Published online Oct 26, 2015. doi: 10.4330/wjc.v7.i10.685
Peer-review started: June 16 2015
First decision: July 3, 2015
Revised: July 17, 2015
Accepted: September 16, 2015
Article in press: September 16, 2015
Published online: October 26, 2015
AIM: To compare the performance of the re-expressed Modification of Diet in Renal Disease equation vs the new Chronic Kidney Disease Epidemiology Collaboration equation in patients with non-valvular atrial fibrillation.
METHODS: We studied 911 consecutive patients with non-valvular atrial fibrillation on vitamin-K antagonist. The performance of the re-expressed Modification of Diet in Renal Disease equation vs the new Chronic Kidney Disease Epidemiology Collaboration equation in patients with non-valvular atrial fibrillation with respect to either a composite endpoint of major bleeding, thromboembolic events and all-cause mortality or each individual component of the composite endpoint was assessed using continuous and categorical ≥ 60, 59-30, and < 30 mL/min per 1.73 m2 estimated glomerular filtration rate.
RESULTS: During 10 ± 3 mo, the composite endpoint occurred in 98 (10.8%) patients: 30 patients developed major bleeding, 18 had thromboembolic events, and 60 died. The new equation provided lower prevalence of renal dysfunction < 60 mL/min per 1.73 m2 (32.9%), compared with the re-expressed equation (34.1%). Estimated glomerular filtration rate from both equations was independent predictor of composite endpoint (HR = 0.98 and 0.97 for the re-expressed and the new equation, respectively; P < 0.0001) and all-cause mortality (HR = 0.98 for both equations, P < 0.01). Strong association with thromboembolic events was observed only when estimated glomerular filtration rate was < 30 mL/min per 1.73 m2: HR is 5.1 for the re-expressed equation, and HR = 5.0 for the new equation. No significant association with major bleeding was observed for both equations.
CONCLUSION: The new equation reduced the prevalence of renal dysfunction. Both equations performed similarly in predicting major adverse outcomes.
Core tip: In atrial fibrillation, renal dysfunction entails more adverse events. Limited data exist on the performance and prognostic value of the re-expressed Modification of Diet in Renal Disease equation vs the new Chronic Kidney Disease Epidemiology Collaboration equation in atrial fibrillation. We compared the performance of both equations at predicting major outcomes in patients with non-valvular atrial fibrillation. The study encouraged the use of the new equation as it decreased the prevalence of patients with renal dysfunction, in a real world cohort of patients with non-valvular atrial fibrillation and at the same time showed similar prognostic impact like the re-expressed equation.
-
Citation: Abumuaileq RRY, Abu-Assi E, López-López A, Raposeiras-Roubin S, Rodríguez-Mañero M, Martínez-Sande L, García-Seara FJ, Fernandez-López XA, González-Juanatey JR. Renal function assessment in atrial fibrillation: Usefulness of chronic kidney disease epidemiology collaboration
vs re-expressed 4 variable modification of diet in renal disease. World J Cardiol 2015; 7(10): 685-694 - URL: https://www.wjgnet.com/1949-8462/full/v7/i10/685.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i10.685
Renal dysfunction is a common comorbidity observed in patients with atrial fibrillation (AF). Patients with AF and renal dysfunction are more likely to develop thromboembolic (TE) events compared to those with AF and normal renal function[1,2]. The presence and severity of renal dysfunction is also a recognized predictor in the bleeding risk scores used commonly to estimate the hemorrhagic risk in anticoagulated patients with AF[3,4].
Therefore, accurate assessment of renal function is of paramount importance as it will help inform the decision making process aiming for optimizing the management of patients with AF. Current recommendations advocate the estimation of renal function by means of estimated glomerular filtration rate (eGFR) using the validating prediction equations instead of serum creatinine[5].
Until recently, the two most commonly used creatinine based equations estimating GFR were the 4 variable Modification of Diet in Renal Disease (MDRD-4) Study[6] and the Cockcroft-Gault (C-G) equation[7]. The MDRD-4 equation was re-expressed to be used in the current era of standardized serum creatinine assay, whereas the C-G equation was not updated, and its use is not recommended currently[8]. More recently, a new equation, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation[9], has been proposed as an alternative equation to replace the widely used re-expressed MDRD-4 formula in routine clinical use, on the basis that it estimates measures of GFR more accurate than the re-expressed MDRD-4 equation.
Several studies have demonstrated the higher accuracy of the new CKD-EPI at estimating the true renal function, thus enabling it to provide better clinical risk prediction in different disease contexts[10-12]. However, it is currently unknown if the better estimates from the new CKD-EPI would be translated into better risk prediction in the particular context of patients with AF, since very few patients in the derivation cohort of the new CKD-EPI formula had AF[9].
In this study, we aimed to comparatively evaluate the re- expressed MDRD-4 and the new CKD-EPI formulas at predicting the occurrence of major adverse outcomes in a real world cohort of patients with non- valvular AF (NVAF) who are recently on vitamin K antagonists (VKA).
Retrospectively, we identified all consecutive patients of ≥ 18 years of age with a confirmed diagnosis of AF on VKAs attending outpatient cardiology consultations of a tertiary hospital between January 2011 and February 2013. Only patients who fulfilled the following criteria were included in this study: Patients with permanent or paroxysmal AF recently started on VKAs (i.e., not more than 8 mo passed since the beginning of their VKAs therapy), and who have regular visits for INR measurements. Patients with prosthetic valve (n = 452), rheumatic heart disease (n = 43), active cancer (n = 41), dementia (n = 26), and/or interrupted vitamin K antagonist > 3 d (n = 73) were excluded. Thus, the final analyzed cohort consisted of 911 patients. A detailed medical history was recorded for each patient, and the basal clinical characteristics at study entry together with information on follow up were carefully gathered by cardiologists.
The vast majority of patients were on acenocoumarol (93%; and the remaining patients were on warfarin).
The study was approved by the Clinical Research Ethics Committee of our hospital.
For each patient, Serum creatinine was measured by the modified kinetic Jaffe method in a single clinical laboratory in our institution. All creatinine measurements were performed with an isotope dilution mass spectroscopy (IDMS)-traceable enzymatic assay that has previously been shown to provide very reliable eGFR results compared with the measured GFR[13]; these measurements were analyzed automatically using the ADVIA 2400 Chemistry System (Siemens Diagnostics, Tarrytown, NY, United States).
We calculated the eGFR using the IDMS-traceable version of the MDRD-4 equation[8]: 175 × [standardized serum creatinine (mg/dL)]-1.154× age-0.203× (0.742 if female) × (1.212 if black).
The new CKD-EPI equation was also used[9]: 141 × (minimum of standardized serum creatinine (mg/dL)/κ or 1)α× [maximum of standardized serum creatinine (mg/dL)/κ or 1]-1.209× 0.993age× (1.018 if female) × (1.159 if black). Where κ is 0.7 for females and 0.9 for males and α is -0.329 for females and -0.411 for males.
We categorized the eGFR obtained from each formula into three categories: ≥ 60 mL/min per 1.73 m2 (normal or mild renal dysfunction), 30-59 mL/min per 1.73 m2 (moderate renal dysfunction) and < 30 mL/min per 1.73 m2 (severe renal dysfunction). No patients were on renal replacement therapy.
Patients were followed up to 1-year after the enrolment. The primary endpoint of the present study was a composite endpoint of major bleeding, TE complications, or death; whichever comes first. The secondary endpoint was each individual component of the composite endpoint.
Data on major bleeding, and TE complications were gathered from the cardiology clinic visits and records, and through hospital files as well as through primary care centers reports.
We used the 2005 International Society on Thrombosis and Haemostasis (ISTH) criteria to define major bleeding[14]. Thus, a major bleeding event was adjudicated if one of the following criteria was met: fatal bleeding and/or symptomatic bleeding in a critical area or organ (e.g., such as intracranial, intraspinal, intraocular, retroperitoneal, atraumatic intraarticular, pericardial, or intramuscular with compartment syndrome); and/or bleeding causing drop of hemoglobin of ≥ 2 g/dL, or leading to transfusion of ≥ 2 units of whole blood or packed red blood cells.
A TE complication was defined as the occurrence of ischemic stroke, transient ischemic attack, or peripheral embolism (including fatal TE events). Diagnosis of stroke or transient ischemic attack required an acute neurological deficit lasting for more or less than 24 h, respectively, which could not be explained by other causes and with at least 1 image test (computed tomography or magnetic resonance) compatible with the diagnosis, as well as confirmation from a neurologist. A diagnosis of peripheral embolism was defined as non-central nervous system embolism leading to an abrupt vascular insufficiency associated with clinical or radiographic evidence of arterial occlusion in absence of another mechanism such as atherosclerosis, instrumentation, or trauma.
Qualitative data were expressed as frequencies and percentages while quantitative data were summarized as mean and standard deviation. Comparison between qualitative data was performed using the χ2 test or the Fisher exact test, as appropriate. The t-Student test was used to compare quantitative data.
The relationship between the primary endpoint and eGFR according to both formulas was evaluated using separate Cox proportional hazard regression models. The candidate variables to construct the multivariate Cox models were those variables presented P < 0.10 in the univariate Cox analysis, or those co-variables of recognized prognostic value in the medical literature. Once the initial Cox models had been established, they were simplified by stepdown elimination. Thus, the final Cox models to determine the adjusted effect of eGFR on the composite endpoint, included: age, sex, previous stroke, basal hemoglobin, chronic obstructive pulmonary disease, diabetes mellitus, congestive heart failure or left ventricular ejection fraction ≤ 40%, history of malignant disease and coronary artery disease.
The association between eGFR formulas and the individual endpoints of either major bleeding or TE events was determined using competing-risks regression based on Fine and Gray’s proportional subhazards models. The Fine and Gray models were adjusted for HAS-BLED score[4] in the case of testing the relationship between eGFR formulas and major bleeding, and for CHA2DS2-VASc score[15] in the case of testing the relationship between eGFR formulas and TE events. For all-cause mortality, we used a Cox regression model. Once the initial Cox model for predicting all-cause mortality had been established, it was simplified by stepdown elimination; and finally included the following covariables: age, sex, diabetes mellitus, and history of malignant disease, previous stroke, basal hemoglobin, and congestive heart failure or ejection fraction ≤ 40%.
The discriminatory capacity of each formula at predicting either the primary or secondary endpoint was determined by calculating the c- statistic. We used the Delong test to compare the c-statistic values from each formula.
The calibration of the model was assessed with the Grønnesby and Borgan goodness-of-fit test. This test determines how closely the predicted event rate approximates the observed event rate over a range of scores. A significant value of P indicates a lack of fit.
The estimated coefficients were expressed as the hazard ratio (HR) with the respective 95%CI. A 2-sided P < 0.05 was considered statistically significant for all analyses.
Finally, we also assessed the incremental prognostic value of using one equation over another; using the concept of net reclassification improvement (NRI) as described by Pencina et al[16], to determine whether the reclassification of patients by one of the formulas regarding to each other, would result in a more accurate risk estimation.
All the analyses were performed with STATA 13, and by using the MedCalc statistical software version 12.2.1.
The study was reviewed by our expert Biostatistic Emad Abu-Assi, MD, PhD.
Mean age was of 73 ± 11 years, male patients constitute 66.4% of the studied population. Baseline characteristics are summarized in Table 1.
| Age (yr) | 73 ± 11 |
| Men | 605 (66.4) |
| Systolic blood pressure at study entry | 139 ± 28 |
| Hypertension | 678 (74.4) |
| Current smoking | 77 (8.5) |
| Diabetes mellitus | 220 (24.1) |
| Heart failure | 343 (37.7) |
| Peripheral arterial disease | 92 (10.1) |
| History of stroke or TIA | 103 (11.3) |
| Coronary artery disease | 127 (13.9) |
| COPD | 183 (20.1) |
| CHA2DS2-VASc: | |
| = 0 | 62 (6.8) |
| ≥ 1 | 849 (93.2) |
| ≥ 2 | 772 (84.7) |
| History of malignancy | 135 (14.8) |
| HAS-BLED | |
| 0 | 47 (5.2) |
| 1 | 160 (17.6) |
| 2 | 365 (40.1) |
| 3 | 261 (28.6) |
| 4 | 69 (7.6) |
| 5 | 6 (0.7) |
| 6 | 3 (0.3) |
| Alcohol consumption ≥ 40 g/daily | 81 (8.9) |
| Prior bleeding | 115 (12.6) |
| Anemia | 178 (19.5) |
| Abnormal liver function1 | 9 (1) |
| PINRR | 58% ± 18% |
The mean eGFR was higher when computed by the new CKD-EPI than with the re-expressed MDRD-4 (69.8 ± 23, 67.2 ± 19 mL/min per 1.73 m2), respectively (P < 0.0001 for comparison).
There was lower prevalence of eGFR < 60 mL/min per 1.73 m2 with the new CKD-EPI than with the re-expressed MDRD-4 (32.9% vs 34.1%).
During a follow up of 10 ± 3 mo, the composite endpoint occurred in 98 (10.8%) patients: 30 (3.3%) patients developed major bleeding, 18 (2%) had TE events, and 60 (6.6%) patients died.
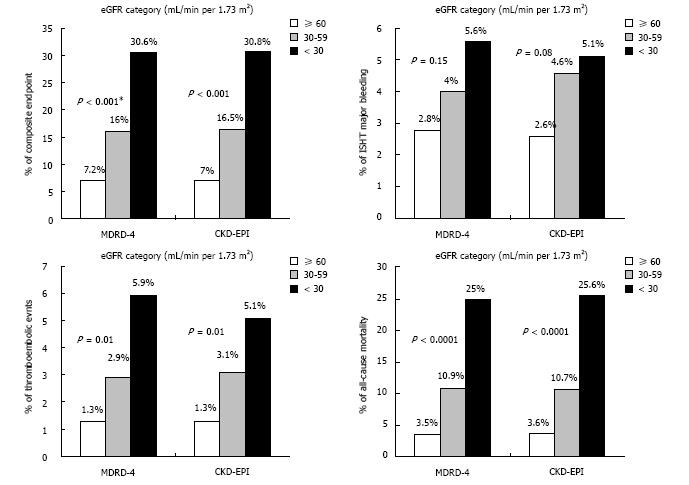
The rate of the composite endpoint increased monotonically from the higher to the lower eGFR categories for both formulas (Figure 1).
Significant association was observed between the eGFR using both formulas as continuous variables and the composite endpoint. The adjusted hazard ratios of eGFR by each formula on the composite endpoint were: 0.98 (95%CI: 0.967-0.988) and 0.97 (95%CI: 0.963-0.987) for the re-expressed MDRD-4 and the new CKD-EPI, respectively (Table 2).
| MDRD-4 | CKD-EPI | |||
| n (%) | Unadjusted HR (95%CI) | Adjusted HR (95%CI) | Unadjusted HR (95%CI) | Adjusted HR (95%CI) |
| Composite endpoint, 98 (10.8) | 0.97 (0.958-0.977) | 0.981 (0.967-0.988) | 0.96 (0.955-0.975) | 0.971 (0.963-0.987) |
| P value | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Major bleeding, 30 (3.3) | 0.97 (0.951-0.985) | 0.982 (0.965-1.000) | 0.97 (0.949-0.984) | 0.982 (0.965-1.000) |
| P value | < 0.0001 | 0.07 | < 0.0001 | 0.07 |
| Thromboembolism, 18 (2) | 0.98 (0.959-1.003) | 0.983 (0.965-1.000) | 0.97 (0.948-0.996) | 0.983 (0.965-1.001) |
| P value | 0.09 | 0.15 | < 0.0001 | 0.22 |
| All-cause mortality, 60 (6.6) | 0.96 (0.948-0.973) | 0.984 (0.965-0.995) | 0.96 (0.947-0.971) | 0.984 (0.965-0.995) |
| P value | < 0.0001 | < 0.0001 | 0.02 | 0.001 |
Similarly, the eGFR as a categorical variable was a strong independent predictor of the occurrence of the composite endpoint regardless of the formula used (Table 3).
| MDRD-4 | CKD-EPI | ||||
| n (%) | Unadjusted HR (95%CI) | Adjusted HR (95%CI) | Unadjusted HR (95%CI) | Adjusted HR (95%CI) | |
| Composite endpoint, 98 (10.8) | ≥ 60 | 1.00 (Reference) | |||
| 30-59 | 2.43 (1.592-3.703) | 1.71 (1.11-2.78) | 2.51 (1.642-3.827) | 1.81 (1.1-2.8) | |
| P < 0.0001 | P = 0.02 | P < 0.0001 | P = 0.02 | ||
| < 30 | 6.99 (3.585-13.649) | 3.3 (1.6-6.9) | 7.4 (3.871-14.125) | 3.6 (1.8-7.4) | |
| P < 0.0001 | P = 0.001 | P < 0.0001 | P < 0.0001 | ||
| Major bleeding, 30 (3.3) | ≥ 60 | 1.00 (Reference) | |||
| 30-59 | 1.53 (0.715-3.260) | 1.012 (0.46-2.25) | 1.87 (0.883-3.948) | 1.22 (0.58-2.75) | |
| P = 0.30 | P = 0.95 | P = 0.1 | P = 0.58 | ||
| < 30 | 3.56 (0.811-15.580) | 1.03 (0.22-4.95) | 3.65 (0.827-16.074) | 1.1 (0.25-5.35) | |
| P = 0.09 | P = 0.93 | P = 0.08 | P = 0.9 | ||
| Thromboembolism, 18 (2) | ≥ 60 | 1.00 (Reference) | |||
| 30-59 | 2.04 (0.734-5.649) | 1.43 (0.49-4.15) | 2.13 (0.767-5.917) | 1.43 (0.50-4.25) | |
| P = 0.17 | P = 0.15 | P = 0.15 | P = 0.50 | ||
| < 30 | 8.01 (1.664-38.555) | 5.1 (1.04-25.4) | 7.84 (1.625-37.825) | 5 (1.0-24.9) | |
| P = 0.009 | P = 0.045 | P = 0.01 | P = 0.04 | ||
| All-cause mortality, 60 (6.6) | ≥ 60 | 1.00 (Reference) | |||
| 30-59 | 3.34 (1.909-5.827) | 2.64 (1.4-2.7) | 3.14 (1.793-5.481) | 2.44 (1.3-4.5) | |
| P < 0.0001 | P = 0.002 | P < 0.0001 | P = 0.005 | ||
| < 30 | 10.64 (4.843-23.359) | 4.9 (2.0-11.9) | 10.89 (5.122-23.166) | 5.2 (2.2-12.3) | |
| P < 0.0001 | P < 0.0001 | P < 0.0001 | P < 0.0001 | ||
The discriminative capacity of both formulas at predicting the composite endpoint, were quite similar, regardless of the eGFR was used as continuous (0.683 vs 0.695 for the re-expressed MDRD-4 and the new CKD-EPI, respectively; P = 0.748) or categorical variable (0.632 vs 0.639 for the re-expressed MDRD-4 and the new CKD-EPI, respectively; P = 0.45) (Table 4).
| MDRD-4 | CKD-EPI | P value | |||
| Composite endpoint | Calibration, χ2 (P value) | 1.7 (0.79) | 3.5 (0.48) | ||
| c-statistic (95%CI) | eGFR continuous | 0.683 (0.629-0.737) | 0.695 (0.643-0.747) | 0.748 | |
| eGFR categorical | 0.632 (0.600-0.664) | 0.639 (0.607-0.670) | 0.452 | ||
| Major bleeding | Calibration, χ2 (P value) | 5.9 (0.20) | 5.4 (0.25) | ||
| c-statistic (95%CI) | eGFR continuous | 0.666 (0.581-0.751) | 0.677 (0.596-0.759) | 0.8548 | |
| eGFR categorical | 0.550 (0.443-0.658) | 0.571 (0.465-0.679) | 0.7872 | ||
| Thromboembolism | Calibration, χ2 (P value) | 0.13 (0.99) | 1.9 (0.76) | ||
| c-statistic (95%CI) | eGFR continuous | 0.616 (0.584-0.648) | 0.644 (0.612-0.675) | 0.2736 | |
| eGFR categorical | 0.617 (0.585-0.649) | 0.622 (0.590-0.654) | 0.7582 | ||
| All-cause mortality | Calibration, χ2 (P value) | 0.83 (0.94) | 1.5 (0.82) | ||
| c-statistic (95%CI) | eGFR continuous | 0.715 (0.684-0.744) | 0.722 (0.691-0.750) | 0.5227 | |
| eGFR categorical | 0.679 (0.647-0. 709) | 0.678 (0.646-0.708) | 0.911 | ||
There was a step increase in the major bleeding rate, as the eGFR declines, independently of the formula used to calculate the eGFR (Figure 1).
After adjusting for HAS-BLED bleeding risk score, the re-expressed MDRD-4 eGFR as well as the new CKD-EPI eGFR, as continuous variables, showed a tendency to predict major bleeding: HR for both formulas = 0.98 (95%CI: 0.965-1.000; P = 0.07) (Table 2).
No significant association was observed between categorical eGFR from both formulas and major bleeding, either in the unadjusted or by using the adjusted competing-risk models (Table 3).
At predicting major bleeding, the discriminative ability of the continuous re-expressed MDRD-4 eGFR was modest: 0.666; quite similar to that obtained from using the continuous new CKD-EPI eGFR: c-statistic = 0.677 (P = 0.85).
When eGFR was considered as a categorical variable, the discriminative capacity of each formula at predicting major bleeding was of 0.550 and of 0.571 for the re-expressed MDRD-4 and the new CKD-EPI, respectively (P = 0.79) (Table 4).
As shown in Figure 1, the distribution of the TE event rate in the different eGFR categories, demonstrated a consistent gradient of risk, regardless of the formula used.
After adjusting for the CHA2DS2-VASc risk score, no significant association was observed between eGFR as a continuous variable and TE events: HR = 0.98 (95%CI: 0.965-1.000) and 0.98 (95%CI: 0.965-1.001), for the re-expressed MDRD-4 and the new CKD-EPI, respectively (Table 2).
When eGFR was considered as a categorical variable, only significant association existed between eGFR < 30 mL/min per 1.73 m2 and the TE complications, after controlling for CHA2DS2-VASc score: HR = 5.1 (95%CI: 1.04-25.4) for the re-expressed MDRD-4, and HR = 5.0 (95%CI: 1.0-24.9) for the new CKD-EPI (Table 3).
The discriminative power of GFR estimates determined by both formulas was also modest. For continuous eGFR, the c-statistic values were of 0.616 and 0.644 for the re-expressed MDRD-4 and the new CKD-EPI, respectively, (P = 0.27), and for categorical eGFR, the c-statistic values were 0.617 and 0.622 when using the re-expressed MDRD-4 and the new CKD-EPI, respectively, (P = 0.76) (Table 4).
The rate of all-cause mortality increased progressively from the higher to the lower eGFR values for both formulas (Figure 1).
Continuous eGFR calculated by either the reexpressed MDRD-4 or the new CKD-EPI was an independent predictor of all-cause mortality; adjusted HR = 0.98; (P < 0.01) (Table 2).
A strong association was also found between categorical eGFR and all-cause mortality after adjusting for several confounders (Table 3).
Good discrimination was obtained from continuous eGFR: c-statistic = 0.715 for the re-expressed MDRD-4 and 0.722 for the new CKD-EPI (P = 0.52).
The discriminative power of eGFR as a categorical variable in terms of c-statistic was: 0.679 and 0.678 when using the re-expressed MDRD-4 and the new CKD-EPI, respectively, (P = 0.91) (Table 4).
Estimated GFR from both formulas demonstrated good calibration for the major cardiovascular events with P value > 0.1 (Table 4).
The NRI analysis did not significantly favor the new CKD-EPI over the re-expressed MDRD-4 whether for predicting the composite endpoint, major bleeding and all-cause mortality (NRI = 2.13%, 4.35%, and 0.9%, with P = 0.27, 0.19, and 0.7, respectively).
However, at predicting the TE event, the NRI favored the new CKD-EPI formula with NRI of 1% (95%CI: -0.08 to +2.0, P = 0.07) indicating a strong tendency to reclassify better the patients according to their risk of developing TE event, compared with the re-expressed MDRD-4.
In this real world cohort of patients with NVAF on VKAs, the new CKD-EPI formula classified lower percentage of patients as having eGFR < 60 mL/min per 1.73 m2 than the re-expressed MDRD-4 equation did. This means that the use of the new CKD-EPI formula results in lower prevalence of renal dysfunction. We also found that renal dysfunction assessed either by the re-expressed MDRD-4 or the new CKD-EPI was strongly associated with the composite endpoint of major bleeding, TE event and all-cause mortality, and with all-cause mortality, as well.
Patients with NVAF are often elderly with multiple comorbidities which require pharmacotherapy of growing complexity, and this makes the reliable estimation of renal function to be undeniably a critical issue. Moreover, the availability of the new oral anticoagulants have renewed the great interest toward the accurate evaluation of renal function in patients with NVAF[17,18].
Up to our knowledge, this is the first study comparing the prognostic performance of the re-expressed MDRD-4 and the new CKD-EPI formulas used for estimating GFR in a real world population of patients with NVAF on VKAs who have a full range of eGFR.
In this cohort, the new CKD-EPI formula classified lower percentage of patients as having eGFR < 60 mL/min per 1.73 m2 (32.9% with new CKD-EPI vs 34.1% with re-expressed MDRD-4). This reasonable ability of the new CKD-EPI formula to reduce the rate of patients with renal dysfunction could be highly appreciated by the clinicians in daily clinical practice which usually needs close attention to the status of renal function to reach the optimal management, and more safe use of renally excreted medications and nephrotoxic contrast agents, in patients with NVAF. Our finding is consistent with that found in the derivation cohort of the new CKD-EPI[9] and to the findings obtained from multiple studies in different clinical settings[12,19-21].
In our analysis, renal dysfunction determined by GFR estimates using both formulas was a significant predictor of the composite endpoint and all-cause mortality. Similar findings have been shown in previous study used the MDRD-4[22], but until now, no study has compared the prognostic usefulness of these formulas in a real world patients with NVAF. In this study, we did not find any significant difference in the prognostic impact between the new CKD-EPI and the re-expressed MDRD-4 at predicting major adverse cardiovascular outcomes.
In our analysis, we found that both formulas with the eGFR as a continuous variable and after controlling for HAS-BLED risk score[4], showed a tendency to predict major bleeding. Previous association between renal dysfunction and major bleeding were found in AF studies[22,23]. However, the prior tendency was lost when the eGFR using both formulas was tested as categorical variables; this may be explained by the small number of events (30 events, 3.3%) that could limit the detection of significant relationship from the data.
TE prevention remains the primary cornerstone in the management of patients with NVAF. In dealing with this great aim, there are conflicting data about the ability of renal dysfunction to predict this major catastrophe. Several studies demonstrated significant association between reduced eGFR and TE event[22-24], conversely, in other studies, decreased eGFR did not show significant relationship with TE event[25,26]. These differences could be explained by the differences in the formula used to estimate GFR, sample size, patients characteristics (i.e., from a real world or clinical trial population), and/or the disparities in duration of follow up between the studies. Therefore, there is a strong need for further evaluation of that uncertainty in a real world population. Regarding this important issue, in our real world cohort of patients with NVAF, and after adjusting for the CHA2DS2-VASc risk score[15] there was a significant association between eGFR as categorical variable and TE event only when the eGFR was < 30 mL/min per 1.73 m2 (i.e., severe renal dysfunction category) with similar prognostic impact of both the re-expressed MDRD-4 and the new CKD-EPI. Furthermore, the NRI analysis showed a tendency of the new CKD-EPI to reclassify better the patients according to their risk of developing TE event, compared with the re-expressed MDRD-4.
It should be kept in mind that the eGFR formulas were designed to most accurately estimate renal function and not to predict major adverse outcomes. Indeed, the relative performance of the two different GFR estimating equations in our study can be explained by their respective compositions (i.e., the difference of mathematical modeling and how specific variables are coded and weighted by each equation). Also, the relative variance in performance between both formulas can be explained by the differences in their respective derivation populations. The MDRD-4 formula was originally developed in patients with established renal dysfunction[6]; for this, the re-expressed MDRD-4 formula may be less applicable to patients from the real world with full range of GFR. In contrast, the new CKD-EPI equation could be more precise in our community-based cohort of patients with NVAF, as the new CKD-EPI was developed in population with and without renal dysfunction[9].
Although, many laboratories are preparing their installation to use the new CKD-EPI equation instead of the re-expressed MDRD-4 formula according to the current guideline[27] and a consensus document[28], however, old habits die hard. Our assessment of the prognostic performance of both formulas in the particular clinical context of AF might be of great importance as it could help convince the clinicians and mitigate the doubts and obstacles regarding the adoption of the new CKD-EPI.
Really, patients with NVAF and renal dysfunction continue to represent a complex management problem in relation to decision making for thromboprophylaxis. With respect to the overall concept, the data obtained from our analysis, state that the new CKD-EPI formula reduced the prevalence of patients with renal dysfunction (i.e., eGFR < 60 mL/min per 1.73 m2), and at the same time continued to have prognostic impact similar to that of the re-expressed MDRD-4 equation at predicting the major adverse events. Taken together, our notable results from a real world cohort encourage the use of the new CKD-EPI equation to assess renal function in patients with NVAF and reinforce the current recommendation[9,27,28] for the use of the new CKD-EPI formula in all clinical situations.
It is clear that our study presents an analysis of a modest sized cohort of patients with NVAF on VKAs from the real world, and the prevalence of patients with eGFR < 60 ml/min/1.73 m2 was just reduced by 1.2% when using the new CKD-EPI formula. However, our cohort might give a good reflection of the general population with millions of patients having NVAF, in whom the percentage of 1.2% would be highly significant.
The main limitation of our study is its retrospective design, but it has interesting strong points as it reflects real world practice by enrolment of consecutive patients with NVAF who have full range of eGFR and were attending our outpatient cardiology clinics with the advantage of careful follow up and data collection by cardiologists.
The sample size might be another limitation of our study that could limit the likelihood of detecting small effects or significant relationships from the data. Important to mention here that we did not have the direct measured GFR, so we cannot determine the extent to which the two formulas reflect the GFR as determined by the gold standard method. However, eGFR is the practical way to estimate renal function which has been used in several patient populations. The fact that we have only one serum creatinine measure for every patient could limit the verification of the acute vs chronic nature of the renal dysfunction in some patients, but this limitation was present in several related studies[23-25]. The lack of cystatin C data might be considered a limitation of our study. However, it should be taken into account that all the creatinine measurements in our study cohort were performed with the IDMS-traceable enzymatic assay method, which has been shown to provide very reliable eGFR results[13] and is considered the standard method to assess renal function[29].
Finally, all of the enrolled patients in our cohort have Caucasian race, so the applicability of our findings in other populations with different races should be addressed in other studies.
The new CKD-EPI reduced the prevalence of patients with renal dysfunction, in a real world cohort of patients with NVAF on VKAs. Renal dysfunction reflected by GFR estimates from the re-expressed MDRD-4 or the new CKD-EPI was an independent predictor of the composite endpoint and all-cause mortality. Both formulas had similar prognostic impacts regarding the prediction of composite endpoint, major bleeding, TE events and all-cause mortality. Our analysis indicates that the more widespread adoption of the new CKD-EPI instead of the re-expressed MDRD-4 may improve the management of patients with NVAF.
Renal dysfunction is a frequent comorbidity seen in patients with atrial fibrillation. Moreover, renal dysfunction is a strong predictor of thromboembolic event and also of bleeding event (when the patients are anticoagulated). This reflects the need for more accurate estimate of renal function to guarantee the optimal management of patients with atrial fibrillation. The standard way to assess renal function is the glomerular filtration rate. Among the available equations to estimate the glomerular filtration rate are: the re-expressed Modification of Diet in Renal Disease equation which is still the commonly used equation by many laboratories all over the world and the new Chronic Kidney Disease Epidemiology Collaboration equation which has been recently proposed to be used instead of previous equation in daily practice as the new equation has an assumed ability to reduce the prevalence of patients with renal dysfunction and better reclassification of patients. There is limited information about the performance of both equations in patients with atrial fibrillation.
The authors think that the new Chronic Kidney Disease Epidemiology Collaboration equation to estimate glomerular filtration rate must have a wide diffusion as an alternative to the re-expressed Modification of Diet in Renal Disease equation. In this paper the authors provide support to the hypothesis, reporting the superiority of the new Chronic Kidney Disease Epidemiology Collaboration equation over the re-expressed Modification of Diet in Renal Disease equation in the clinical context of patients with atrial fibrillation on anticoagulation.
The results derived from our analysis, state that the new Chronic Kidney Disease Epidemiology Collaboration equation reduced the prevalence of patients with renal dysfunction (i.e., estimated glomerular filtration rate < 60 ml/min per 1.73 m2), and at the same time continued to have the prognostic impact similar to the re-expressed Modification of Diet in Renal Disease equation at predicting the major adverse events. Although there are still some concerns about the performance of the new equation in subgroups of elderly and obese patients, the study from a real world cohort encourages the cardiologists to use of the new Chronic Kidney Disease Epidemiology Collaboration equation to assess renal function in patients with atrial fibrillation and increase the confidence to use it in all clinical situations.
The millions of patients with atrial fibrillation will get benefit and better management if there is wide spread adoption of the new Chronic Kidney Disease Epidemiology Collaboration equation instead of the re-expressed Modification of Diet in Renal Disease equation, giving the ability of the new equation to correctly reclassify patients in comparison with the re-expressed equation.
The Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) was published in May 2009 as a reliable tool to estimate glomerular filtration rate. It was developed in an effort to create an equation more accurate than the re-expressed Modification of Diet in Renal Disease equation. Researchers pooled data from multiple studies to develop and validate this new equation. They used 10 studies that included 8254 participants, randomly using 2/3 of the data sets for development and the other 1/3 for internal validation. Sixteen additional studies, which included 3896 participants, were used for external validation. The CKD-EPI equation performed better than the Modification of Diet in Renal Disease equation, as the prevalence of chronic kidney disease was 11.5% vs 13.1% according to the National Health and Nutrition Examination Survey data in the United States of America.
First of all I would like to congratulate the authors with their achievement. In this retrospective study including relatively limited sample size of Caucasian subjects, the findings encourage the use and application of the new CKD-EPI equation for assessment not only of renal function in patients with non-valvular atrial fibrillation but also in all clinical situations. For the first time, Abumuaileq RRY et al evaluated the re- expressed MDRD-4 and the new CKD-EPI formulas at predicting the occurrence of major adverse outcomes in a real world cohort of patients with non-valvular atrial fibrillation on anticoagulation. The study was well conducted and clinically relevant.
P- Reviewer: Liu T, Said SAM S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J. 2010;159:1102-1107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 2. | Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, Warnock DG, Muntner P. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 591] [Cited by in F6Publishing: 611] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 4. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2971] [Cited by in F6Publishing: 3113] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 5. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-S266. [PubMed] [Cited in This Article: ] |
| 6. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [PubMed] [Cited in This Article: ] |
| 7. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [PubMed] [Cited in This Article: ] |
| 8. | Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1278] [Cited by in F6Publishing: 1370] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 9. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15626] [Cited by in F6Publishing: 17702] [Article Influence: 1180.1] [Reference Citation Analysis (0)] |
| 10. | Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:648-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Matsushita K, Tonelli M, Lloyd A, Levey AS, Coresh J, Hemmelgarn BR. Clinical risk implications of the CKD Epidemiology Collaboration (CKD-EPI) equation compared with the Modification of Diet in Renal Disease (MDRD) Study equation for estimated GFR. Am J Kidney Dis. 2012;60:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Choi JS, Kim CS, Bae EH, Ma SK, Ahn YK, Jeong MH, Kim YJ, Cho MC, Kim CJ, Kim SW. Predicting outcomes after myocardial infarction by using the Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease study equation: results from the Korea Acute Myocardial Infarction Registry. Nephrol Dial Transplant. 2012;27:3868-3874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, Poggio ED, Schmid CH, Steffes MW, Zhang YL. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non surgical patients. J Thromb Haemost. 2005;3:692-694. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3234] [Cited by in F6Publishing: 3416] [Article Influence: 179.8] [Reference Citation Analysis (0)] |
| 15. | Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4381] [Cited by in F6Publishing: 4794] [Article Influence: 319.6] [Reference Citation Analysis (0)] |
| 16. | Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157-172; discussion 207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4325] [Cited by in F6Publishing: 4789] [Article Influence: 299.3] [Reference Citation Analysis (0)] |
| 17. | Hohnloser SH, Connolly SJ. Atrial fibrillation, moderate chronic kidney disease, and stroke prevention: new anticoagulants, new hope. Eur Heart J. 2011;32:2347-2349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kooiman J, van de Peppel WR, van der Meer FJ, Huisman MV. Incidence of chronic kidney disease in patients with atrial fibrillation and its relevance for prescribing new oral antithrombotic drugs. J Thromb Haemost. 2011;9:1652-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Stevens LA, Li S, Kurella Tamura M, Chen SC, Vassalotti JA, Norris KC, Whaley-Connell AT, Bakris GL, McCullough PA. Comparison of the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study equations: risk factors for and complications of CKD and mortality in the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2011;57:S9-S16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | White SL, Polkinghorne KR, Atkins RC, Chadban SJ. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis. 2010;55:660-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 21. | van den Brand JA, van Boekel GA, Willems HL, Kiemeney LA, den Heijer M, Wetzels JF. Introduction of the CKD-EPI equation to estimate glomerular filtration rate in a Caucasian population. Nephrol Dial Transplant. 2011;26:3176-3181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Roldán V, Marín F, Fernández H, Manzano-Fernández S, Gallego P, Valdés M, Vicente V, Lip GY. Renal impairment in a “real-life” cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding). Am J Cardiol. 2013;111:1159-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Apostolakis S, Guo Y, Lane DA, Buller H, Lip GY. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013;34:3572-3579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 25. | Banerjee A, Fauchier L, Vourc’h P, Andres CR, Taillandier S, Halimi JM, Lip GY. Renal impairment and ischemic stroke risk assessment in patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. J Am Coll Cardiol. 2013;61:2079-2087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Bos MJ, Koudstaal PJ, Hofman A, Breteler MM. Decreased glomerular filtration rate is a risk factor for hemorrhagic but not for ischemic stroke: the Rotterdam Study. Stroke. 2007;38:3127-3132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:S6-S308. [Cited in This Article: ] |
| 28. | Martínez-Castelao A, Górriz JL, Segura-de la Morena J, Cebollada J, Escalada J, Esmatjes E, Fácila L, Gamarra J, Gràcia S, Hernánd-Moreno J. Consensus document for the detection and management of chronic kidney disease. Nefrologia. 2014;34:243-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 13] [Reference Citation Analysis (0)] |
| 29. | Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 817] [Article Influence: 43.0] [Reference Citation Analysis (0)] |