalvo M, Driscoll H, Green A, Biederman I, Woodworth KY, Faraone SV. A novel digital health intervention to improve patient engagement to stimulants in adult ADHD in the primary care setting: Preliminary findings from an open label study. Psychiatry Res. 2020;291:113158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Spencer T, Noyes E, Biederman J. Telemedicine in the Management of ADHD: Literature Review of Telemedicine in ADHD. J Atten Disord. 2020;24:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 126. | Myers K, Vander Stoep A, Lobdell C. Feasibility of conducting a randomized controlled trial of telemental health with children diagnosed with attention-deficit/hyperactivity disorder in underserved communities. J Child Adolesc Psychopharmacol. 2013;23:372-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 127. | Myers K, Vander Stoep A, Zhou C, McCarty CA, Katon W. Effectiveness of a telehealth service delivery model for treating attention-deficit/hyperactivity disorder: a community-based randomized controlled trial. J Am Acad Child Adolesc Psychiatry. 2015;54:263-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 128. | Dose C, Hautmann C, Buerger M, Schuermann S, Woitecki K, Doepfner M. Telephone-assisted self-help for parents of children with attention-deficit/hyperactivity disorder who have residual functional impairment despite methylphenidate treatment: a randomized controlled trial. J Child Psychol Psychiatry. 2017;58:682-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 129. | Ingoldsby EM. Review of Interventions to Improve Family Engagement and Retention in Parent and Child Mental Health Programs. J Child Fam Stud. 2010;19:629-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 130. | Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd ed. New York: Guilford Press, 2002. [Cited in This Article: ] |
| 131. | Miller WR, Rollnick S. Motivational interviewing: Helping people change. New York: Guilford Press, 2013. [Cited in This Article: ] |
| 132. | Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C, Kunkel LE, Mikulich-Gilbertson SK, Morgenstern J, Obert JL, Polcin D, Snead N, Woody GE; National Institute on Drug Abuse Clinical Trials Network. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: a multisite effectiveness study. Drug Alcohol Depend. 2006;81:301-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 284] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 133. | VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 134. | Fabiano GA, Pelham WE, Cunningham CE, Yu J, Gangloff B, Buck M, Linke S, Gormley M, Gera S. A waitlist-controlled trial of behavioral parent training for fathers of children with ADHD. J Clin Child Adolesc Psychol. 2012;41:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 135. | Sibley MH, Comer JS, Gonzalez J. Delivering Parent-Teen Therapy for ADHD through Videoconferencing: A Preliminary Investigation. J Psychopathol Behav Assess. 2017;39:467-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 136. | Sibley MH, Graziano PA, Coxe S, Bickman L, Martin P. Effectiveness of Motivational Interviewing-Enhanced Behavior Therapy for Adolescents With Attention-Deficit/Hyperactivity Disorder: A Randomized Community-Based Trial. J Am Acad Child Adolesc Psychiatry. 2021;60:745-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 137. | Hamrin V, Iennaco JD. Evaluation of Motivational Interviewing to Improve Psychotropic Medication Adherence in Adolescents. J Child Adolesc Psychopharmacol. 2017;27:148-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 138. | Coghill D, Seth S. Effective management of attention-deficit/hyperactivity disorder (ADHD) through structured re-assessment: the Dundee ADHD Clinical Care Pathway. Child Adolesc Psychiatry Ment Health. 2015;9:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 139. | Brinkman WB, Hartl Majcher J, Poling LM, Shi G, Zender M, Sucharew H, Britto MT, Epstein JN. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 140. | Brinkman WB, Baum R, Kelleher KJ, Peugh J, Gardner W, Lichtenstein P, Langberg J, Epstein JN. Relationship Between Attention-Deficit/Hyperactivity Disorder Care and Medication Continuity. J Am Acad Child Adolesc Psychiatry. 2016;55:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 141. | Moore JA, Karch K, Sherina V, Guiffre A, Jee S, Garfunkel LC. Practice procedures in models of primary care collaboration for children with ADHD. Fam Syst Health. 2018;36:73-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Myers K, Stoep AV, Thompson K, Zhou C, Unützer J. Collaborative care for the treatment of Hispanic children diagnosed with attention-deficit hyperactivity disorder. Gen Hosp Psychiatry. 2010;32:612-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 143. | MGMA. Integrated behavioral health in a clinical primary care setting. [cited 26 March 2021]. Available from: https://www.mgma.com/resources/quality-patient-experience/integrated-behavioral-health-in-a-clinical-primary. [Cited in This Article: ] |
| 144. | Bettencourt AF, Plesko CM. A Systematic Review of the Methods Used to Evaluate Child Psychiatry Access Programs. Acad Pediatr. 2020;20:1071-1082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 145. | Ofonedu ME, Belcher HME, Budhathoki C, Gross DA. Understanding Barriers to Initial Treatment Engagement among Underserved Families Seeking Mental Health Services. J Child Fam Stud. 2017;26:863-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 146. | Petts RA, Shahidullah JD. Engagement interventions delivered in primary care to improve off-site pediatric mental health service initiation: A systematic review. Fam Syst Health. 2020;38:310-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 147. | Pyle KK, Artis NJ, Vaughan RS, Fabiano GA. Impact of pediatrician invitation on enrollment in behavioral parent training. Clin Pract Pediatr Psychol. 2019;7:192-197. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Shingleton RM, Palfai TP. Technology-delivered adaptations of motivational interviewing for health-related behaviors: A systematic review of the current research. Patient Educ Couns. 2016;99:17-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 149. | Anton MT, Jones DJ. Adoption of Technology-Enhanced Treatments: Conceptual and Practical Considerations. Clin Psychol (New York). 2017;24:223-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 150. | Oppenheimer J, Ojo O, Antonetty A, Chiujdea M, Garcia S, Weas S, Loddenkemper T, Fleegler E, Chan E. Timely Interventions for Children with ADHD through Web-Based Monitoring Algorithms. Diseases. 2019;7:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 151. | DuPaul GJ, Kern L, Belk G, Custer B, Daffner M, Hatfield A, Peek D. Face-to-Face Versus Online Behavioral Parent Training for Young Children at Risk for ADHD: Treatment Engagement and Outcomes. J Clin Child Adolesc Psychol. 2018;47:S369-S383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 152. | Gross D, Belcher HME, Budhathoki C, Ofonedu ME, Dutrow D, Uveges MK, Slade E. Reducing Preschool Behavior Problems in an Urban Mental Health Clinic: A Pragmatic, Non-Inferiority Trial. J Am Acad Child Adolesc Psychiatry. 2019;58:572-581.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 153. | Baweja R, Verma S, Pathak M, Waxmonsky JG. Development of a Child and Adolescent Tele-Partial Hospitalization Program (tele-PHP) in Response to the COVID-19 Pandemic. Prim Care Companion CNS Disord. 2020;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 154. | Cortese S, Asherson P, Sonuga-Barke E, Banaschewski T, Brandeis D, Buitelaar J, Coghill D, Daley D, Danckaerts M, Dittmann RW, Doepfner M, Ferrin M, Hollis C, Holtmann M, Konofal E, Lecendreux M, Santosh P, Rothenberger A, Soutullo C, Steinhausen HC, Taylor E, Van der Oord S, Wong I, Zuddas A, Simonoff E; European ADHD Guidelines Group. ADHD management during the COVID-19 pandemic: guidance from the European ADHD Guidelines Group. Lancet Child Adolesc Health. 2020;4:412-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 155. | Cortese S, Coghill D, Santosh P, Hollis C, Simonoff E; European ADHD Guidelines Group. Starting ADHD medications during the COVID-19 pandemic: recommendations from the European ADHD Guidelines Group. Lancet Child Adolesc Health. 2020;4:e15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 156. | Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2-4. [PubMed] [Cited in This Article: ] |
| 157. | Byrd-Bredbenner C, Finckenor M. Putting the transtheoretical model into practice with type 2 diabetes mellitus patients. Top Clin Nutr. 2000;15:44-58. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 158. | Chronis-Tuscano A, Raggi VL, Clarke TL, Rooney ME, Diaz Y, Pian J. Associations between maternal attention-deficit/hyperactivity disorder symptoms and parenting. J Abnorm Child Psychol. 2008;36:1237-1250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 159. | Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47:1102-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 1238] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 160. | Cunningham CE. Readiness for change: applications to the design and evaluation of interventions for children with ADHD. ADHD Report. 1997;5:6-9. [Cited in This Article: ] |
| 161. | Prochaska JO. Assessing how people change. Cancer. 1991;67:805-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 162. | Miller WR, Moyers TB. Eight stages in learning motivational interviewing. J Teaching Addictions. 2006;5:3-17. [DOI] [Cited in This Article: ] |
| 163. | Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: Twenty-five years of empirical studies. Res Soc Work Pract 2010; 20: 137-160. [DOI] [Cited in This Article: ] |
| 164. | Bertholet N, Gaume J, Faouzi M, Gmel G, Daeppen JB. Predictive value of readiness, importance, and confidence in ability to change drinking and smoking. BMC Public Health. 2012;12:708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 165. | Chung T, Maisto SA, Mihalo A, Martin CS, Cornelius JR, Clark DB. Brief assessment of readiness to change tobacco use in treated youth. J Subst Abuse Treat. 2011;41:137-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 166. | DiClemente CC, Nidecker M, Bellack AS. Motivation and the stages of change among individuals with severe mental illness and substance abuse disorders. J Subst Abuse Treat. 2008;34:25-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 167. | Mühlbacher AC, Rudolph I, Lincke HJ, Nübling M. Preferences for treatment of Attention-Deficit/Hyperactivity Disorder (ADHD): a discrete choice experiment. BMC Health Serv Res. 2009;9:149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 168. | Waschbusch DA, Cunningham CE, Pelham WE, Rimas HL, Greiner AR, Gnagy EM, Waxmonsky J, Fabiano GA, Robb JA, Burrows-Maclean L, Scime M, Hoffman MT. A discrete choice conjoint experiment to evaluate parent preferences for treatment of young, medication naive children with ADHD. J Clin Child Adolesc Psychol. 2011;40:546-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 169. | Bussing R, Meyer J, Zima BT, Mason DM, Gary FA, Garvan CW. Childhood ADHD Symptoms: Association with Parental Social Networks and Mental Health Service Use during Adolescence. Int J Environ Res Public Health. 2015;12:11893-11909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 170. | Lundahl B, Risser HJ, Lovejoy MC. A meta-analysis of parent training: moderators and follow-up effects. Clin Psychol Rev. 2006;26:86-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 656] [Cited by in F6Publishing: 465] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 171. | Gardner F, Montgomery P, Knerr W. Transporting Evidence-Based Parenting Programs for Child Problem Behavior (Age 3-10) Between Countries: Systematic Review and Meta-Analysis. J Clin Child Adolesc Psychol. 2016;45:749-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |