the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The response of ostracod faunal assemblages to hydrology, lake level, and carbon cycling in a Jamaican marl lake: a palaeolimnological investigation
Hannah Greenway
Jonathan Holmes
Michael Burn
Ostracod taxa from shallow freshwater lakes are sensitive to a range of limnological factors including temperature, hydrological habitat, lake level, and the distribution of aquatic plants. Ostracod assemblages preserved in Quaternary lake sediments can be used to reconstruct limnological change and are therefore potentially valuable palaeoenvironmental proxies. However, lack of autecological information about some taxa may limit the validity of such reconstructions. We use fossil ostracod assemblages recovered from radiocarbon-dated late Holocene sediments from Wallywash Great Pond, a small, shallow freshwater lake in southwestern Jamaica, to reconstruct limnological change over the past ∼ 1800 years. We circumvent ongoing taxonomic and ecological uncertainties associated with the identification of fossil ostracod taxa by drawing on observations of the ecology of ostracods found living in Jamaican water bodies. By combining this information with limnological data from the extant lake, and with sedimentological and isotopic data from the lake sediments, we show that a published interpretation of ostracod assemblages for the late Quaternary of Wallywash Great Pond is simplistic, at least for the late Holocene section of the sediment record. We conclude that changes in ostracod assemblages are linked to variations in the input of undersaturated groundwater to the northern part of the lake from which the core was recovered. These variations, which were driven by changes in the precipitation / evaporation ratio (effective moisture), also controlled sedimentation, with reduced effective moisture and a decline in undersaturated groundwater input favouring marl precipitation, whereas organic sediments are linked to increased effective moisture and enhanced groundwater input. Our findings suggest that the dramatic shifts in ostracod assemblages at this site are a complex response to changes in hydrology, sedimentology, and carbonate saturation rather than being a simple indicator of lake-level change. Combining ostracod assemblage data with the results of other palaeolimnological analyses also allows more detailed reconstructions to be made for this lake, and such a multiproxy approach is recommended for similar lakes elsewhere.
- Article
(5807 KB) - Full-text XML
- BibTeX
- EndNote
Ostracod shells preserved in Holocene lake sediments are valuable indicators of limnological change (Holmes, 1992; De Deckker, 2002; Smith and Horne, 2002). However, the factors controlling ostracod species assemblages may not always be easy to isolate, meaning that palaeolimnological reconstructions can be speculative and poorly constrained. Taxonomic uncertainties and scant information about the ecological preferences of individual species are additional complications. In freshwater lakes that have not undergone significant changes in water chemistry, factors including lake level (Alin and Cohen, 2003), seasonal permanence (Absolon, 1973), temperature (Horne, 2007), spring and groundwater influence (Taylor et al., 1994; Holmes et al., 2010), and the distribution of aquatic plants (Roca and Danielopol, 1991; Frenzel et al., 2005) have all been invoked as possible controls on ostracod species assemblages, although interpretations may sometimes be based on anecdotal evidence. Here, we present an ∼ 1800-year record of fossil ostracod assemblages preserved in the sediments of Wallywash Great Pond, a small, shallow freshwater lake in southwestern Jamaica. This is a follow-up investigation from a study of ostracod assemblages from a much longer, 9.23 m long sediment record from the lake covering the past ∼ 125 000 years (Holmes, 1998). We use information about ostracods living in Jamaican lakes and ponds (Holmes, 1997) to provide ecological data for the ostracod species and to address several taxonomic uncertainties that have become apparent since the publication of the long-core record. We combine this information with modern limnological data and with supporting sedimentological and isotopic data to derive a palaeoenvironmental record for Wallywash Great Pond for the past ∼ 1800 years.
A reasonably large body of literature details the geographical distribution and taxonomy of modern ostracods across the northern Neotropics and Caribbean regions (Broodbakker 1982, 1983a, b, c, d, 1984a, b, c; Holmes, 1997; Peréz et al., 2012, 2013a, b; Cohuo et al., 2017; Marcario-Gonzalez et al., 2018; Galindo et al., 2019). Moreover, ostracods have been used as palaeoecological indicators in several freshwater lakes across the region (Holmes, 1998; Bridgwater et al., 1999; Cohuo et al., 2020; Peréz et al., 2011). However, despite this rich source of information, palaeoecological interpretations may be hampered by taxonomic uncertainties within the faunas from this region. Indeed, ecological tolerance ranges established for taxa in one locality may not be applicable to the tolerance ranges of similar taxa elsewhere unless identifications can be confirmed (Marcario-Gonzalez et al., 2018).
In the previous study of fossil ostracod assemblages from the 9.23 m core from Wallywash Great Pond (Holmes, 1998), variations in ostracod assemblages were explained primarily by changes in the spatial distribution of aquatic plant communities within the lake, which were in turn thought to have been driven by climate-induced lake-level change. Here, we evaluate fossil ostracod assemblages recovered from a short sediment core at higher stratigraphic resolution. Previously published sedimentological, chronological, and stable isotope determinations from the same core show that the record covers the past ∼ 1800 years and shows evidence of significant changes in hydrology and sedimentation over this period, providing a good basis for investigating their impact on the ostracod assemblages. We evaluate the original interpretations from the long core in light of our new findings. We also assess the contribution of a multiple-proxy approach to palaeoenvironmental reconstruction along with contemporary limnological information to the interpretation of the ostracod assemblages.
Wallywash Great Pond is a small (0.76 km2), shallow (maximum depth of 5 m and mean depth of 2.8 m) hard-water lake located in a fault-bounded basin in Oligo–Miocene limestone in southwestern Jamaica (Fig. 1) (Street-Perrott et al., 1993). Although situated < 3 km from the coast, it is entirely fresh and of Ca–Mg–HCO3 to Mg–Ca–HCO3 composition (Street-Perrott et al., 1993; Holmes et al., 1995a). The lake is fed by direct precipitation, spring flow, and diffuse groundwater flow. Outputs are mainly from evaporation, although some water is undoubtedly lost from groundwater outflow and from a small, hand-dug channel at the lake's northern end (Holmes et al., 1995a, b) (Fig. 1). The lake's catchment supports a dry limestone semi-deciduous scrub forest to the northeast, and the lake itself is largely surrounded by freshwater wetland vegetation. The lake margins support reed swamps and floating macrophytes, whereas the deepest parts are dominated by dense stands of submerged macrophytes, including Potamogeton spp. and Chara spp. (Street-Perrott et al., 1993). The southwestern part of Jamaica experiences a seasonally wet subtropical climate, with total annual precipitation at Black River (Fig. 1) of 1234 mm (1901–2016 average), with most of that precipitation falling during the hurricane season between May and October. The mean annual temperature is 26 °C, with seasonal variation of 2–7 °C.
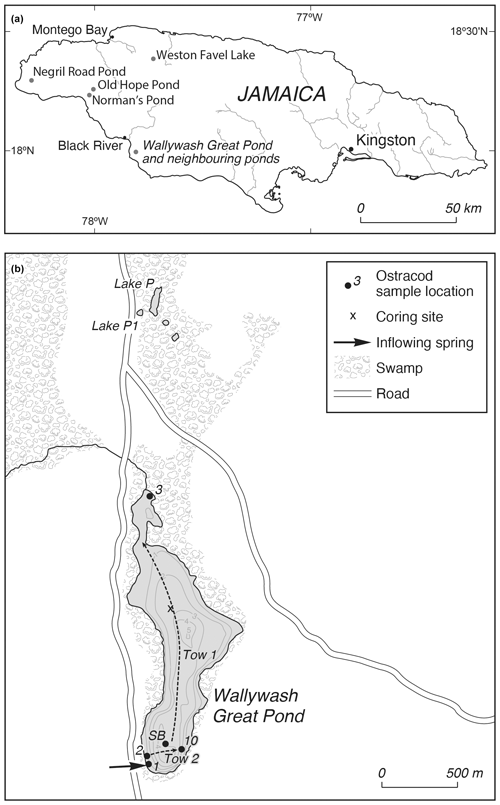
Figure 1(a) Location of Wallywash Great Pond and other sites referred to in the text. (b) Wallywash Great Pond and neighbouring lakes, showing core and modern sample locations. Dashed lines indicate the approximate lines of the two net tows taken close to the lake surface. SB is the southern basin surface sample.
This study is based on a 2.16 m long sediment sequence (core WAGP) recovered from the northern basin of Wallywash Great Pond through 3.15 m of water using a 5 cm diameter Colinvaux–Vohnout piston corer and, for the uppermost adjoining unconsolidated 52 cm of sediment, a Perspex® tube fitted with a piston. Samples of 1 cm stratigraphic thickness were taken from selected levels in WAGP, dispersed in tap water and sieved through a 250 µm mesh. The > 250 µm residues were oven-dried at 40 °C, and ostracod shells were picked from the dried coarse fractions under a low-power binocular microscope and stored on micropalaeontological slides. Carapaces were counted as two valves, and broken specimens were counted when sufficiently whole to allow identification. All of the ostracods were picked from the sediment residues, except those from 48–49 and 141–142 cm, which were exceptionally rich in material. For these two samples, all of the ostracods were picked from between approximately 75 % and 80 % of the original residue. Although similar amounts of sediment were processed for each level, exact weights were not recorded. Separate samples were therefore subsequently weighed, processed in the same way as the original sample, and the ostracod content was counted to estimate ostracod concentrations in valves per gram of dry sediment. These data were then used to estimate the concentration of each species, in valves per gram of dry sediment, which was then used for plotting purposes. Qualitative evaluations of the proportions of adult and juvenile moults were made at each level. In addition, length and height measurements of specimens were made at selected levels using a calibrated eyepiece reticule under a low-power stereomicroscope to confirm these evaluations. Ostracod identifications followed Holmes (1997, 1998). Faunal assemblage zones were established using the constrained agglomerative cluster analysis technique CONISS in the R package “rioja” (Juggins, 2015), which uses the incremental sum of squares method of Grimm (1987). The resulting dendrogram was inspected visually to determine the ostracod assemblage zones (OAZs). In addition to the ostracod faunal assemblage data presented here, we draw on data published in Holmes et al. (2023), including sediment composition, stable isotope data from ostracod shells, and the age model constructed for core WAGP, to inform our interpretations. Information relating to the modern occurrences of ostracod taxa found in core WAGP was used to help interpret the faunal assemblages in the core. This included previously published collections from Wallywash Great Pond itself (5 sites and two nekton net tows) and from other waterbodies in Jamaica (10 sampling sites from seven waterbodies) (Holmes, 1997). In addition, ostracod assemblages present in surface samples from the deepest parts of the northern and southern basins of Wallywash were examined. For the northern basin, the uppermost sample recovered from core WAGP (2–3 cm) was used; for the southern basin, a core-top sediment sample was collected from a second sediment core recovered from the southern basin. We also draw on information from studies of ostracod distribution elsewhere in the northern Neotropics, whilst exercising caution over potential taxonomic uncertainty, as discussed further below. Key references for the identification and ecology of the ostracod taxa from Wallywash are Furtos (1934), Ferguson (1959, 1964), Keyser (1977), Broodbakker (1983a, b, c, d, 1984a, b, c), Holmes (1997, 1998), Peréz et al. (2010a, b, 2011, 2013a, b, 2015), Saldarriaga and Martinez (2010), Cohuo et al. (2017, 2020), Marcario-Gonzalez et al. (2018), and Galindo et al. (2019).
Ostracods are present mainly in the upper 160 cm of core WAGP, although a few valves were found between 160 and 180 cm. A total of six taxa were recovered from the sequence, which, in order of decreasing abundance, are Cypretta brevisaepta (Furtos), Cytheridella ilosvayi (Daday), Candonopsis sp., Cypridopsis vidua (O. F. Müller), Strandesia pistrix (Broodbakker), and Darwinula sp. cf. D. stevensoni (Brady and Robertson). Cypretta brevisaepta is the most abundant taxon by a considerable margin, being more than 3.5 times more abundant overall than Cytheridella ilosvayi and having a maximum abundance (at 40–41 cm) more than double that of the maximum abundance of the latter (at 105–106 cm). The taxa are distributed unevenly through the sequence with Cypretta brevisaepta and Candonopsis sp. present throughout, although the former is more dominant above 100 cm (Fig. 2). Cypridopsis vidua and S. pistrix only occur below 100 cm, and Darwinula sp. cf. D. stevensoni occurs rarely. Cytheridella ilosvayi is more abundant below 100 cm and above 3 cm.
Altogether, six ostracod assemblage zones (OAZs) were established (Fig. 2). Zone 1 (181–161 cm) contains low concentrations of Cytheridella ilosvayi along with a few specimens of Cypridopsis vidua and Candonopsis sp. Ostracod abundance increases in OAZ2 (161–148 cm), with all taxa present except Darwinula sp. cf. D. stevensoni. Cytheridella ilosvayi and Candonopsis sp. are co-dominant for much of the zone with a spike in Cypridopsis vidua in the lower part. OAZ3 (148–99 cm) shows a further increase in abundance with Cypretta brevisaepta and Cytheridella ilosvayi co-dominant and all other taxa present except Darwinula sp. cf. D. stevensoni. OAZ4 (99–27 cm) is dominated by Cypretta brevisaepta and contains moderate concentrations of Candonopsis sp. and Darwinula sp. cf. D. stevensoni in one level. Concentrations are at a maximum in this zone, although variation between levels is considerable. For example, concentrations of Cypretta brevisaepta range from slightly above 250 to more than 2300 : concentrations of Candonopsis sp. are also variable but much lower (from 0 to more than 230 ). OAZ5 (27–11 cm) shows reduced abundance overall (< 200 throughout), and Cypretta brevisaepta dominates the assemblage. Finally, OAZ6 (above 11 cm) shows an increased abundance (> 600 in both levels within the zone), co-dominance by Cypretta brevisaepta and Cytheridella ilosvayi, and lesser occurrences of Candonopsis sp. and Darwinula sp. cf. D. stevensoni. In most levels, assemblages are represented by adult and juvenile moults (shown for Cypretta brevisaepta in Fig. 3). Surface samples from the northern and southern basins of the lake differ markedly. The northern basin, represented by samples from 2–3 cm depth in core WAGP, is co-dominated by Cypretta brevisaepta and Cytheridella ilosvayi with carbonate-rich marl sediment, whereas the southern basin is characterized by a wider diversity of taxa (Cypretta brevisaepta, Cytheridella ilosvayi, Cypridopsis vidua, and Candonopsis sp.), but much lower concentrations than in the north, and organic sediment.
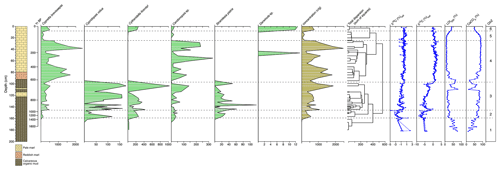
Figure 2Ostracod assemblages from core WAGP (University College London, 2024a). Abundance of each taxon, in valves per gram of dry sediment, is shown by depth in the core alongside sediment lithology, chronology (years before present, where present is 2012 CE), and ostracod-shell stable isotope values. A dendrogram, produced using CONISS, was used to determine the ostracod assemblage zone (OAZ). Chronology, loss on ignition, calcium carbonate content, and stable isotope data are from Holmes et al. (2023) (University College London, 2024b).
4.1 Taxonomic and ecological notes on the ostracod taxa recorded
-
Cypretta brevisaepta (Furtos) (Plate 1)
The species was originally described inhabiting small, fresh, and oligosaline waterbodies in Florida (Furtos, 1934; Keyser, 1977) and has subsequently been found in both shallow and deeper (up to 15 m deep) waterbodies on the Yucatán Peninsula. In Jamaica, it is abundant in the deeper open waters of Wallywash Great Pond (> 1 m below the lake surface), where it swims amongst dense submerged macrophytes, indicating that it is nektobenthic and strongly phytophilous (Holmes, 1997). It is present, although less common, in the shallower parts of the lake and in smaller waterbodies in Jamaica, including sites P and P1 to the north of Wallywash Great Pond (Fig. 1), which show influence of minor intrusion of marine–saline groundwater in their chemistry (higher total dissolved solids and higher Na and Cl concentrations compared with Wallywash Great Pond itself; Holmes et al., 1995a). Modern specimens of the species include males and females, although sexual dimorphism is not apparent in carapace morphology under a binocular light microscope at 75 × magnification. Modern adult specimens collected from the Wallywash Great Pond outlet (Site 3 in Fig. 1) have a length of 0.88 ± 0.02 mm and a height of 0.68 ± 0.04 mm (n=6) for specimens that were living at the time of collection: both left and right valves were measured. Fossil adult specimens from Wallywash Great Pond are similar in size (length of 0.87 ± 0.05 mm and height of 0.68 ± 0.05 mm; n = 14) to the modern Jamaican material and to specimens from Florida (length of 0.82–0.85 mm and height of 0.60–0.67 mm for male and female specimens, respectively, and the number of individuals measured is not stated; Furtos, 1934) but larger than specimens from Lake Petén Itzá (length of 0.54–0.67 mm and height of 0.40–0.52 mm; n = 4; Pérez et al., 2010). Marcario-Gonzalez et al. (2018) suggested that populations referred to as Cypretta brevisaepta from different localities cannot be assumed to be of the same species on the basis of carapace morphology without further investigation; thus they cautioned against the uncritical application of ecological preferences derived from specimens of this species collected in one region to draw palaeoecological inferences from material elsewhere. Moreover, based on valve overlap, it seems likely that material referred to here as Cypretta brevisaepta, in which the left valve overlaps the right, may in fact belong to a different genus: Ferreira et al. (2023) proposed that only species in which the right valve overlaps the left anteriorly should be placed in the genus Cypretta. Accordingly, we base our interpretations of this species solely on the material collected in Jamaica but, in the absence of formal taxonomic revision, retain the name Cypretta brevisaepta for the Jamaican specimens.
-
Cytheridella ilosvayi (Daday) (Plate 2)
This species is widely distributed across the northern Neotropics and Caribbean (Peréz et al., 2013a, b; Cohou et al., 2017; Galindo et al., 2019). It is a sexual species with strong sexual dimorphism in carapace shape. Galindo et al. (2019) found the species in Guatemala and Mexico in oligosaline water (EC = 1–3 mS cm−1) and noted its tolerance of high temperatures (≤ 32 °C). It is a benthic species mainly found in water 10–15 m deep (Peréz et al., 2013a, b). In Jamaica, Holmes (1997) found the species in the shallower parts of Wallywash Great Pond, close to the spring and the lake outlet (Fig. 1), where it crawls over organic calcareous mud substrate.
-
Cypridopsis vidua (O. F. Müller) (Plate 1)
Specimens of this species were found in two small ponds in Jamaica but referred to as Cypridopsis viduella (Sars, 1895). Ferguson (1964) provided a taxonomic key to North American Cypridopsis spp. in which he noted the similarity between Cypridopsis viduella and Cypridopsis vidua but argued that they differed in their height–length () ratio, with the former having a ratio of < 0.5 and the latter > 0.5. Living specimens from Black River, Jamaica, have a length of 0.55 ± 0.01 mm, a height of 0.33 ± 0.02 mm, and an ratio of 0.60 ± 0.02 (n = 4). Adult disarticulated valves from core WAGP exhibit similar dimensions (length of 0.57 ± 0.02 mm and height of 0.35 ± 0.02 mm; n = 34). Moreover, Cypridopsis viduella has since been synonymized with Cypridopsis vidua (Martens, 2001; Meisch et al., 2019), with differences in ratio attributed to intraspecific morphological variability. Koen Martens (personal communication, 2021) suggests that the Cypridopsis vidua species “complex” probably behaves similarly to other non-marine species complexes, such as Eucypris virens, for which no significant ecological differences have been noted for the different cryptic species (Bode et al., 2010). In the present study, the ecological preferences for the cosmopolitan Cypridopsis vidua can be used with reasonable confidence to interpret the occurrence of the species in Wallywash Great Pond. This contrasts with Cypretta brevisaepta, for which taxonomic uncertainties associated with different geographical populations have not yet been resolved. Cypridopsis vidua is a nektobenthic species that is closely associated with aquatic plants (Roca and Danielopol, 1991; Roca et al., 1993; Meisch, 2000). In addition to its occurrence in Jamaica, it has been widely reported from elsewhere in the northern Neotropics (Galindo et al., 2019; Pérez et al., 2011, 2013a, b, 2015).
-
Candonopsis sp. (Plate 1)
This taxon is common in core WAGP and has been found in the modern lake but has not been formally described and is therefore left in open nomenclature. As a genus, Candonopsis is a non-swimmer and is often found living in the littoral zone of lakes. This accords with the occurrence of Candonopsis sp. in the littoral zone of Wallywash Great Pond, where it occupies the boundary between the reed swamp and the open water of the lake, living on an organic mud substrate (Holmes, 1997).
-
Strandesia pistrix (Broodbakker) (Plates 1 and 2)
This distinctive species has only been found in a vegetation-rich freshwater spring in Haiti (Broodbakker, 1983) and in the marginal reed swamp of Wallywash Great Pond (Holmes, 1997); information about its ecology is therefore limited. Other species of the genus show association with aquatic macrophytes and shallow water, although with wide variation in habitat conditions (Higuti et al., 2013; Ferreira et al., 2020).
-
Darwinula sp. cf. Darwinula stevensoni (Brady and Robertson) (Plate 2)
This species is rare in the sediment record from Wallywash Great Pond. In the modern lake, it was found in shallow water (< 1 m deep) close to the inlet spring (Holmes, 1997). Darwinula stevensoni sensu stricto is a widespread cosmopolitan species often found in the shallow littoral zone of lakes. It is a freshwater species, although it can tolerate slightly elevated salinity (Ranta, 1979; Neale, 1988; Meisch, 2000). It is common in lakes in the northern Neotropics (Peréz et al., 2010, 2013a, b, 2015; Marcario-Gonzalez et al., 2018; Galindo et al., 2019; Cohuo et al., 2020). Broodbakker (1984c) also encountered the species in association with springs on Caribbean islands.
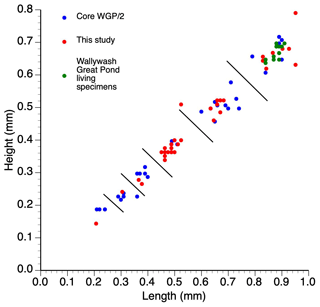
Figure 3Length–height measurements of single specimens of Cypretta brevisaepta from Wallywash Great Pond. Material from this study was from core WAGP, while core WGP/2 ostracods are described in Holmes (1997) – the measured specimens come from the late Holocene section of the core. The living specimens were collected from Site 3 (see Fig. 1 for location) of the lake.
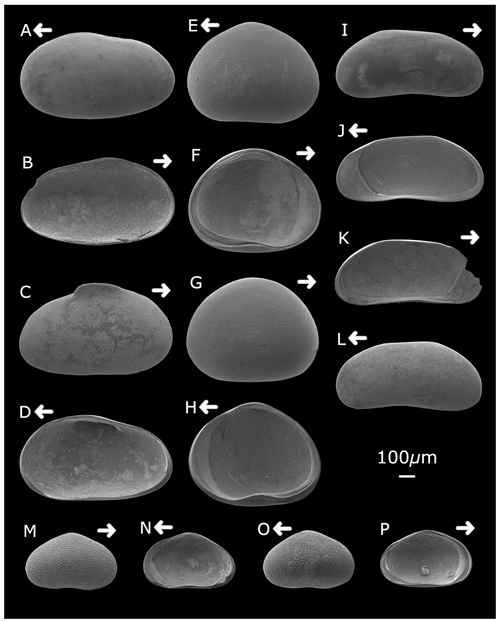
Plate 1Strandesia pistrix (A) left valve, external lateral view; (B) left valve, internal lateral view; (C) right valve, external lateral view; and (D) right valve, internal lateral view. Cypretta brevisaepta (E) right valve, external lateral view; (F) left valve, internal lateral view; (G) left valve, external lateral view; and (H) right valve, internal lateral view. Candonopsis sp. (I) right valve, external lateral view; (J) right valve, internal lateral view; (K) left valve, internal lateral view; and (L) left valve, external lateral view. Cypridopsis vidua (M) right valve, external lateral view; (N) right valve, internal lateral view; (O) left valve, external lateral view; and (P) left valve, internal lateral view. Arrows point to the anterior of the shell. Figured specimens are held in the Department of Geography, University College London, UK.
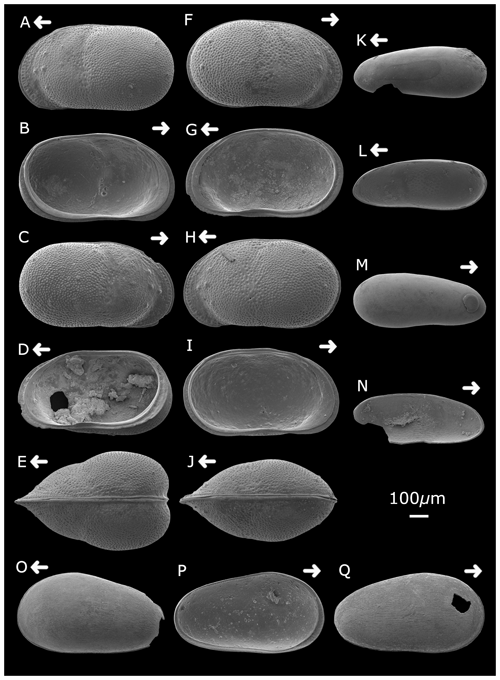
Plate 2Cytheridella ilosvayi female (A) left valve, external lateral view; (B) left valve, internal lateral view; (C) right valve, external lateral view; (D) right valve, internal lateral view; and (E) dorsal view. Cytheridella ilosvayi male (F) right valve, external lateral view; (G) right valve, internal lateral view; (H) left valve, external lateral view; (I) left valve, internal lateral view; and (J) dorsal view. Darwinula stevensoni (K) left valve, external lateral view; (L) right valve, internal lateral view; (M) right valve, external view; and (N) left valve, internal view. Strandesia pistrix juvenile (O) left valve, external lateral view; (P) left valve, internal lateral view; and (Q) right valve, external lateral view. Arrows point to the anterior of the shell. Figured specimens are held in the Department of Geography, University College London, UK.
4.2 Interpretation of the faunal assemblages
In the earlier, low-resolution study of ostracod assemblages from the much longer Late Pleistocene to Holocene core WGP/2, also recovered from the northern basin of the lake, Holmes (1998) highlighted alternations between a Cypretta-dominated assemblage and a Candonopsis-dominated assemblage, the former associated with pure marl facies and the latter with organic muds. Based on the association between ostracod assemblage and sediment type, switches between the two assemblages were interpreted to represent lake-level change, with marl sediments linked with deep open waters and the organic muds with shallow conditions. Associations between sediment type and oxygen isotope ratios (referred to hereafter in standard delta units as δ18O values) further suggested that these changes were driven by hydroclimate variability. If correct, this interpretation of the recent sediment record (pure marl facies and an ostracod assemblage rich in Cypretta brevisaepta, both purportedly reflecting deep water) appears inconsistent with the elevated δ18O values because the oxygen isotope values indicate that effective moisture was reduced at this time. The higher-resolution ostracod record from core WAGP presented here, along with detailed isotopic and sedimentological data and additional information about the modern ecology of key ostracod taxa, indicates that the original interpretation of the ostracod assemblages in Holmes (1998) requires re-evaluation, at least for the part of the sequence relating to core WAGP.
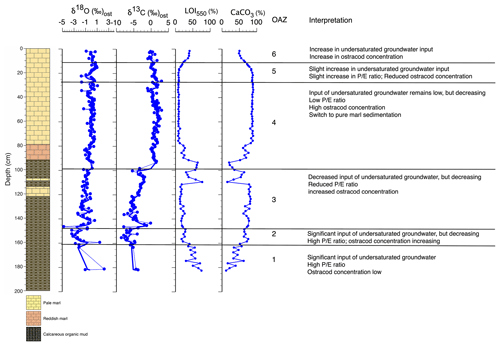
Figure 4Palaeolimnological changes inferred from core WAGP based on ostracod assemblages and other information. is the ratio of precipitation to evaporation. For core legend, see Fig. 2.
The carbonate isotope record from WAGP, which is important to the interpretation of the ostracod assemblages, is described in detail in Holmes et al. (2023), and we summarize only the key points here. The δ18O value of lacustrine carbonate in Wallywash is controlled primarily by the isotopic composition of lake water, which in turn is determined by the balance of inflow by direct precipitation and groundwater to evaporative losses (Holmes et al., 2023). Almost half of the input to the lake's water budget is from groundwater, and a significant portion of that inflow comes from one or more springs that discharge into the southern basin (Holmes et al., 1995a). This water evolves as it moves northward in the lake because of changes in temperature, aquatic photosynthesis, physical degassing of CO2, and evaporative enrichment, leading to an increase in the δ18O of water, the 13C 12C (δ13C) of dissolved inorganic carbon (DIC), and calcite saturation (Holmes et al., 1995a). This evolution leads to a marked south-to-north gradient in physical, chemical, and isotopic characteristics of the lake water and to a contrast between the two basins. Moreover, calcite saturation in the northern basin explains why marl precipitates here, whereas undersaturation in the southern basin in the modern lake leads to formation of organic-rich sediment with low carbonate content. The contrasting sediment types are also associated with differing ostracod assemblages in the modern lake. The marls that are characteristic of the northern basin are dominated by Cypretta brevisaepta and Cytheridella ilosvayi in high concentrations, whereas the organic-rich facies in the southern basin support a more diverse fauna but with much lower concentration. Modern nekton tows provide some evidence that Cypretta brevisaepta is rarer in the undersaturated parts of the lake, given its paucity in samples collected from the southern basin and abundance in the north (Holmes, 1997). However, the samples from the two basins are not directly comparable: the one from the northern basin was taken from amongst the dense stands of submerged aquatic plants, whereas that from the southern basin came from just below the water surface and above the plants. However, modern ostracod samples from elsewhere in western Jamaica also provide support for a link between the occurrence of Cypretta brevisaepta and calcite saturation. Despite the species not occurring exclusively in calcite-saturated waterbodies (Holmes et al., 1995a; Holmes 1997), it is generally more common and more abundant at such sites, although the fact that the ostracod collections and water chemistry determinations are effectively snapshot samples must be considered.
Ostracod assemblage zone (OAZ) 1 has sporadic occurrence of ostracods from a range of species (Fig. 2). Cytheridella ilosvayi is the only species to feature throughout OAZ1, with concentrations increasing later in the zone. Although Cypretta brevisaepta, Cypridopsis vidua, Candonopsis sp., and Strandesia pistrix are not present in the early-mid zone, their concentrations increase towards the boundary with OAZ2, most notably Cypridopsis vidua, which shows a marked increase. Low δ18O and δ13C values coupled with high organic content suggest enhanced input of undersaturated groundwater during this time, although the rarity of ostracods means that the number of stable isotope determinations in this zone is limited. Anoxia of the benthos could also explain low ostracod numbers, although this seems a less likely explanation given the presence of benthic ostracods in the zone. OAZ2 is marked by an increase in ostracod concentration with a significant increase in the abundance of Cypridopsis vidua, Cytheridella ilosvayi, and Strandesia pistrix (Fig. 2). Cypridopsis vidua exhibits the most notable increase before overall concentrations begin to fall towards the latter part of the zone. Low δ18O and δ13C values represented by a good number of data points indicate a continuation of enhanced input of undersaturated groundwater, although the steady rise in the carbonate content of the organic sediments and concomitant decrease in organic matter content (Fig. 2) suggest that the northern basin was becoming progressively saturated over the period represented by this zone. OAZ3 shows a further rise in ostracod concentration while diversity remains relatively high, along with a slight increase in carbonate content and corresponding slight decrease in organic content up to near the top of the zone, above which a reversal of these trends is accompanied by a decline in ostracod concentration. More generally, the rise in both δ18O and δ13C values over much of this zone points to a reduction in input of undersaturated groundwater. Cypridopsis vidua, Cytheridella ilosvayi, and Strandesia pistrix all reach their highest concentrations within OAZ3, with each showing significant variability throughout the zone. Concentrations of Cypretta brevisaepta and Candonopsis sp. remain relatively consistent here. The boundary between OAZ3 and OAZ4 marks the most notable change in ostracod assemblages, with a further increase in concentration but a marked decrease in diversity, with assemblages dominated by high concentrations of Cypretta brevisaepta and a sudden drop in the abundance of Cypridopsis vidua, Cytheridella ilosvayi, and Strandesia pistrix. The stable isotope values increase at the start of OAZ4 (slightly in the case of δ18O and substantially in the case of δ13C) and then remain high for the rest of the zone, suggesting that the input of undersaturated groundwater remained relatively low. This is supported by the switch to marl sediment above the base of the zone. In addition to the rise in Cypretta brevisaepta concentrations in this zone, there is a complete disappearance of Cytheridella ilosvayi, Strandesia pistrix, and Cypridopsis vidua. Cypretta brevisaepta clearly dominated the nektic environment in this zone and appears to be well adapted to carbonate saturation, perhaps outcompeting Cypridopsis vidua, another nektobenthic species. A clear explanation for the disappearance of Cytheridella ilosvayi and S. pistrix is lacking, although it is possible that the decline in organic content of the sediment reflected changes in the plant type and/or abundance. OAZ5 is marked by a reduction in ostracod concentration accompanied by a slight decrease in δ18O, possibly from a short-lived increase in groundwater input. As in OAZ1, although anoxia of the benthos could also explain the low ostracod numbers, the presence of benthic ostracods in the zone suggests that this is unlikely. δ18O and δ13C values remain high in the uppermost part of core WAGP coinciding with OAZ6. Here, increases in ostracod concentration and occurrences of Cytheridella ilosvayi and Darwinula stevensoni along with Cypretta brevisaepta coincide with a significant rise in the organic content of the marl, despite this not registering in the visible stratigraphy of the core. A summary of interpretations of the WAGP record is presented in Fig. 4.
The ostracod assemblages in core WAGP are a complex response to changes in calcite saturation, sediment type, and carbon cycling within the lake driven primarily by changes in hydrology, especially the varying contribution of calcite-undersaturated groundwater. In contrast to Holmes (1998), they are not a simple response to changing lake level, at least for the past ∼ 1800 years of the lake's history. More generally, we have demonstrated the importance of a detailed understanding of the modern limnology of a lake to the effective use of fossil ostracod assemblages in palaeoenvironmental reconstruction. Our limited knowledge of the distribution of modern ostracods in Wallywash Great Pond is also important, although additional sampling would provide a more detailed understanding of the distribution of ostracod taxa in the lake. The employment of additional palaeoenvironmental proxies places reconstructions on a much firmer footing than by using ostracod assemblages alone: ostracod carbonate oxygen- and carbon isotope values were especially useful in this instance. Finally, taxonomic uncertainties for one key species – in this case, Cypretta brevisaepta – were circumvented by using only material collected in Jamaica to support palaeoenvironmental reconstructions from Wallywash Great Pond.
Data from core WAGP presented in this paper are available from the UK National Geoscience Data Centre (NGDC) (ostracod assemblage data: item ID 182631 https://webapps.bgs.ac.uk/services/ngdc/accessions/index.html?simpleText=Jamaica#item182631, University College London, 2024a; stable isotope and sedimentological data: item ID 176996 https://webapps.bgs.ac.uk/services/ngdc/accessions/index.html?simpleText=Jamaica#item176996, University College London, 2024b).
HG: laboratory analyses, formal analysis, writing (original draft), and visualization. JH: conceptualization, laboratory analyses, formal analysis, writing (original draft), visualization, and funding acquisition. MB: conceptualization, fieldwork, and writing (original draft).
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank Koen Martens for invaluable discussions on taxonomy; Rachel Gwynn, Eileen Cheng, and Bonnie Atkinson for technical support; and Jim Davy for SEM photography. We are grateful to Steffen Mischke and the anonymous reviewer for their constructive comments on an earlier version of the paper.
This research has been supported by the UK Research and Innovation (grant no. NE/K00610X/1).
This paper was edited by Moriaki Yasuhara and reviewed by Steffen Mischke and one anonymous referee.
Absolon, A.: Ostracoden aus einigen Profilen spät-und postglazialer Karbonatablagerungen in Mitteleuropa, Mitt. Bayer. Staatssamml. Paläontol. Hist. Geol., 13, 47–94, 1973.
Alin, S. R. and Cohen, A. S.: Lake-level history of Lake Tanganyika, East Africa, for the past 2500 years based on ostracode-inferred water-depth reconstruction, Palaeogeogr. Palaeocl., 99, 31–49, 2003.
Bode, S. N. S., Adolfsson, S., Lamatsch, D. K., Martins, M. J. F., Schmit, O., Vandekerkhove, J., Mezquita, F., Namiotko, T., Rossetti, G., Schön, I., Butlin, R. K., and Martens, K.: Exceptional cryptic diversity and multiple origins of parthenogenesis in a freshwater ostracod, Mol. Phylogenet. Evol., 54, 542–552, https://doi.org/10.1016/j.ympev.2009.08.022, 2010.
Bridgwater, N. D., Heaton, T. H. E., and O'Hara, S. L.: A late Holocene palaeolimnological record from central Mexico, based on faunal and stable-isotope analysis of ostracod shells, J. Paleolimnol., 22, 383–397, 1999.
Broodbakker, N. W.: Amsterdam expeditions to the West Indian Islands, report 20, The genus Heterocypris (Crustacea, Ostracoda) in the West Indies, Part I: Taxonomic characteristics, Bijdr. Dierkd., 52, 207–227, 1982.
Broodbakker, N. W.: Amsterdam expeditions to the West Indian Island, report 24, The genus Heterocypris (Crustacea, Ostracoda) in the West Indies, Part II: Carapace length, ecology and zoogeography, Bijdr. Dierkd., 53, 115–134, 1983a.
Broodbakker, N. W.: Amsterdam expeditions to the West Indian Islands, report 25, The genus Hermicypris (Crustacea, Ostracoda) in the West Indies, Bijdr. Dierkd., 53, 135–157, 1983b.
Broodbakker, N. W.: Amsterdam expeditions to the West Indian Islands, Report 34, The subfamily Candoninae (Crustacea, Ostracoda) in the West Indies, Bijdr. Dierkd., 53, 287–326, 1983c.
Broodbakker, N. W.: Amsterdam expeditions to the West Indian Islands, report 35, The genus Strandesia and other Cypricercini (Crustacea, Ostracoda) in the West Indies, Part I: taxonomy, Bijdr. Dierkd., 53, 327–368, 1983d.
Broodbakker, N. W.: The genus Strandesia and other Cypricercini (Crustacea, Ostracoda) in the West Indies, Part II: Carapace length: ecology and distribution of two Strandesia species, Bijdr. Dierkd., 54, 1–14, 1984a.
Broodbakker, N. W.: The genus Tanycypris (Crustacea, Ostracoda) in the West Indies, Bijdr. Dierkd., 54, 15–24, 1984b.
Broodbakker, N. W.: The distribution and zoogeography of fresh-water Ostracoda (Crustacea) in the West Indies with emphasis on species inhabiting wells, Bijdr. Dierkd., 54, 25–50, 1984c.
Cohuo, S., Macario-Gonzalez, L., Perez, L., and Schwalb, A.: Overview of Neotropical-Caribbean freshwater ostracode fauna (Crustacea, Ostracoda): identifying areas of endemism and assessing biogeographical affinities, Hydrobiologia, 786, 5–21, 2017.
Cohuo, S., Macario-González, L., Wagner, S., Naumann, K., Echeverría-Galindo, P., Pérez, L., Curtis, J., Brenner, M., and Schwalb, A.: Influence of late Quaternary climate on the biogeography of Neotropical aquatic species as reflected by non-marine ostracodes, Biogeosciences, 17, 145–161, https://doi.org/10.5194/bg-17-145-2020, 2020.
De Deckker, P.: Ostracod palaeoecology, in: The Ostracoda: Applications in Quaternary Research, edited by: Holmes, J. A. and Chivas, A. R., American Geophysical Union, Geophysical Monograph, AGU, 121–134, ISBN: 0-875-90990-6, 2002.
Ferguson, E.: A synopsis of the ostracod (Crustacea) genus Cypridopsis with the description of a new species, Proc. Biol. Soc. Wash., 72, 59–68, 1959.
Ferguson, E.: The Ostracod (Crustacea) genus Cypridopsis in North America and a description of Cypridopsis howei sp. nov., Trans. Am. Mic. Soc., 83, 380–384, 1964.
Ferreira, V. G., Higuti, J., and Martens, K.: Taxonomic revision of Strandesia s.s. (Crustacea, Ostracoda) from four Brazilian floodplains, with the description of three new species, Zootaxa, 4760, 1–74, https://doi.org/10.11646/zootaxa.4760.1.1, 2020.
Ferreira, V. G., Higuti, J., and Martens, K.: Redescription of the type species of the genus Cypretta (Ostracoda, Crustacea), with notes on the taxonomy of the genus, Zootaxa, 5231, 79–92, https://doi.org/10.11646/zootaxa.5231.1.6, 2023.
Frenzel, P., Henkel, D., Siccha, M., and Tschendel, L.: Do ostracod associations reflect macrophyte communities? A case study from the brackish water of the southern Baltic Sea coast, Aquat. Sci., 67, 142–155, 2005.
Furtos, N. C.: Two new species of Cypretta from the Marquesas Islands and Florida with notes on the distribution of the species, Bernice P Bishop Mus. Bull., 114, 279–286, 1934.
Galindo, P. G. E., Peréz, L., Correa-Metrio, A., Avendano, C. E., Moguel, B., Brenner, M., Cohuo, S., Macario, L., Caballero, M., and Schwalb, A.: Tropical freshwater ostracodes as environmental indicators across an altitude gradient in Guatemala and Mexico, Rev. Biol. Trop., 67, 1037–1058, 2019.
Grimm, E. C.: CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares, Comput. Geosci., 13, 13–35, 1987.
Higuti, J., Schön, I., Audenaert, L., and Martens, K.: On the Strandesia obtusata/elliptica lineage (Ostracoda, Cyprididae) in the alluvial valley of the Upper Parana River (Brazil), with the description of three new species, Crustaceana, 86, 182–211, https://doi.org/10.1163/15685403-00003160, 2013.
Holmes, J. A.: Nonmarine ostracods as Quaternary palaeoenvironmental indicators, Prog. Phys. Geogr., 16, 405–431, 1992.
Holmes, J. A.: Recent non-marine Ostracoda from Jamaica, West Indies, J. Micropal., 16, 137–143, 1997.
Holmes, J. A.: A late Quaternary ostracod record from Wallywash great pond, a Jamaican marl lake, J. Paleolimnol., 19, 115–128, 1998.
Holmes, J. A., Street-Perrott, F. A., Heaton, T., Darbyshire, D. P. F., Davies, N. C., and Hales, P. E.: Chemical and isotopic composition of karstic lakes in Jamaica, West Indies, Hydrobiologia, 312, 121–138, 1995a.
Holmes, J. A., Street-Perrott, F. A., Ivanovich, M., and Perrott, R. A.: A late Quaternary palaeolimnological record from Jamaica based on trace-element chemistry of ostracod shells, Chem. Geol., 124, 143–160, 1995b.
Holmes, J. A., Atkinson, T., Darbyshire, D. P. F., Horne, D. J., Joordens, J., Roberts, M. B., Sinka, K. J., and Whittaker, J. E.: Middle Pleistocene climate and hydrological environment at the Boxgrove hominin site (West Sussex, UK) from ostracod records, Quaternary Sci. Rev., 29, 1515–1527, https://doi.org/10.1016/J.Quascirev.2009.02.024, 2010.
Holmes, J., Burn, M., Cisneros-Dozal, L. M., Jones, M., and Metcalfe, S.: An 1800-year oxygen-isotope record of short- and long-term hydroclimate variability in the northern neotropics from a Jamaican marl lake, Quaternary Sci. Rev., 301, 107930, https://doi.org/10.1016/j.quascirev.2022.107930, 2023.
Horne, D. J.: A Mutual Temperature Range method for Quaternary palaeoclimatic analysis using European nonmarine Ostracoda, Quaternary Sci. Rev., 26, 1398–1415, 2007.
Juggins, S.: rioja: Analysis of Quaternary Science Data, R package version (0.9-9), http://cran.r-project.org/package=rioja (last access: 10 May 2024), 2015.
Keyser, D.: Ecology and zoogeography of recent brackish-water Ostracoda (Crustacea) from South-West Florida, in: Aspects of Ecology and Zoogeography of Recent and Fossil Ostracoda, edited by: Löffler, H. and Danielopol, D., 207–222, Dr W. Junk b.v., ISBN: 9-061-93581-4, 1977.
Macario-Gonzalez, L., Cohuo, S., Elias-Gutierrez, M., Vences, M., Perez, L., and Schwalb, A.: Integrative taxonomy of freshwater ostracodes (Crustacea: Ostracoda) of the Yucatan Peninsula, implications for paleoenvironmental reconstructions in the northern Neotropical region, Zool. Anz., 275, 20–36, https://doi.org/10.1016/j.jcz.2018.04.002, 2018.
Martens, K.: Ostracoda, in: Guides to the Freshwater Invertebrates of Southern Africa, edited by: Day, J. A., de Moor, I. J., Stewart, B. A., and Louw, A. E., 3 Crustacea II, 9–77, Pretoria, ISBN: 1-86845-703-6, 2001.
Meisch, C.: Freshwater Ostracoda of western and central Europe, in: Süsswasserfauna von Mitteleuropa, 8/3, Spektrum Akademischer Verlag Gustav Fischer, Heidelberg, Berlin, ISBN: 3-8274-1001-0, 2000.
Meisch, C., Smith, R. J., and Martens, K.: A subjective global checklist of the extant non-marine Ostracoda (Crustacea), Eur. J. Taxon., 492, 1–135, https://doi.org/10.5852/ejt.2019.492, 2019.
Neale, J. W.: Ostracods and palaeosalinity reconstruction, in: Ostracoda in the Earth Sciences, edited by: De Deckker, P., Colin, J.-P., and Peypouquet, J.-P., Elsevier, Amsterdam, 125–155, ISBN: 0-444-43011-3, 1988.
Pérez, L., Lorenschat, J., Brenner, M., Scharf, B., and Schwalb, A.: Non-marine ostracodes (Crustacea) of Guatemala, edited by: Cano, E. and Schuster, J., Biodiversidad de Guatemala, Vol. 2, 121–131, ISBN: 978-9929-40-239-3, 2012.
Pérez, L., Lorenschat, J., Brenner, M., Scharf, B., and Schwalb, A.: Extant freshwater ostracodes (Crustacea: Ostracoda) from Lago Petén Itzá, Guatemala, Rev. Biol. Trop., 58, 871–895, https://doi.org/10.15517/rbt.v58i2.5252, 2010a.
Pérez, L., Lorenschat, J., Bugja, R., Brenner, M., Scharf, B., and Schwalb, A.: Distribution, diversity and ecology of modern freshwater ostracodes (Crustacea), and hydrochemical characteristics of Lago Petén Itzá, Guatemala, J. Limnol., 69, 146–159, 2010b.
Pérez, L., Frenzel, P., Brenner, M., Escobar, J., Hoelzmann, P., Scharf, B., and Schwalb, A.: Late Quaternary (24–10 ka BP) environmental history of the Neotropical lowlands inferred from ostracodes in sediments of Lago Peten Itza, Guatemala, J. Paleolimnol., 46, 59–74, https://doi.org/10.1007/s10933-011-9514-0, 2011.
Pérez, L., Lorenschat, J., Massaferro, J., Pailles, C., Sylvestre, F., Hollwedel, W., Brandorff, G.-O., Brenner, M., Islebe, G., Lozano, M. S., Scharf, B., and Schwalb, A.: Bioindicators of climate and trophic state in lowland and highland aquatic ecosystems of the northern Neotropics, Rev. Biol. Trop., 61, 603–644, https://doi.org/10.15517/rbt.v61i2.11164, 2013a.
Pérez, L., Curtis, J., Brenner, M., Hodell, D., Escobar, J., Lozano, S., and Schwalb, A.: Stable isotope values (δ18O and δ13C) of multiple ostracode species in a large Neotropical lake as indicators of past changes in hydrology, Quaternary Sci. Rev., 66, 96–111, 2013b.
Pérez, L., Lozano-García, S., and Caballero, M.: Non-marine ostracodes from highland lakes in east-central Mexico, Rev. Biol. Trop., 63, 401–425, https://doi.org/10.15517/rbt.v63i2.15240, 2015.
Ranta, E.: Population biology of Darwinula stevensoni (Crustacea, Ostracoda) in an oligotrophic lake, Ann. Zool. Fenn., 16, 28–35, 1979.
Roca, J. R. and Danielopol, D. L.: Exploration of interstitial habitats by the phytophilous ostracod Cypridopsis vidua (O. F. Müller): experimental evidence, Ann. Limnol., 27, 243–252, 1991.
Roca, J. R., Baltanás, A., and Uiblein, F.: Adaptive responses in Cypridopsis vidua (Crustacea, Ostracoda) to food and shelter offered by a macrophyte (Chara fragilis), Hydrobiologia, 262, 127–131, 1993.
Saldarriaga, A. T. and Martínez, J. I.: Ecology of non–marine Ostracoda from La Fe reservoir (El Retiro, Antioquia) and their potential application in paleoenvironmental studies, Rev. Acad. Col. Cienc., 34, 397–409, 2010.
Smith, A. J. and Horne, D. J.: Ecology of Marine, Marginal Marine and Nonmarine Ostracodes, in: The Ostracoda: Applications in Quaternary Research, edited by: Holmes, J. A. and Chivas, A. R., American Geophysical Union, Geophysical Monograph, AGU, 37–64, ISBN: 0-87590-990-6, 2002.
Street-Perrott, F. A., Hales, P. E., Perrott, R. A., Fontes, J. C., Switsur, V. R., and Pearson, A.: Late Quaternary palaeolimnology of a tropical marl lake: Wallywash Great Pond, Jamaica, J. Paleolimnol., 9, 3–22, 1993.
Taylor, D. M., Griffiths, H. I., Pedley, H. M., and Prince, I.: Radiocarbon-dated Holocene pollen and ostracod sequences from barrage tufa-dammed fluvial systems in the White Peak, Derbyshire, UK, Holocene, 4, 356–364, 1994.
University College London: Ostracod assemblage data from Wallywash Great Pond, Jamaica, University College London [data set], https://webapps.bgs.ac.uk/services/ngdc/accessions/index.html?simpleText=Jamaica#item182631, last access: 10 May 2024a.
University College London: Stable isotope and sedimentological data from Wallywash Great Pond, Jamaica, University College London [data set], https://webapps.bgs.ac.uk/services/ngdc/accessions/index.html?simpleText=Jamaica#item176996, last access: 10 May 2024b.





