HEME OXYGENASES
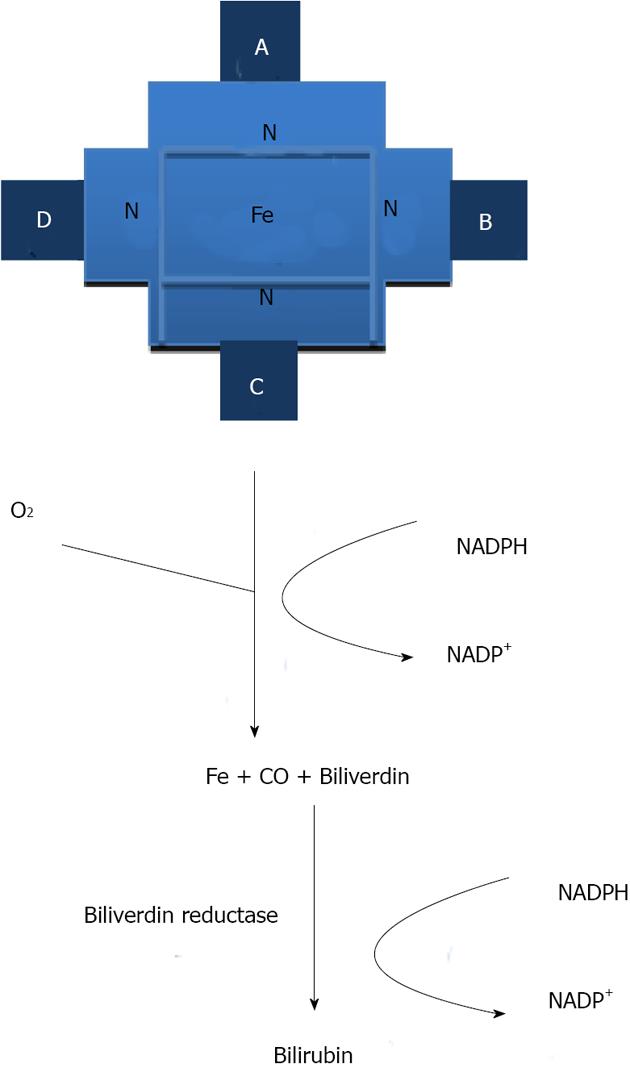
Heme oxygenases (HOs) are ubiquitous and essential enzymes for all eukaryotic organisms that depend on aerobic oxidation and electron transport via heme-containing proteins[1,2]. HOs were first recognized as catalyzing the rate-limiting step in the principal degradative mechanism of heme (iron protoporphyrin IX)[2,3] catabolism. In a reaction that requires oxygen and nicotinamide adenine dinucleotide phosphate (NADP), the heme ring is cleaved by HO to yield biliverdin, along with the concomitant release of iron and the emission of carbon monoxide (CO) (in equimolar quantities). Biliverdin (BV) is then reduced to bilirubin (BR) by biliverdin reductase[4] (Figure 1).
Figure 1 The heme oxygenase enzyme reaction.
Heme is enzymatically degraded to yield carbon monoxide, iron and biliverdin (which is converted into bilirubin in a coupled reaction). CO: Carbon monoxide; NADP: Nicotinamide adenine dinucleotide phosphate; NADPH: β-Nicotinamide adenine dinucleotide 2'-phosphate reduced tetrasodium salt.
Two distinct isoforms of HO (the products of different genes) have been identified. HO-1 is a single transmembrane inducible protein found in endoplasmic reticulum, caveola, nuclei and mitochondria. It is ubiquitously present in mammalian tissues such as liver, spleen, pancreas, intestine, kidney, heart, retina, prostate, lung, skin, brain, spinal cord, vascular smooth muscle cells and endothelial cells. Its expression is relatively low under physiological conditions, except in the spleen where the action of HO-1 is critical to the recycling of iron from senescent erythrocytes[5]. HO-2 shares 40% amino acid homology with HO-1; it is constitutively expressed and may provide an additional temporary buffering function against pro-oxidant heme by means of sequestration (via additional heme binding sites located on the enzyme). It is localized to the mitochondria where it likely regulates a variety of cellular functions. The existence of a third distinct isoform of HO encoded (HO-3) has been postulated but it is now clear that this is a non-coding pseudogene[1]. Both isoforms, HO-1 and HO-2, of this enzyme catalyze the same enzymatic reaction, resulting in the degradation of heme[6].
The role that HO-1 plays in the modulation of the immune response has been increasingly studied within the field of immunology and it is now recognized that HO-1 may act as a molecular brake on the activation, recruitment and amplification of immune responses[7]. Over-expression of HO-1 results in reduced expression of endothelial-leukocyte adhesion molecules and reduced activity of the nuclear factor-κB (NF-κB) pathway; conversely, HO-1-deficient animals exhibit increased levels of monocyte chemo-attractant protein-1. In humans, HO-1 deficiency is associated with susceptibility to oxidative stress and an increased pro-inflammatory state correlated with severe endothelial damage[8]. Mice lacking HO-1 develop progressive inflammatory diseases[9] and show enhanced sensitivity to lipopolysaccharide (LPS)-induced toxemia. HO-1 deficiency shows partial embryonic lethality. HO-1 knockout mice display a progressive chronic inflammatory disease characterized by enlarged spleens and hepatic inflammatory lesions. Additionally, the protective properties of HO-1 have been studied in a variety of inflammatory models[6].
EXPRESSION AND TRANSCRIPTIONAL REGULATION OF HO-1
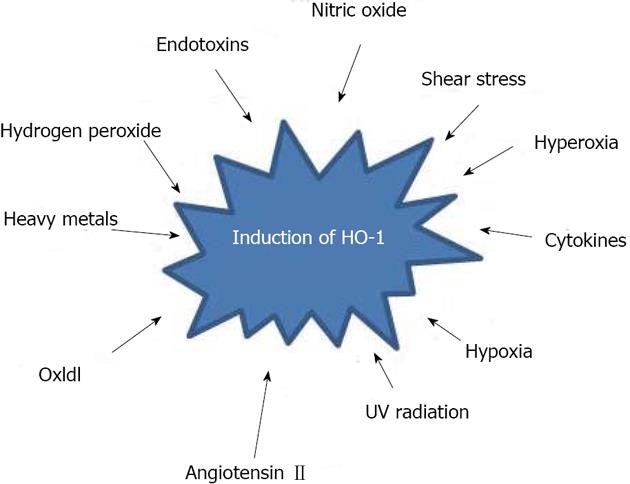
The human HO-1 gene is located on chromosome 22q12; it is approximately 14 kb long and contains 5 exons[8]. Control of HO-1 transcription is complex and tightly regulated, with differences in expression found between tissues, as well as between species[9]. A wide variety of stimuli have been shown to induce HO-1 expression, including heme, heavy metals, hydrogen peroxide, oxidized low density lipoproteins, hyperoxia, hypoxia, endotoxins, nitric oxide (and nitric oxide donors), cytokines, angiotensin II, shear stress and ultraviolet radiation[3] (Figure 2). Its biological function is to provide a specific regulatory mechanism for control of the level of many heme proteins. Multiple regulatory elements control human HO-1 gene transcription. These elements contain numerous transcription factor consensus binding sites in both the proximal and distal 5’ promoter sequences, as well as in an internal enhancer region. HO-1 gene expression can be up-regulated through its multiple stress response and cadmium response elements, as well as by numerous important transcription factors, including Jun B, activator proteins 1 and 2, and NF-κB[10]. Conversely, the transcription factors Bach1 and Jun D act as negative regulators of human HO-1 gene expression[2,11].
Figure 2 A variety of stimuli induce heme oxygenase-1 expression, a wide array of hepatotoxic chemicals and conditions conferring an adaptive cytoprotective response.
HO-1: Heme oxygenase-1; UV: Ultraviolet.
Hypoxia induces HO-1 expression in multiple rodent, bovine and monkey cell lines, but interestingly, hypoxia represses expression of the human HO-1 gene in a variety of human cell types (endothelial cells, epithelial cells, T cells)[5]. The short guanosine/thymine (GT)n[12] repeat in the HO-1 gene promoter may provide protective effects against carotid atherosclerosis in individuals with a high level of arsenic exposure. Therefore, the translation of HO-1 research from “bench to bedside” must be carried out carefully, with the appreciation that the response of HO-1 gene expression to certain stimuli may be different in humans and rodent models[9].
HEME OXYGENASE-1 PROMOTER POLYMORPHISMS
There are 3 possible polymorphisms in the 5’ flanking region: a (GT)n dinucleotide length polymorphism and two single nucleotide polymorphisms (SNP). Functional polymorphic dinucleotide guanosine/thymine (GT) repeat regions are present in the proximal promoter region of HO-1 approximately encompassing nucleotides -248 to -198, relative to the transcription start repeats regions in the HO promoter. The reported number of GT-repeats range from 12 to 40, although a bimodal distribution exists in most populations, with the main alleles being composed of 23 or 30 repeats. HO-1 mRNA expression and enzyme expression are greater when GT-repeat lengths are short and lesser when they are long[9]. There is some evidence that links H2O2 or other HO-1 transcriptional activators and the number of GT repetitions and it has been postulated that conformational changes account for the modulation of transcriptional activity. For instance, a left-handed double-helix structure (Z-DNA conformation) may be formed by the alternating purine-pyrimidine sequences, an arrangement that has been correlated with diminished transcriptional activity in other genes[3]. Indeed, Yamada et al[13] observed that GT-dinucleotide repeat polymorphisms in the promoter region of the HO-1 gene were frequently associated with increased HO-1 responses to exogenous stimuli.
The inducibility of HO-1 is affected by (GT)n polymorphisms[14], as well as the SNP A(-413)T, in the promoter of HO-1[8]. Both short (GT)n alleles and the A-allele have been associated with increased HO-1 promoter activity[4]. For instance, in 308 liver transplantations, HO-1 patient genotypes were correlated with outcome variables[15]. With respect to their (GT)n genotype, liver samples were divided into two classes - those containing short alleles (less than 25 repeats; class S) and those containing long alleles (greater than or equal to 25 repeats; class L). Haplotype analyses confirmed the dominance of the A (-413)T SNP over (GT)n polymorphism[16].
The results of transient transfection assays using luciferase promoter constructs were consistent with in vitro assays carried out in lymphoblastoid cell lines containing HO-1 GT repeats of various lengths[9]. Cells homozygous for short GT-stretches had significantly greater HO-1 expression and resistance to oxidant-induced apoptosis compared with cells homozygous for long GT-stretches, demonstrating that polymorphisms in GT-stretch length are functionally significant[9].
Hyperbilirubinemia is associated with multiple repeats of TA nucleotides, as well as with the 211 G mutation, which are polymorphisms found in the UGT1A1 gene promoter[15] and coding regions, respectively[1]. However, it is not clear how these polymorphisms influence overall bilirubin levels and whether polymorphisms in other genes involved in the bilirubin metabolism pathway also influence overall bilirubin levels[3]. Although the short repeats found in certain HO alleles (< 25 repeats) have been found to have beneficial effects on the serum bilirubin levels and lipid profiles of patients with coronary artery disease (CAD), few studies have examined the effects of these repeats in healthy patients with normal bilirubin levels or patients who carry short GT-repeats (within in the HO-1 promoter) who are at a high risk for developing unconjugated hyperbilirubinemia[17]. Considering the antioxidant and anti-atherogenic properties of bilirubin, the beneficial effects of the S allele on serum bilirubin levels in carriers might be sufficient to exert a protective effect against the development and clinical severity of CAD[3].
Taha et al[18] have shown that pentoxifylline (PTX, a drug used to improve blood flow in patients with circulation problems) in fibrosarcoma L292 cells strongly induces the expression of HO-1. The potential involvement of HO-1 in the regulation of PTX signaling is of particular importance, especially with respect to human HO-1 promoter polymorphisms[19]. The presence of alleles with lower activity (resulting in lower HO-1 expression) was demonstrated to be a contributing factor to increased risk of cardiovascular complications, at least in some populations of patients[5]. The cytoprotective effects of PTX treatment were reproduced by incubation with hemin (an HO-1 activator)[5] or with CORM (a compound that causes the release of CO)[15]. These effects were blocked by treatment with ZnPPIX (zinc protoporphyrin-IX)[20], an HO-1 inhibitor[15]. Thus, both PTX and HO-1 appear to have anti-inflammatory properties, as exemplified by the inhibition of interleukin (IL)-1β, IL-6, IL-8, TNF-α and granulocyte-macrophage colony-stimulating factor expression, and the induction of IL-10 expression[15,21]. Furthermore, the expression of both genes has been shown to reduce proliferation, migration and adhesion of leukocytes to endothelial cells, through inhibition of CD25 and ICAM-1[6].
Thus, one can expect that the efficacy of PTX treatment is likely to be influenced by these polymorphisms and the efficacy of this treatment is likely to be low in patients with less active HO-1 alleles[15].
The HO-1 genotype is associated with liver transplantation outcome and these findings suggest that HO-1 mediates graft survival following liver transplantation[1,5]. Therefore, the expression of the HO-1 gene may be altered by the number of (GT)n repeats and could play an important role in modulating vascular tone under different pathological situations[3].
MODULATORY AND CYTOPROTECTIVE EFFECTS OF HO-1
Induction of HO-1 and its metabolites has a protective effect in a large number of seemingly unrelated pathologies, including sepsis, malaria[20], endotoxic shock, ischemic reperfusion injury (IRI)[4], organ transplant rejection[22], induction of tolerance myocardial infarction, type 2 diabetes[15] and obesity[23]. This wide spectrum of protective effects has been attributed to multi-level mechanisms of cytoprotection and inflammatory modulation. Three catabolic products, BV, BR and CO[22], and ferritin (which is induced by free iron[2]) have been implicated in HO-1 control of apoptosis and cell proliferation. However, the effects of BV/BR and CO do not overlap in terms of their effects on cell signaling and target molecules. The beneficial effects of the three products generated by HO-1 differ not only in their inherent molecular mechanisms, but also in their downstream cellular targets. To date, this is the only enzymatic system known to exhibit such characteristics; a good example of this is the manner in which HO-1 regulates the cell cycle - it increases endothelial cell (EC) proliferation but simultaneously decreases airway and vascular smooth muscle cell cycle progression[4]. A number of intracellular signaling molecules have been identified as being involved in regulating the induction of HO-1; among which upstream signaling kinases, extracellular signal-regulated protein kinase (ERK), c-Jun N terminal kinase and p38 mitogen activated protein kinase (MAPK) have been considered to play major roles in controlling up-regulation of HO-1[24]. Activation of either one or more of these MAPKs by external stimuli triggers HO-1 gene expression. ERK1/2 and p38 MAPK are involved in the induction of HO-1 gene transcription by sodium arsenite in the hepatoma cell line.
CO acts through a variety of pathways, including increased cycling of guanosine monophosphate through the activation of guanylate cyclase[15], modulation of inducible nitric oxide synthase[5] and regulation of protein kinases C (which have effects on vascular smooth muscle cells)[6]. Exogenous CO can be delivered in both an in vivo model and in vitro model, as either a gaseous molecule or in the form of innovative CO-releasing molecules (CORM) that are currently being developed by HemoCORM and Alfama Inc[3,19].
HO-1 expression protective effects are linked to a combination of factors, such as removal of the reactive substrate heme, the biological effects of the reaction products, the suppressive effects on monocyte chemo-attractant protein 1, and modulation of cell-cycle regulators[7]. It has been suggested that activated Kupffer cells play an important role in the pathogenesis of fulminant hepatic failure, as reflected by the activation of both pro and anti-inflammatory cascades in the innate immune system[8]. Recent studies have identified serum HO-1 levels as a novel diagnostic marker for macrophage activation under conditions including sepsis and hemophagocytic syndrome, which is useful for predicting the severity and prognosis of acute liver injury[1,5].
Kawakami et al[22] have confirmed that T helper type 17 (Th17) cells IL-17-producing CD4+ T cells[25] have a fundamental role in the immunopathogenesis of experimental autoimmune encephalomyelitis (EAE). Hammerich et al[26] demonstrated that myeloid HO-1 deficiency exacerbated EAE in mice and enhanced infiltration of activated macrophages and Th17 cells to the central nervous system, thereby establishing HO-1 as a critical early mediator of the innate immune response in EAE[3].
The liver is a primary source of the IL-7 response to Toll-like receptor (TLR) stimulation, although IL-7 expression in the liver is limited under steady state conditions[27]. TLR-mediated systemic cytokine expression alters protein expression[9,15]. Profiles in hepatocytes, which produce a number of acute phase response proteins, show markers of systemic inflammation, including ferritin[28], plasminogen and complement components[9] in ischemia-reperfusion injury (IRI)[5]. IL-7 is an important survival factor for T cells and is expressed in the liver as an acute phase reactant in response to inflammation, which allows the immune system to respond to pathogens[9]. IL-7 expression is increased in the liver and kidney tissues only following TLR stimulation, whereas it decreased in the spleen and lymph nodes[27,29]. These results suggest that systemic hepatocyte-mediated IL-7 expression (but not expression mediated by the lymphoid organs) plays an important role in IL-7 mediated T cell homeostasis in response to infection[9].
In recent years, the immunomodulatory capacity of the adaptive responses by HO-1 has received more attention. It has been reported that HO-1 can promote graft tolerance by activation/production of CD4+/CD25+ regulatory T cells (Tregs) or by promoting the activation-induced cell death of T cells[8]. CD4+/CD25+/Foxp3+ Tregs are a functionally distinct subset of mature T cells with broad suppressive activity[9]. Tregs play a key role in the maintenance of peripheral tolerance and Treg deficiencies can lead to progressive autoimmune disorders[5]. Similarly, increased Treg function can prevent allograft rejection and suppress tumor immunity[15] during allogeneic hematopoietic stem cell transplantation (HSCT)[9]. Tregs have been shown to play an important role in establishing tolerance between recipient tissues and donor-derived immunity. This was initially demonstrated in murine studies in which the depletion of Tregs from stem cell grafts resulted in increased graft-versus-host disease (GVHD) and increased Treg levels resulted in the suppression of GVHD following transplant[5]. Patients with active chronic GVHD have lower Treg levels when compared with patients without chronic GVHD[15].
Recent studies have suggested that IL-7 and IL-15 are the primary homeostatic cytokines that drive CD4+ T cell proliferation[25]. Previous analyses found that plasma IL-7 levels are consistently higher following allogenic HSCT, but IL-7 levels do not correlate with the degree of CD4+ T-cell recovery. Thus, IL-7 may promote T-cell expansion following transplants, but high plasma levels of IL-7 persist after lymphopenia has resolved[15]. Unbalanced Th1/Th2 T-cell responses in the liver are a characteristic of hepatic inflammation and subsequent liver fibrosis. The recently discovered Th17 cells, a subtype of CD4(+) T-helper cells mainly producing IL-17 and IL-22, have initially been linked to host defense against infections and to autoimmunity.
HO-1 is not essential for TLR4/MyD88-induced NF-κB/MAPK activation and the subsequent pro-inflammatory cytokine production, but it is required for TLR4/TLR3/IRF3-induced production of IFN-γ[30], as well as the expression of primary IRF3-target genes in macrophages. IRF3 activation and subsequent IFN-γ production is severely impaired in HO-1 knockout macrophages infected with Sendai virus (SeV) used to down regulate HO-1 expression[31]. In the presence of polyI:C, myeloid HO-1 knockout mice infected with Listeria monocytogenes (an infection model showing enhanced severity that is dependent on IFN-γ production) showed increased levels of bacterial clearance, whereas control mice succumbed to infection[1,30].
Lee and Bai et al[12] identified a potential interplay between IL-10 and HO-1 in the inhibition of LPS-induced inflammatory responses in macrophages. They also provided evidence that HO-1 mediates the anti-inflammatory function of IL-10, both in vivo, and that IL-10 and HO-1 activate a positive feedback circuit to amplify the anti-inflammatory response[15]. IL-10 and HO-1 were up-regulated in patients receiving norfloxacin and correlated with norfloxacin in a concentration-dependent manner, whereas proinflammatory inducible nitric oxide synthase, cyclooxygenase-2 and NF-κB behaved inversely. Higher IL-10 levels correlated with lower white blood cell count and higher mean arterial pressure. No correlations were found between IL-10 and disease clinical scores or liver function markers in blood[9].
THE PHYSIOLOGICAL ROLE OF THE HO-1 GENE IN THE LIVER
The liver is the largest gland in the body and is situated slightly below the diaphragm and anterior to the stomach. It consists of two lobes that are wedge-shaped. Two blood vessels enter the liver, namely the hepatic portal vein, with dissolved food substances from the small intestine, and the hepatic artery, with oxygenated blood from the lungs[8,32]. Two ducts originate in the liver and these unite to form the common hepatic duct which opens with the pancreatic duct in the hollow side of the duodenum (the first section of the small intestine). The gall bladder lies inside the liver and is the storage place for bile, which is formed by the liver cells[8].
The hepatic microvasculature is a unique and well-organized system of microvessels, which are composed of parenchymal hepatocytes and a variety of non-parenchymal cells, such as sinusoidal endothelial cells, Kupffer cells[33] and Ito cells. Cytochrome P450[34] is a major contributor to production of bilirubin derived from non-hemoglobin sources in the liver[1].
HO-1 is expressed at low to undetectable levels in hepatocytes and is expressed mainly in Kupffer cells under basal conditions[33]. However, HO-1 undergoes a rapid transcriptional activation in both Kupffer cells and hepatocytes in response to noxious stimuli[35]. A physiological role for HO-1 gene expression has also been demonstrated in HO-1 deficient mice and in one case of human genetic HO-1 deficiency. Both murine and human HO-1 deficiencies have systemic manifestations associated with iron metabolism, such as hepatic overload (with signs of a chronic hepatitis) and iron-deficiency anemia (with paradoxical increased levels of ferritin)[7]. Determining the role that HO-1 plays in such regulatory mechanisms has become increasingly relevant in recent years because its induction has been shown to prevent ethanol-induced inflammation in the intestine[36] and liver[4], as well as in the prevention of oxidative damage to hepatocytes[5].
MECHANISMS OF ACTION OF HMOX-1 IN THE LIVER
Hepatic ischemia and IR are the leading causes of clinical liver damage and occur[5] during major trauma concomitant with blood loss, extensive liver resection during tumor removal and liver transplantation[37]. Steatosis is a major risk factor for liver surgery because steatotic livers respond poorly to IRI. The predominant form of hepatocyte death is caused by apoptosis in ischemic non-steatotic livers, whereas steatotic livers develop massive necrosis following ischemic injury[1]. Mitochondria are the main cellular source of reactive oxygen species (ROS), which are observed to be dramatically increased in steatotic livers during the reperfusion period[38]. Increased ROS generation results in increased hepatic lipid peroxidation and oxidative stress, as well as decreased adenosine triphosphate generation, which may partially explain the poor tolerance of steatotic livers to IRI. Increased neutrophil accumulation causes significant sinusoidal endothelial cell injury through the release of free radicals and proteases, which may further aggravate hepatic microcirculation via the dysregulation of vasoactive mediators[1]. Kupffer cells activation is also reported to be involved in the poor outcomes of patients with steatotic livers[4]. Oxidative injury leads to hepatocyte damage followed by activation of Kupffer cells and results in the production of a number of inflammatory cytokines, including TNF-α and IL-6. These results suggest that the HO system has a potent protective effect on acute liver injury induced by carbon tetrachloride, CCl4. In rats, CXCL14 expression was increased in the liver injury phase and returned to normal after[2] liver regeneration, suggesting its involvement in the liver injury or regeneration regulation[5,9]. Inhibition of HO-1 has been shown to protect against tissue injury in livers exposed to CCl4[11,39]. Animals pretreated with hemin and then exposed to CCl4 showed a reduced leukocyte infiltration. Elevated HO activity has also been observed in damaged liver tissue, most likely due to interference with the HO pathway by tetrachloride-dependent metabolism triggered by heme overload-associated toxicity. Hepatocyte apoptosis and liver injury were both attenuated in rats pretreated with hemin[15]. Basal HO-1 levels appear to be more critical than the ability to up-regulate HO-1 in response to IRI and they may also be able to predict the success of pharmacologically induced cytoprotection. Consistent with this, cobalt protoporphyrin (CoPP)[40]-induced HO-1 up-regulation suppresses the type-1 interferon pathway downstream of the Toll-like receptor (TLR) 4 system in hepatic IRI models[8,17].
Orthotopic liver transplantation (OLT) is the best available treatment for patients with end-stage liver failure, during which the liver is exposed to some stressful stimuli, such as ischemia and reperfusion injury. HO-1 has been shown to provide cytoprotection during liver ischemia and reperfusion[41].
HO-1 expression has been suggested to have an immune-modulatory effect[2] and up-regulation of HO-1 has been shown to protect livers from IRI and improve graft survival[1]. All of the products formed during this process have potential beneficial effects in transplant settings. CO has vasodilatory effects, which help to maintain microvascular hepatic blood flow[3], BV and BR, and possess potent antioxidant properties[1]. Free iron is itself highly reactive; however, the cellular Fe2+ released during heme degradation up-regulates the expression of the Fe2+ sequestering protein ferritin, as well as an Fe2+ pump, thereby limiting the amount of free iron and preventing the generation of reactive oxygen species[8]. HO-1 participates in iron homeostasis. Javanmard et al[42] generated mice with targeted HO-1 null mutations and analyzed parameters of iron metabolism and they discovered that adult HO-1-deficient animals develop both serum iron deficiency and pathological iron-loading, indicating that HO-1 is crucial for the expulsion of iron from tissue stores.
HO-1 expression protects transplanted organs from IRI and immune rejection[8]. This review has demonstrated that persistent over-expression of HO-1 in donor livers can improve survival by expanding T regulatory cells in a model of OLT[43]. The possible mechanisms by which CO protects the liver against cold ischemia-reperfusion do not appear to be associated with down-regulation of the NF-κB-signaling pathway. ROS generation following re-oxygenation causes tissue damage and initiates a cellular cascade leading to inflammation, cell death and, ultimately, organ failure. A growing body of evidence suggests that Kupffer cells and T cells mediate the activation of neutrophil inflammatory responses[33]. The activation of TLRs in Kupffer cells may provide the triggering signal for pro-inflammatory responses in the IRI sequence[8]. Dissecting the signaling pathways that link HO-1 and TLR activation may be important in devising novel therapeutic strategies for combating IRI[4]. The evolutionarily conserved sentinel TLR belong to the IL-1R family and recognize bacterial/viral-specific pathogen-associated molecular patterns[5]. TLRs trigger host inflammatory responses that are mediated by macrophages, neutrophils and complement[44]. The induced cytokine mediators may then activate systemic responses to recruit leukocytes to sites of inflammation. The cellular and molecular mechanisms by which hepatic microvascular flow is regulated during normal and disease conditions are being actively investigated[2].
Intraportal delivery of an adeno-associated virus (AAV) expression vector encoding rat HO-1 (AAV-HO-1) resulted in persistent expression of HO-1 in hepatocytes (and increased HO-1 activity)[45] in transplanted livers, leading to prolonged survival in recipients[15]. Over-expression of HO-1 reduced the Banff rejection activity index, measured by ELISA, by inhibiting the production of IL-2 and TNF-α, decreasing infiltration of CD4+ and CD8+ cells, and increased infiltration of Treg cells into donor livers[5,46].
The spleens of recipients expressed higher levels of Foxp3, TGF-β and IL-10 compared with control rats and the transplanted livers expressed higher levels of Foxp3 and TGF-β[47]. Splenocytes from tolerant recipients had higher levels of Treg cells and responded poorly to allogeneic donor splenocytes. Persistent expression of HO-1 in donor livers by intraportal delivery of AAV-HO-1 improves survival by expanding the number of Treg cells. The HO-1-based therapies described here are promising new strategies for the prevention of liver transplant rejections[48].
During maturation, dendritic cells (DCs) inhabit a variety of microenvironments that contain different levels of chemokines and chemokine receptors. Immature DCs express CCR1, CCR2, CCR5 and CXCR1, while mature DCs express high levels of CCR7, CCL22, CCL5, CCL2 and CXCL10[39]. In CoPPIX-treated mice, CCR7 was observed to be markedly down-regulated in liver DCs[49]. CoPPIX treatment reduced the expression of CCL2 and CCL22, while the expression levels of CXCL10 and CCL5 were unchanged[50]. These results indicate that HO-1 inhibits the phenotypic and functional maturation of DCs, and thus reduces the ability of DCs to stimulate CD4+ T cells[46], and the modulation of Th2 cytokine production by donor-derived DC may contribute to the comparative immune privilege of hepatic allografts[49,51].
HO-1 AS A POTENTIAL THERAPEUTIC TARGET IN LIVER DISEASES
Hepatitis C virus (HCV) represents a major public health burden in both industrialized and developing countries. HO-1 over-expression has been observed to decrease HCV replication, reduce pro-oxidant production in replicon cells, and increase resistance to oxidative injury[52]. These findings strongly suggest potential therapeutic roles for HO-1[53]. Nevertheless, iron was recently shown to inhibit the activity of HCV RNA-dependent RNA polymerase, as well as inhibiting replication in non-structural replicons[54]. However, a potential antiviral role for iron must be considered in the context of clinical data showing that mild increases in iron storage correlate with progressive HCV liver disease and hepatic decompensation. Therefore, a potential role for biliverdin and carbon monoxide as inhibitors of viral replication (via their antioxidative effects) requires further study[53].
Recent advances in molecular biology have provided new approaches to reducing hepatic IRI with gene therapy[55]. Liver transplantation[56] (a commonly employed treatment for hepatic failure) is still a limited means of therapy and has many drawbacks, including high monetary costs for the patient and donor shortages[8]. Presently, there are a limited number of strategies for protecting liver cells from damage and degeneration[57]. Keeping in mind the increasing amount of experimental evidence demonstrating the antioxidant and anti-inflammatory effects of HO-1 products, induction of this enzyme (or its catalytic activity) by either natural or synthetic compounds may represent an effective strategy to intervene in liver carcinogenesis and other hepatic disorders[11]. A wide variety of chemopreventive agents that elicit cytoprotective, anti-inflammatory and/or antioxidant effects by the induction of HO-1 expression have been recognized[58]. While continuing efforts should be geared towards the search for novel hepatoprotective[59] agents targeting HO-1, certain synthetic or naturally occurring substances already identified appear to induce HO-1 expression as part of their chemoprevention/chemoprotective effects against hepatocarcinogenesis[37] and should be further investigated.
Constitutive HO-1 expression by the Kupffer cells of the liver has been shown to be beneficial in hepatic IRI treatment. The consequences of HO-1 production have been shown to have antioxidant, anti-inflammatory, anti-proliferative, anti-apoptotic, immunomodulatory and vasorelaxant effects[60].
CONCLUSION
The HO-1 system may play an important role in various pathophysiological conditions. Thus, pharmacological modulation of the HO-1 system may represent an effective and cooperative strategy to mitigate liver injury in HCV, although the exact effects are likely to differ depending on the type of disease. Therefore, down-regulating the HO-1 system by pharmacological or genetic means may represent a new therapeutic approach for the management of liver injury. A comprehensive understanding of the mechanisms underlying the observed effects of HO-1 and its products will be needed before their use can be evaluated in clinical applications for the prevention and/or treatment of human diseases, such as HCV.
There are wide-ranging biological implications for HO-1 expression, extending far beyond its initial identified role as the rate-limiting enzyme in heme degradation. The tight regulation of HO-1 gene transcription and the lack of high levels of constitutive expression reflect the great potential of this “protective” gene for inducing injury under certain conditions. Each HO-1 reaction product can be harmful and (in sufficient quantities) cause tissue injury, with resulting enhanced susceptibility to oxidative stress. Thus, HO-1 is not exclusively cytoprotective or cytotoxic, but it is involved in a complex equilibrium of inflammatory and reparative cellular processes.