Published online Dec 8, 2016. doi: 10.4254/wjh.v8.i34.1511
Peer-review started: August 13, 2016
First decision: September 13, 2016
Revised: September 26, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 8, 2016
To investigate potential predictors for treatment response to nucleos(t)ide analogues (NAs) in hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB) patients.
Seventy-six HBeAg-positive CHB patients received 96-wk NAs optimized therapy (lamivudine and adefovir dipivoxil) were studied retrospectively. Serum hepatitis B surface antigen, HBeAg, hepatitis B core antibody, hepatitis B virus (HBV) DNA and alanine aminotransferase levels were quantitatively measured before and during the treatment at 12 and 24 wk. Stepwise logistic regression analyses were performed to identify predictors for treatment response, and areas under the receiver operating characteristic curves (AUROC) of the independent predictors were calculated.
Forty-three CHB patients (56.6%) achieved virological response (VR: HBV DNA ≤ 300 copies/mL) and 15 patients (19.7%) developed HBeAg seroconversion (SC) after the 96-wk NAs treatment. The HBeAg level (OR = 0.45, P = 0.003) as well as its declined value (OR = 2.03, P = 0.024) at 24-wk independently predicted VR, with the AUROC of 0.788 and 0.736, respectively. The combination of HBeAg titer < 1.3 lg PEIU/mL and its decreased value > 1.6 lg PEIU/mL at 24-wk predicted VR with a sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) of 85%, 100%, 100% and 83%, respectively, and the AUROC increased to 0.923. The HBeAg level (OR = 0.37, P = 0.013) as well as its declined value (OR = 2.02, P = 0.012) at 24-wk also independently predicted HBeAg SC, with the AUROC of 0.828 and 0.814, respectively. The HBeAg titer < -0.5 lg PEIU/mL combined with its declined value > 2.2 lg PEIU/mL at 24-wk predicted HBeAg SC with a sensitivity, specificity, PPV, NPV of 88%, 98%, 88% and 98%, respectively, and the AUROC reached 0.928.
The combination of HBeAg level and its declined value at 24-wk may be used as a reference parameter to optimize NAs therapy.
Core tip: Few studies have systematically evaluated quantitative hepatitis B surface antigen, hepatitis B e antigen (HBeAg), hepatitis B core antibody, hepatitis B virus DNA and alanine aminotransferase for predicting treatment response to nucleos(t)ide analogues (NAs) in HBeAg-positive chronic hepatitis B (CHB). In this study, on-treatment HBeAg level as well as its declined value at 24-wk were identified to be the best predictors not only for 96-wk virological response (VR) but also for HBeAg seroconversion (SC). The combination of HBeAg level and its decline at 24-wk strongly predicted 96-wk VR and HBeAg SC with the AUROC of 0.923 and 0.928, respectively. Thus monitoring an early on-treatment HBeAg level and its decline may help to optimize NAs therapy for CHB patients.
- Citation: Gao YH, Meng QH, Zhang ZQ, Zhao P, Shang QH, Yuan Q, Li Y, Deng J, Li T, Liu XE, Zhuang H. On-treatment quantitative hepatitis B e antigen predicted response to nucleos(t)ide analogues in chronic hepatitis B. World J Hepatol 2016; 8(34): 1511-1520
- URL: https://www.wjgnet.com/1948-5182/full/v8/i34/1511.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i34.1511
Hepatitis B virus (HBV) infection is a global public health problem and an estimated 240 million persons are chronically infected worldwide, among which 20%-30% will develop cirrhosis and hepatocellular carcinoma (HCC)-the major complications of chronic hepatitis B (CHB)[1]. Antiviral treatment has been proved to be an effective and potent way to reverse the process of liver fibrosis or cirrhosis and decrease the incidence rate of liver complications[2]. However, due to the persistence of HBV covalently closed circular DNA (cccDNA) in the nucleus of infected hepatocytes, HBV cannot be completely eradicated by current antiviral drugs, and a long-term treatment are necessary for most patients. It is now clear that sustained viral suppression and hepatitis B e antigen (HBeAg) seroconversion (SC) are two important markers of treatment response for CHB patients receiving antiviral therapy, which is usually associated with a good long-term outcome[3]. Generally, after a 1-year course of the current available nucleos(t)ide analogues (NAs) or peginterferon (Peg-IFN) therapy, 7%-76% of patients achieved undetectable serum HBV DNA and 16%-32% developed HBeAg SC for patients with HBeAg-positive CHB[3]. Therefore, it is crucial to identify pre-treatment and early on-treatment biomarkers that can effectively predict long-term treatment response and use these biomarkers to choose appropriate antiviral drugs and treatment regimens to optimize therapy and improve efficacy.
Serum HBV DNA is the most widely used virological marker in the management of CHB patients[2,3]. A study from Zeuzem et al[4] reported that both baseline ALT ≥ 2 × upper limit of normal (ULN) (OR = 2.47, P = 0.0012) and non-detectable serum HBV DNA at treatment week 24 (OR = 2.61, P < 0.001) were associated with HBeAg SC after 2-year telbivudine (LdT) treatment, and among patients with non-detectable serum HBV DNA at 24-wk as well as favorable pretreatment characteristics [alanine aminotransferase (ALT) ≥ 2 × ULN and HBV DNA < 9 lg copies/mL], 52% obtained HBeAg SC at 2-year of therapy[4]. However, the detection of serum HBV DNA is costly and may not always objectively serve as a reliable indicator of sustained response to antiviral therapy[5]. Unlike HBV DNA, serum hepatitis B surface antigen (HBsAg), HBeAg and hepatitis B core antibody (anti-HBc) are classical serological markers for HBV infection and are used in clinical diagnosis routinely. The level of HBsAg was identified as an outcome predictor for Peg-IFN therapy among HBeAg-positive CHB patients[6]; however, its predictive value in NAs treatment was inconsistent based on the reported data[7,8]. Serum HBeAg level was proposed to be a better outcome predictor for NAs treatment according to recent studies[8-12]. However, most of studies applied the semi-quantitative measurement of HBeAg, and some had a limited sample size or a short period of follow-up. Thus the predictive value of the quantitative HBeAg level needs to be further evaluated. In addition, benefiting from a newly developed double-sandwich anti-HBc immunoassay, anti-HBc quantification was identified as a novel biomarker for predicting treatment response[13]. Nevertheless, very few studies have systematically evaluated the predictive power of these biomarkers for NAs treatment response.
In the current study, HBeAg-positive CHB patients received 96-wk NAs optimized therapy [lamivudine (LAM) and adefovir dipivoxil (ADV)] were retrospectively investigated. Serum HBsAg, HBeAg, anti-HBc, HBV DNA and ALT were quantitatively tested, and the baseline as well as early on-treatment levels of these parameters were analyzed using logistic regression model to assess their functions in predicting 96-wk virological response (VR) and HBeAg SC.
We retrospectively analyzed a cohort of HBeAg-positive CHB patients who underwent the 96-wk LAM and ADV optimized therapy between 2011 and 2014 in China. The treatment was continued for CHB patients after week 96, and the data were not available from the patients after 96 wk. The inclusion criteria of patients enrolled for antiviral therapy were briefly summarized as follows: 18-65 years old, HBsAg positive for at least 6 mo, HBeAg positive and hepatitis B e antibody (anti-HBe) negative, 105 copies/mL ≤ HBV DNA ≤ 109 copies/mL, ALT ≥ 2 × ULN, and no history of antiviral therapy with NAs or interferon within previous six months. The patients were treated with LAM 100 mg/d, and ADV (10 mg/d) was added on when serum HBV DNA > 300 copies/mL at week 24 or a virological breakthrough (> 1 lg increase of serum HBV DNA from nadir or re-detectable after achieving an undetected level) occurred during the 96-wk treatment. Laboratory measurements were done every 12 wk before week 24, and every 24 wk from week 24 to week 96. The main endpoints were VR (defined as HBV DNA ≤ 300 copies/mL) and HBeAg SC at 96-wk. A total of 76 patients completed the 96-wk follow-up and finally included in the analyses. The study was approved by the Institutional Review Board of Peking University Health Science Center and conducted in accordance with the ethical standards of the Helsinki Declaration. The informed consents were obtained from recruited patients.
HBV DNA was quantified by Roche COBAS TaqMan HBV test (Roche Diagnostics, Mannheim, Germany) with a linear range of 20-108 IU/mL (1 IU/mL = 5.82 copies/mL). Serological HBV markers (HBsAg, anti-HBs, HBeAg, anti-HBe) were measured by Chemiluminescent Microparticle ImmunoAssay using ARCHITECT i2000SR analyzer (Abbott Diagnostics, North Chicago, IL, United States). HBeAg level was quantified by World Health Organization (WHO) HBeAg reference standard (Paul-Ehrlich-Institute, Germany) also using ARCHITECT i2000SR analyzer[14]. Anti-HBc quantification was conducted by using a newly developed double-sandwich immunoassay (Wantai, Beijing, China) validated by WHO anti-HBc standards[15]. Biochemical tests (ALT, AST) were detected by the department of laboratory in four hospitals.
Categorical variables were compared using χ2 or Fisher’s exact tests. Continuous variables were compared using the Student’s t test or Mann-Whitney test. Stepwise logistic regression analysis was performed to identify independent predictors for VR and HBeAg SC. The predictive value of the independent predictor was further evaluated using areas under the receiver operating characteristic curve (AUROC). The best cut-off value was determined in the condition of the highest Youden index (the sum of sensitivity and specificity minus 1). And the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated at the specified cut-off value. All analysis was done using SPSS 19.0 (SPSS, Chicago, IL, United States). A P value < 0.05 was considered as statistically significant.
After the 96-wk NAs treatment, 56.6% (43/76) CHB patients achieved VR and 19.7% (15/76) patients developed HBeAg SC. Only 1 patient eliminated HBsAg. The baseline parameters such as age, HBsAg, HBeAg, anti-HBc, HBV DNA and ALT were comparable between patients achieved HBeAg SC and those did not. Compared to patients without VR, those obtained VR had significantly higher baseline ALT level (247.95 ± 150.58 U/L vs 169.13 ± 156.56 U/L, P = 0.029), while HBsAg, HBeAg, anti-HBc, HBV DNA levels were not significantly different between two groups (Table 1).
| Parameters | Overall | VR (+) | VR (-) | P value | SC (+) | SC (-) | P value |
| n (%) | 76 | 43 (56.6) | 33 (43.4) | - | 15 (19.7) | 61 (80.3) | - |
| Gender, female/male | 20/56 | 15/28 | 5/28 | 0.053 | 5/10 | 15/46 | 0.491 |
| Age, yr | 32.63 ± 9.69 | 32.3 ± 9.83 | 33.06 ± 9.63 | 0.738 | 31.87 ± 11.6 | 32.82 ± 9.26 | 0.735 |
| HBsAg, lg IU/mL | 3.95 ± 0.83 | 3.96 ± 0.68 | 3.94 ± 1.00 | 0.881 | 3.93 ± 0.60 | 3.96 ± 0.88 | 0.385 |
| HBeAg, lg PEIU/mL | 2.24 ± 1.31 | 2.18 ± 1.38 | 2.32 ± 1.22 | 0.679 | 2.36 ± 1.41 | 2.21 ± 1.29 | 0.527 |
| anti-HBc, lg IU/mL | 4.77 ± 0.46 | 4.82 ± 0.43 | 4.71 ± 0.50 | 0.311 | 4.85 ± 0.44 | 4.75 ± 0.47 | 0.464 |
| ALT, U/L | 213.73 ± 157.17 | 247.95 ± 150.58 | 169.13 ± 156.56 | 0.029 | 216.49 ± 153.18 | 213.05 ± 159.38 | 0.94 |
| ALT strata, ≥/< 5ULN | 31/45 | 25/18 | 6/27 | < 0.001 | 9/6 | 22/39 | 0.091 |
| HBV DNA, lg copies/mL | 8.16 ± 1.34 | 8.06 ± 1.45 | 8.3 ± 1.19 | 0.608 | 8.55 ± 0.91 | 8.07 ± 1.41 | 0.324 |
| Genotype, C/non-C | 53/23 | 31/12 | 22/11 | 0.61 | 10/5 | 43/18 | 0.773 |
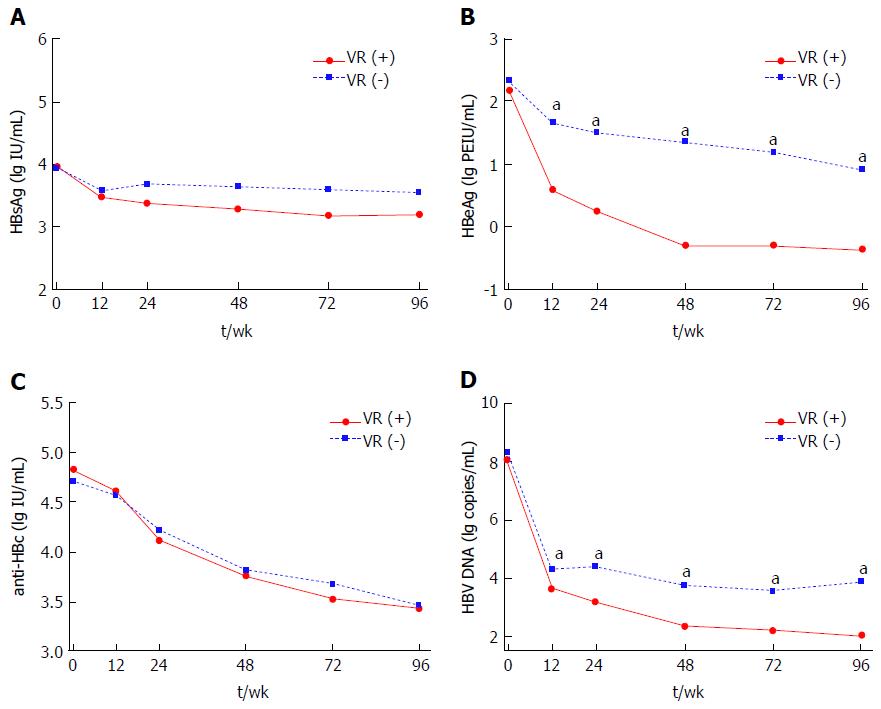
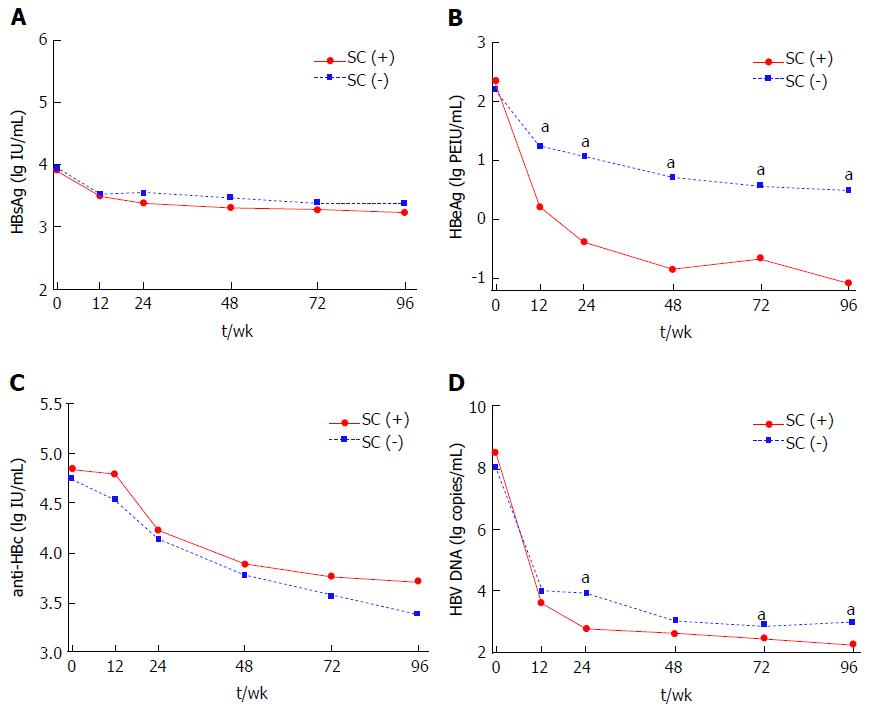
Serum HBsAg, HBeAg, anti-HBc and HBV DNA levels were all significantly decreased from baseline to week 96 of therapy (HBsAg, 3.95 ± 0.83 lg IU/mL to 3.36 ± 0.86 lg IU/mL; HBeAg, 2.24 ± 1.31 lg PEIU/mL to 0.20 ± 1.13 lg PEIU/mL; anti-HBc, 4.77 ± 0.46 lg IU/mL to 3.45 ± 0.65 lg IU/mL; HBV DNA, 8.16 ± 1.34 lg copies/mL to 2.85 ± 1.36 lg copies/mL; all P < 0.001). Significant lower HBeAg and HBV DNA levels were found in patients with VR as compared with patients without VR at every follow-up time-point except baseline (P < 0.05), while HBsAg and anti-HBc levels were comparable between two groups (Figure 1). Similarly, HBsAg and anti-HBc levels between patients with and without HBeAg SC were also comparable from baseline to week 96 (Figure 2A and C). However, HBeAg levels were significant lower in patients with HBeAg SC than in patients without HBeAg SC at every follow-up time-point except baseline (P < 0.05) (Figure 2B). And a significant difference in HBV DNA levels between patients with and without HBeAg SC was only observed at week 24, 72 and 96 (P < 0.05), as shown in Figure 2D.
At baseline, ALT and ALT ≥ 5 × ULN were associated with VR according to univariate analysis, and multivariate analysis indicated that sex (OR = 3.76, 95%CI: 1.09-13.01, P = 0.037) and ALT ≥ 5 × ULN (OR = 7.09, 95%CI: 2.32-21.67, P < 0.001) independently predicted VR, respectively. At week 12, univariate analysis revealed that HBeAg, HBeAg decline, ALT decline, and HBV DNA were associated with VR, and multivariate analysis identified that HBeAg (OR = 0.62, 95%CI: 0.40-0.95, P = 0.03) and HBeAg decline (OR = 2.58, 95%CI: 1.25-5.33, P = 0.01) independently predicted VR, respectively. At week 24, HBeAg, HBeAg decline, ALT decline, HBV DNA and HBV DNA decline were associated with VR via univariate analysis, and multivariate analysis found that HBeAg (OR = 0.45, 95%CI: 0.27-0.77, P = 0.003) and HBeAg decline (OR = 2.03, 95%CI: 1.10-3.74, P = 0.024) independently predicted VR, respectively (Table 2).
| Factors | VR | SC | ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Baseline | ||||||||
| Age | 0.99 (0.95-1.04) | 0.734 | - | - | 0.99 (0.93-1.05) | 0.731 | - | - |
| Sex, female/male | 3 (0.96-9.38) | 0.059 | 3.76 (1.09-13.01) | 0.037 | 1.53 (0.45-5.20) | 0.493 | - | - |
| HBsAg (lg IU/mL) | 1.04 (0.60-1.81) | 0.879 | - | - | 0.96 (0.49-1.88) | 0.904 | - | - |
| HBeAg (lg PEIU/mL) | 0.92 (0.65-1.31) | 0.633 | - | - | 1.1 (0.70-1.72) | 0.682 | - | - |
| Anti-HBc (lg IU/mL) | 1.68 (0.62-4.59) | 0.308 | - | - | 1.6 (0.46-5.50) | 0.459 | - | - |
| HBV DNA (lg copies/mL) | 0.87 (0.62-1.24) | 0.451 | - | - | 1.38 (0.82-2.31) | 0.221 | - | - |
| ALT (U/L) | 1.004 (1.000-1.007) | 0.038 | - | - | 1 (0.99-1.01) | 0.939 | - | - |
| ALT strata, ≥/< 5ULN | 6.25 (2.14-18.26) | < 0.001 | 7.09 (2.32-21.67) | < 0.001 | 2.66 (0.84-8.46) | 0.098 | - | - |
| Genotype, C/non-C | 1.29 (0.48-3.46) | 0.61 | - | - | 0.84 (0.25-2.80) | 0.773 | - | - |
| Week 12 | ||||||||
| HBsAg (lg IU/mL) | 0.83 (0.45-1.53) | 0.551 | - | - | 0.95 (0.47-1.92) | 0.882 | - | - |
| HBsAg decline (lg IU/mL) | 1.43 (0.72-2.84) | 0.301 | - | - | 1.04 (0.46-2.35) | 0.919 | - | - |
| HBeAg (lg PEIU/mL) | 0.5 (0.33-0.76) | 0.001 | 0.62 (0.40-0.95) | 0.03 | 0.5 (0.30-0.85) | 0.011 | - | - |
| HBeAg decline (lg PEIU/mL) | 3.04 (1.55-5.98) | 0.001 | 2.58 (1.25-5.33) | 0.01 | 2.47 (1.46-4.16) | < 0.001 | 2.47 (1.46-4.16) | 0.001 |
| Anti-HBc (lg IU/mL) | 1.14 (0.49-2.64) | 0.764 | - | - | 2.56 (0.83-7.93) | 0.102 | - | - |
| Anti-HBc decline (lg IU/mL) | 1.43 (0.45-4.59) | 0.546 | - | - | 0.3 (0.06-1.51) | 0.145 | - | - |
| ALT (U/L) | 0.99 (0.98-1.01) | 0.221 | - | - | 1 (0.98-1.02) | 0.876 | - | - |
| ALT decline (U/L) | 1.004 (1.00-1.01) | 0.03 | - | - | 1 (0.99-1.01) | 0.918 | - | - |
| HBV DNA (lg copies/mL) | 0.57 (0.36-0.90) | 0.016 | - | - | 0.72 (0.42-1.25) | 0.249 | - | - |
| HBV DNA decline (lg copies/mL) | 1.36 (0.90-2.03) | 0.141 | - | - | 2.11 (1.16-3.84) | 0.015 | - | - |
| Week 24 | ||||||||
| HBsAg (lg IU/mL) | 0.61 (0.31-1.21) | 0.155 | - | - | 0.84 (0.48-1.46) | 0.535 | - | - |
| HBsAg decline (lg IU/mL) | 1.79 (0.94-3.42) | 0.076 | - | - | 1.21 (0.62-2.37) | 0.571 | - | - |
| HBeAg (lg PEIU/mL) | 0.39 (0.24-0.64) | < 0.001 | 0.45 (0.27-0.77) | 0.003 | 0.28 (0.14-0.58) | < 0.001 | 0.37 (0.17-0.81) | 0.013 |
| HBeAg decline (lg PEIU/mL) | 2.37 (1.41-3.99) | 0.001 | 2.03 (1.10-3.74) | 0.024 | 2.8 (1.64-4.78) | < 0.001 | 2.02 (1.17-3.49) | 0.012 |
| Anti-HBc (lg IU/mL) | 0.73 (0.33-1.63) | 0.448 | - | - | 1.29 (0.48-3.45) | 0.608 | - | - |
| Anti-HBc decline (lg IU/mL) | 3.11 (0.99-3.79) | 0.053 | - | - | 1.11 (0.31-3.99) | 0.874 | - | - |
| ALT (U/L) | 0.99 (0.98-1.01) | 0.477 | - | - | 0.99 (0.96-1.02) | 0.516 | - | - |
| ALT decline (U/L) | 1.004 (1.00-1.01) | 0.031 | - | - | 1 (0.99-1.01) | 0.822 | - | - |
| HBV DNA (lg copies/mL) | 0.55 (0.37-0.80) | 0.002 | - | - | 0.45 (0.23-0.86) | 0.016 | - | - |
| HBV DNA decline (lg copies/mL) | 1.39 (1.02-1.88) | 0.035 | - | - | 2.39 (1.39-4.11) | 0.002 | - | - |
At week 12, HBeAg, HBeAg decline, and HBV DNA decline were associated with HBeAg SC through univariate analysis, and HBeAg decline (OR = 2.47, 95%CI: 1.46-4.16, P = 0.001) independently predicted HBeAg SC via multivariate analysis. At week 24, univariate analysis presented that HBeAg, HBeAg decline, HBV DNA and HBV DNA decline were associated with HBeAg SC, and multivariate analysis identified that HBeAg (OR = 0.37, 95%CI: 0.17-0.81, P = 0.013) and HBeAg decline (OR = 2.02, 95%CI: 1.17-3.49, P = 0.012) independently predicted HBeAg SC, respectively (Table 2).
Based on the results of above analysis, both of the HBeAg level and its on-treatment declined value at 12-wk (or 24-wk) as independent predictors were further evaluated using AUROC for predicting 96-wk VR and HBeAg SC.
At week 12, the HBeAg titer and its declined value predicted VR with an AUROC of 0.733 (95%CI: 0.617-0.849, P = 0.001) and 0.709 (95%CI: 0.590-0.827, P = 0.002), respectively, and the best cut-off value for the HBeAg titer and its decline was 0.8 lg PEIU/mL and 0.84 lg PEIU/mL, respectively. Twenty-two patients achieved HBeAg titer < 0.8 lg PEIU/mL as well as the declined value > 0.84 lg PEIU/mL at 12-wk, and among them 91% (20/22) reached VR, whereas only 29% obtained VR among 28 patients without meeting the above two standards. HBeAg titer combined with on-treatment decline at 12-wk predicted VR with an AUROC of 0.812 (95%CI: 0.687-0.936, P < 0.001) and the sensitivity, specificity, PPV, NPV was 71%, 91%, 91% and 71%, respectively.
At week 24, the HBeAg titer and its declined value predicted VR with an AUROC of 0.788 (95%CI: 0.683-0.892, P < 0.001) and 0.736 (95%CI: 0.620-0.851, P < 0.001), respectively, and the best cut-off value for the HBeAg titer and its decline was 1.3 lg PEIU/mL and 1.6 lg PEIU/mL, respectively. All the 22 patients with HBeAg titer < 1.3 lg PEIU/mL and the declined value > 1.6 lg PEIU/mL at 24-wk achieved VR, whereas only 17% of 23 patients without meeting the above two standards obtained VR. HBeAg titer combined with its decline at 24-wk strongly predicted VR with an AUROC of 0.923 (95%CI: 0.838-1.000, P < 0.001) and the sensitivity, specificity, PPV, NPV was 85%, 100%, 100% and 83%, respectively (Table 3).
| Factors | ROC | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | |
| AUROC (95%CI) | P value | |||||
| Week 12 | ||||||
| HBeAg < 0.8 lg PEIU/mL | 0.733 (0.617-0.849) | 0.001 | 0.63 (0.48-0.78) | 0.81 (0.67-0.96) | 0.82 (0.68-0.96) | 0.62 (0.47-0.77) |
| HBeAg decline > 0.84 lg PEIU/mL | 0.709 (0.590-0.827) | 0.002 | 0.65 (0.50-0.80) | 0.75 (0.59-0.91) | 0.78 (0.64-0.92) | 0.62 (0.46-0.78) |
| Combined the above | 0.812 (0.687-0.936) | < 0.001 | 0.71 (0.54-0.89) | 0.91 (0.78-1.00) | 0.91 (0.78-1.00) | 0.71 (0.54-0.89) |
| Week 24 | ||||||
| HBeAg < 1.3 lg PEIU/mL | 0.788 (0.683-0.892) | < 0.001 | 0.88 (0.78-0.98) | 0.64 (0.46-0.81) | 0.76 (0.63-0.88) | 0.81 (0.65-0.97) |
| HBeAg decline > 1.6 lg PEIU/mL | 0.736 (0.620-0.851) | < 0.001 | 0.55 (0.39-0.70) | 0.94 (0.85-1.00) | 0.92 (0.81-1.00) | 0.62 (0.48-0.76) |
| Combined the above | 0.923 (0.838-1.000) | < 0.001 | 0.85 (0.70-0.99) | 1 | 1 | 0.83 (0.66-0.99) |
At week 12, the HBeAg declined value predicted HBeAg SC with an AUROC of 0.767 (95%CI: 0.623-0.911, P = 0.001), and the best cut-off value for HBeAg decline was 1.8 lg PEIU/mL. The HBeAg declined value > 1.8 lg PEIU/mL at 12-wk predicted HBeAg SC with a sensitivity, specificity, PPV, NPV of 60%, 87%, 53% and 90%, respectively.
At week 24, the HBeAg titer and its declined value predicted HBeAg SC with an AUROC of 0.828 (95%CI: 0.712-0.944, P < 0.001) and 0.814 (95%CI: 0.676-0.953, P < 0.001), respectively, and the best cut-off value for the HBeAg titer and its decline was -0.5 lg PEIU/mL and 2.2 lg PEIU/mL, respectively. Eight patients achieved HBeAg titer < -0.5 lg PEIU/mL and the declined value > 2.2 lg PEIU/mL at 24-wk, among them 88% (7/8) achieved HBeAg SC; whereas only 2% of 51 patients who did not meet the above two standards obtained HBeAg SC. HBeAg titer combined with its declined value at 24-wk strongly predicted HBeAg SC with an AUROC of 0.928 (95%CI: 0.791-1.000, P < 0.001) and the sensitivity, specificity, PPV, NPV was 88%, 98%, 88% and 98% (Table 4).
| Factors | ROC | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | |
| AUROC (95%CI) | P value | |||||
| Week 12 | ||||||
| HBeAg decline > 1.8 lg PEIU/mL | 0.767 (0.623-0.911) | 0.001 | 0.6 (0.32-0.88) | 0.87 (0.78-0.96) | 0.53 (0.26-0.79) | 0.9 (0.82-0.98) |
| Week 24 | ||||||
| HBeAg < -0.5 lg PEIU/mL | 0.828 (0.712-0.944) | < 0.001 | 0.67 (0.40-0.94) | 0.92 (0.84-0.99) | 0.67 (0.40-0.94) | 0.92 (0.84-0.99) |
| HBeAg decline > 2.2 lg PEIU/mL | 0.814 (0.676-0.953) | < 0.001 | 0.73 (0.48-0.99) | 0.9 (0.82-0.98) | 0.65 (0.39-0.90) | 0.93 (0.86-1.00) |
| Combined the above | 0.928 (0.791-1.000) | < 0.001 | 0.88 (0.58-1.00) | 0.98 (0.94-1.00) | 0.88 (0.58-1.00) | 0.98 (0.94-1.00) |
Achieving a long-term suppression of serum HBV DNA through antiviral therapy is one of important targets of treatment for CHB patients. Several studies tried to investigate the value of an early on-treatment change of HBV markers such as HBeAg in predicting VR to NAs therapy. A study presented that the HBeAg titer decreased by 1 lg PEIU/mL at 12-wk predicted VR (HBV DNA < 20 IU/mL) after 48-wk entecavir (ETV) therapy with a sensitivity, specificity, PPV, NPV of 67.6%, 87.9%, 86.2% and 70.7%, respectively[10]. However, the predictive value of HBeAg titer and its declined value at 12-wk for a long-term treatment response (≥ 2 years) was not reported. In this study, we found that HBeAg titer < 0.8 lg PEIU/mL combined with its declined value > 0.84 lg PEIU/mL at 12-wk predicted VR after 96-wk LAM and ADV optimized therapy with a sensitivity, specificity, PPV, NPV of 71%, 91%, 91% and 71%, respectively, and accompanied with an AUROC of 0.812. In addition, although some studies indicated that the on-treatment HBeAg level or declined value at 24-wk was a predictor for NAs treatment response[9,12], the sample size was small or HBeAg was detected using a semi-quantitative method which cannot accurately quantify HBeAg level, thus limited the validity of the prediction. For example, Zhang et al[12] detected serum HBeAg semi-quantitatively and found that the on-treatment declined value of HBeAg (> 65%) at 24-wk was the best predictor for treatment response (HBeAg seroconversion and accompanied by undetectable serum HBV DNA) after 96-wk ETV therapy, and the PPV, NPV, AUROC was 83.3%, 93.6% and 0.885, respectively. However, the finding may have difficulty in applying clinical practice since the absent of accurate quantitative HBeAg levels. In our study, the HBeAg titer (OR = 0.45, P = 0.003) and its declined value (OR = 2.03, P = 0.024) at 24-wk were found to predict 96-wk VR with the AUROC of 0.788 and 0.736, respectively. Moreover, the combination of HBeAg titer < 1.3 lg PEIU/mL and its decrease > 1.6 lg PEIU/mL at 24-wk predicted 96-wk VR with a sensitivity, specificity, PPV, NPV of 85%, 100%, 100% and 83%, respectively, and the AUROC increased to 0.923, which had a better predictive value than the semi-quantitative method reported by Zhang et al[12]. With respect to the cost of semi-quantitative and quantitative tests of HBeAg, it is comparable between them. Twenty-two patients in our cohort matched the combination standard and they all achieved 96-wk VR. The results suggested that if patients reached HBeAg titer < 1.3 lg PEIU/mL and declined > 1.6 lg PEIU/mL at 24-wk, there will be a better viral suppression during the continued NAs therapy.
In addition to achieve long-term suppression in serum HBV DNA, HBeAg SC is another important indicator to evaluate the efficacy of antiviral therapy in HBeAg-positive CHB patients. In our study, the declined value of HBeAg at 12-wk (OR = 2.47, P = 0.001) independently predicted 96-wk HBeAg SC with an AUROC of 0.767, and for the predictive value of HBeAg declined value at 24-wk, an AUROC increased to 0.814. Lee et al[8] found that the decline of HBeAg at month 6 was a strongest predictor for HBeAg SC after 2 years of ETV treatment with an AUROC of 0.820 (P = 0.004), which was similar to our result (AUROC = 0.814). However, Lee’s study used the declined value of HBeAg alone to predict treatment response and did not consider combining other indicators. Our data showed that both HBeAg titer as well as its declined value at 24-wk were independent predictors for 96-wk HBeAg SC, and it strongly predicted HBeAg SC with an AUROC of 0.928 if combining HBeAg titer < -0.5 lg PEIU/mL and declined value > 2.2 lg PEIU/mL at 24-wk. A study from Shin et al[11] revealed that HBeAg titer < 0.62 lg PEIU/mL after 48 wk of ETV therapy was a strongest predictor for HBeAg SC at year 3 with an AUROC of 0.86 (P < 0.001), which was inferior to the combined prediction validity of HBeAg level and declined value at 24-wk in our study (AUROC = 0.928). Our study presented that 88% (7/8) of patients with HBeAg titer < -0.5 lg PEIU/mL and declined value > 2.2 lg PEIU/mL at 24-wk achieved 96-wk HBeAg SC, whereas only 2% (1/51) of patients without meting above standards obtained HBeAg SC, thus got a NPV of 98%. Among the 51 patients with unfavorable 24-wk HBeAg titer, 46 patients were added on ADV at 24-wk due to serum HBV DNA > 300 copies/mL. The results suggested that patients with HBeAg titer > -0.5 lg PEIU/mL as well as declined value < 2.2 lg PEIU/mL after 24 wk of LAM therapy will rarely achieve HBeAg SC during the following NAs treatment, even adding on ADV still cannot improve the efficacy. So the patients with unfavorable 24-wk HBeAg titer should be considered to switch to other drugs or use other regimens for a better treatment outcome.
Results of logistic regression analysis in the current study showed that no baseline parameters were associated with 96-wk HBeAg SC, which was consistent with Lee’s report[8], who did not find correlations between baseline level of HBeAg (or HBsAg, HBV DNA) and HBeAg SC after 2 years of ETV therapy either. However, other studies showed that pre-treatment serum HBeAg was associated with HBeAg SC during ETV treatment[10,12]. The inconsistent results may due to that treatment period was relatively short or HBeAg was detected semi-quantitatively in the latter’s studies[10,12]. Several recent studies revealed that B lymphocytes played a key role in the regulation of host immune responses to HBV, while anti-HBc was produced and secreted by hepatitis B core antigen-specific B lymphocytes, therefore the serum anti-HBc level may be a surrogate marker for the host immune response to HBV[16,17]. Fan et al[13] demonstrated that the baseline level of anti-HBc independently predicted HBeAg SC after 2 years of LdT and ADV optimized therapy (OR = 1.99, P = 0.001). However, our results presented that baseline anti-HBc was not associated with HBeAg SC or VR after 96-wk LAM and ADV optimized therapy (HBeAg SC, OR = 1.60, P = 0.459; VR, OR = 1.68, P = 0.308). The discrepancy may due to the little difference of baseline anti-HBc level between patients with and without HBeAg SC (0.10 lg IU/mL) in our study, while the difference in baseline anti-HBc was 0.24 lg IU/mL in Fan’s study. Besides, previous studies identified that the elevation of ALT was contributed to T lymphocyte mediated hepatolysis occurred in CHB patients, therefore baseline ALT level may reflect T lymphocyte immune response to HBV which is related to the outcome after antiviral treatment[18]. Zeuzem et al[4] reported that the pre-treatment ALT level was associated with VR and baseline ALT ≥ 2 × ULN could independently predict non-detectable serum HBV DNA after 2 years of LdT treatment (OR = 2.00, P = 0.0071). In agreement with these findings, we also found that baseline ALT ≥ 5 × ULN independently predicted 96-wk VR (OR = 7.09, P < 0.001) with the AUROC of 0.700 (P = 0.003, data not shown), which suggested that patients with a higher pre-treatment ALT level maybe situated in a better immune status and will have an active virological response to NAs treatment.
All the parameters (HBsAg, HBeAg, anti-HBc and HBV DNA) presented continuous descent during the 96-wk treatment. Serum HBV DNA dropped significantly from baseline 8.16 lg copies/mL to 96-wk 2.85 lg copies/mL (P < 0.001) and 56.6% (43/76) patients obtained HBV DNA ≤ 300 copies/mL in our cohort, showing a similar data to the GLOBE study[19], in which 55.6% HBeAg-positive patients achieved HBV DNA < 300 copies/mL with serum HBV DNA declined by 6.1 lg copies/mL after 2-year LdT treatment. The antiviral effect of NAs agents was reducing viral replication by the inhibition of HBV DNA polymerase, but having limited impacts on the level of HBsAg[20]. In our study, HBsAg decreased slowly by 0.59 lg IU/mL after 96-wk therapy. Heathcote et al[21] also reported that the mean HBsAg level in HBeAg-positive CHB patients decreased by 0.66 lg IU/mL after 2 years of tenofovir disoproxil (TDF) treatment, which was similar to our data. In addition, anti-HBc level decreased from baseline 4.77 lg IU/mL to 96-wk 3.45 lg IU/mL in our cohort, with an average decrease of 1.32 lg IU/mL. In Fan’s report[13], 1.26 lg IU/mL of an average decrease of anti-HBc level was observed in HBeAg-positive CHB patients after 104-wk LdT and ADV optimized therapy (baseline 4.20 lg IU/mL to 104-wk 2.94 lg IU/mL). The average declines in anti-HBc levels were comparable between the two studies, although the baseline anti-HBc level was relatively higher in our study. Concerning the dynamic change of HBeAg, Shin et al[11] reported that HBeAg level reduced from baseline 2.23 lg PEIU/mL to 0.96 lg PEIU/mL after 96-wk ETV therapy and 17.1% (14/82) achieved HBeAg SC. In the present study, HBeAg level decreased from baseline 2.24 lg PEIU/mL to 96-wk 0.20 lg PEIU/mL and 19.7% (15/76) obtained HBeAg SC, which presented comparable HBeAg reduction and HBeAg SC rate as compared to Shin’s study.
Comparing dynamic changes in HBeAg level between patients with and those without 96-wk VR (or HBeAg SC), our results showed that baseline HBeAg levels were comparable between two groups. Further, HBeAg levels in patients obtained 96-wk VR (or HBeAg SC) were significantly lower than those without VR (or HBeAg SC) from 12-wk to the end of follow-up (P < 0.05, as shown in Figures 1B and 2B). With respect to dynamic changes of HBV DNA levels, the same trend of decline was observed. The patients achieved 96-wk VR had a significant lower HBV DNA level at every follow-up time-point except baseline when compared with those without VR (P < 0.05), while the significant differences in HBV DNA levels between patients with and without HBeAg SC were only observed at week 24, 72 and 96 (Figures 1D and 2D). These results indicated that HBeAg may have a better predictive value than HBV DNA for treatment response in HBeAg-positive CHB. At the same time, our finding showed that both the HBV DNA level as well as its declined value at 24-wk (or 12-wk) were significantly associated with 96-wk VR and HBeAg SC when performed univariate analysis (P < 0.05). However, HBV DNA levels and its declines would not associate with 96-wk VR and HBeAg SC anymore if all parameters including HBeAg levels and its declines were enrolled in multivariate analysis. Other studies also pointed out that HBeAg levels as well as its declines may maintain a better predictive value than HBV DNA to predict NAs treatment response in HBeAg-positive CHB patients[10,11]. HBeAg is generated by transcription and translation of HBV cccDNA, and previous studies had reported that serum HBeAg level was significantly correlated with intrahepatic HBV cccDNA (r = 0.507, P = 0.010)[22]. Thus, the decline in serum HBeAg level may reflect the reduction of HBV cccDNA and represented a good treatment outcome. In addition, viral persistence and the development of CHB was associated with viral manipulation and evasion of the host’s immune system, while HBeAg has been reported to attenuate the host immune response to the nucleocapsid protein and down-regulate the innate and adaptive immune responses[23,24]. Therefore, the decline in HBeAg level might weaken this effect and thus the patients may present a better immune control for HBV infection.
There were some limitations in our study. Firstly, LAM and ADV used in the cohort are no longer the first-line antiviral drugs. However, NAs drugs have a similar antiviral mechanism, and HBeAg SC rates in CHB patients are comparable between ETV (or TDF) therapy and an optimized therapy with LAM and ADV. Hence, we propose that the combination parameter of HBeAg level and its declined value at 24-wk might be used as a reference parameter to predict the efficacy of ETV or TDF treatment. Secondly, treatment endpoint evaluated in our study was on-treatment response. For virological response, off-treatment response may be more important than on-treatment response.
To our knowledge, this is the first report that identified the combination of on-treatment quantitative HBeAg level and its decline as the predictor of positive response to long term NAs therapy among HBeAg-positive CHB patients. In particular, the combination of HBeAg titer and its decline at 24-wk strongly predicted 96-wk VR and HBeAg SC with the AUROC of 0.923 and 0.928, respectively. This combination predictor was identified through a retrospective investigation based on the cohort of HBeAg-positive CHB patients received LAM and ADV optimized therapy. We will conduct a prospective study to evaluate and confirm the predictive validity of the combination parameter in the cohort of ETV or TDF therapy in the future, and anticipate that the combination of on-treatment HBeAg level and its declined value could serve as a reference parameter to guide NAs therapy.
The authors are grateful to Dr. Shumei Yun, from Missouri Department of Health and Senior Services, Jefferson City, MO, United States for proofreading and editing the manuscript.
The antiviral effect of current available nucleos(t)ide analogues (NAs) or peginterferon drugs are not satisfied. To improve the efficacy, it is crucial to explore the pre-treatment and early on-treatment biomarkers to effectively predict long-term treatment response. However, very few studies have systematically evaluated the predictive power of quantitative hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), hepatitis B core antibody (anti-HBc), hepatitis B virus (HBV) DNA and alanine aminotransferase (ALT) for NAs treatment response.
In the current study, serum HBsAg, HBeAg, anti-HBc, HBV DNA and ALT were quantitatively tested during the 96-wk NAs therapy, and the baseline as well as early on-treatment levels of these parameters were comprehensively analyzed to assess their functions in predicting 96-wk virological response (VR) and HBeAg seroconversion (SC).
This is the first report that the combination parameter of on-treatment quantitative HBeAg level and its declined value was found to predict 96-wk treatment response to lamivudine and adefovir dipivoxil optimized therapy for HBeAg-positive chronic hepatitis B (CHB) patients. In particular, the combination of HBeAg titer and its decline at 24-wk strongly predicted 96-wk VR and HBeAg SC with the AUROC of 0.923 and 0.928, respectively.
The combination variable of on-treatment HBeAg level and its declined value may serve as a reference parameter to optimize NAs therapy for HBeAg-positive CHB patients.
HBV is a major cause of chronic liver disease including chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Reactivation of HBV is closely related to acute exacerbation of HBV carriers which sometimes leads to liver failure. Introduction of NAs dramatically changed the landscape of HBV treatment that is useful weapon to suppress HBV replication. NAs can only suppress HBV replication but not eradicate HBV. Therefore several problems remains including setting of endpoint and adequate cessation of NAs.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inoue K S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization 2015; . [PubMed] [Cited in This Article: ] |
| 2. | Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 742] [Cited by in F6Publishing: 759] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 3. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2323] [Cited by in F6Publishing: 2338] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 4. | Zeuzem S, Gane E, Liaw YF, Lim SG, DiBisceglie A, Buti M, Chutaputti A, Rasenack J, Hou J, O’Brien C. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Bowden S. Serological and molecular diagnosis. Semin Liver Dis. 2006;26:97-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 7. | Fung J, Lai CL, Young J, Wong DK, Yuen J, Seto WK, Yuen MF. Quantitative hepatitis B surface antigen levels in patients with chronic hepatitis B after 2 years of entecavir treatment. Am J Gastroenterol. 2011;106:1766-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Lee JM, Ahn SH, Kim HS, Park H, Chang HY, Kim DY, Hwang SG, Rim KS, Chon CY, Han KH. Quantitative hepatitis B surface antigen and hepatitis B e antigen titers in prediction of treatment response to entecavir. Hepatology. 2011;53:1486-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Wang J, Du LY, Zhu X, Chen EQ, Tang H. The predictive value of early indicators for HBeAg seroconversion in HBeAg-positive chronic hepatitis B patients with Telbivudine treatment for 104 weeks. Indian J Med Microbiol. 2015;33 Suppl:20-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Kwon JH, Jang JW, Lee S, Lee J, Chung KW, Lee YS, Choi JY. Pretreatment HBeAg level and an early decrease in HBeAg level predict virologic response to entecavir treatment for HBeAg-positive chronic hepatitis B. J Viral Hepat. 2012;19:e41-e47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Shin JW, Jung SW, Park BR, Kim CJ, Eum JB, Kim BG, Jeong ID, Bang SJ, Lee SH, Kim SR. Prediction of response to entecavir therapy in patients with HBeAg-positive chronic hepatitis B based on on-treatment HBsAg, HBeAg and HBV DNA levels. J Viral Hepat. 2012;19:724-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Lin SM, Ye F, Chen TY, Liu M, Chen YR, Zheng SQ, Zhao YR, Zhang SL. An early decrease in serum HBeAg titre is a strong predictor of virological response to entecavir in HBeAg-positive patients. J Viral Hepat. 2011;18:e184-e190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Fan R, Sun J, Yuan Q, Xie Q, Bai X, Ning Q, Cheng J, Yu Y, Niu J, Shi G. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues. Gut. 2016;65:313-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Zhou B, Liu M, Lv G, Zheng H, Wang Y, Sun J, Hou J. Quantification of hepatitis B surface antigen and E antigen: correlation between Elecsys and architect assays. J Viral Hepat. 2013;20:422-429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Li A, Yuan Q, Huang Z, Fan J, Guo R, Lou B, Zheng Q, Ge S, Chen Y, Su Z. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol. 2010;17:464-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Farci P, Diaz G, Chen Z, Govindarajan S, Tice A, Agulto L, Pittaluga S, Boon D, Yu C, Engle RE. B cell gene signature with massive intrahepatic production of antibodies to hepatitis B core antigen in hepatitis B virus-associated acute liver failure. Proc Natl Acad Sci USA. 2010;107:8766-8771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Oliviero B, Cerino A, Varchetta S, Paudice E, Pai S, Ludovisi S, Zaramella M, Michelone G, Pugnale P, Negro F. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55:53-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30:770-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, Lai CL, Heathcote EJ, Manns M, Bzowej N, Niu J. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136:486-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 20. | Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Heathcote EJ, Marcellin P, Buti M, Gane E, De Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Tangkijvanich P, Komolmit P, Mahachai V, Sa-Nguanmoo P, Theamboonlers A, Poovorawan Y. Comparison between quantitative hepatitis B surface antigen, hepatitis B e-antigen and hepatitis B virus DNA levels for predicting virological response to pegylated interferon-alpha-2b therapy in hepatitis B e-antigen-positive chronic hepatitis B. Hepatol Res. 2010;40:269-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Walsh R, Locarnini S. Hepatitis B precore protein: pathogenic potential and therapeutic promise. Yonsei Med J. 2012;53:875-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Chen MT, Billaud JN, Sällberg M, Guidotti LG, Chisari FV, Jones J, Hughes J, Milich DR. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci USA. 2004;101:14913-14918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |