Published online Oct 27, 2013. doi: 10.4254/wjh.v5.i10.528
Revised: September 5, 2013
Accepted: September 13, 2013
Published online: October 27, 2013
The liver has a central role in regulating inflammation by its capacity to secrete a number of proteins that control both local and systemic inflammatory responses. Chronic inflammation or an exaggerated inflammatory response can produce detrimental effects on target organs. Chronic hepatitis C virus (HCV) infection causes liver inflammation by complex and not yet well-understood molecular pathways, including direct viral effects and indirect mechanisms involving cytokine pathways, oxidative stress and steatosis induction. An increasing body of evidence recognizes the inflammatory response in chronic hepatitis C as pathogenically linked to the development of both liver-limited injury (fibrosis, cirrhosis and hepatocellular carcinoma) and extrahepatic HCV-related diseases (lymphoproliferative disease, atherosclerosis, cardiovascular and brain disease). Defining the complex mechanisms of HCV-induced inflammation could be crucial to determine the global impact of infection, to estimate progression of the disease, and to explore novel therapeutic approaches to avert HCV-related diseases. This review focuses on HCV-related clinical conditions as a result of chronic liver and systemic inflammatory states.
Core tip: Chronic hepatitis C virus (HCV) infection causes liver inflammation by complex and not yet well-understood molecular pathways. HCV-induced inflammation has a significant clinical impact on development of both hepatic disease and HCV-associated extrahepatic manifestations. Knowledge of the complex mechanisms underlying HCV-related inflammation and development of disease as well as individuation of relevant markers of inflammation could be of importance for understanding disease progression, predicting prognosis and, possibly, conceiving new therapeutic approaches targeting the different steps of the inflammatory response.
- Citation: Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, Romano C, Adinolfi LE. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol 2013; 5(10): 528-540
- URL: https://www.wjgnet.com/1948-5182/full/v5/i10/528.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i10.528
Inflammation is a crucial physiological pathway in the homeostatic altered response to a number of exogenous distressing stimuli; however, a chronic inflammatory state or an excessive inflammatory response can produce deleterious effects. The liver plays a central role in regulating inflammation by its capacity to secrete a number of proteins that control both local and systemic inflammatory responses. A number of liver cells, including hepatocytes, hepatic stellate cells (HSCs), Kuppfer cells (KCs), bile duct epithelial cells and sinusoidal endothelial cells, are implicated in the synthesis and secretion of, and response to, inflammatory stimuli. Systemic inflammation is mediated by a number of cytokines released by macrophages as well as by adipocytokines secreted from adipose tissue[1]. The hepatic response to inflammation is characterized by the release of resident soluble mediators that also enter the circulation resulting in a systemic response to hepatic injury.
Chronic hepatitis C virus (HCV) infection[2] causes liver inflammation by complex and not yet well-understood molecular pathways. HCV-induced inflammation has a significant clinical impact on development of both hepatic disease and HCV-associated extrahepatic manifestations. Knowledge of the complex mechanisms underlying HCV-related inflammation and development of disease could be of importance for understanding disease progression, predicting prognosis and, possibly, conceiving new therapeutic approaches targeting the different steps of the inflammatory response. In this respect, it is important to underline that HCV clearance by standard of care does not always mean recovery of all associated pathological conditions.
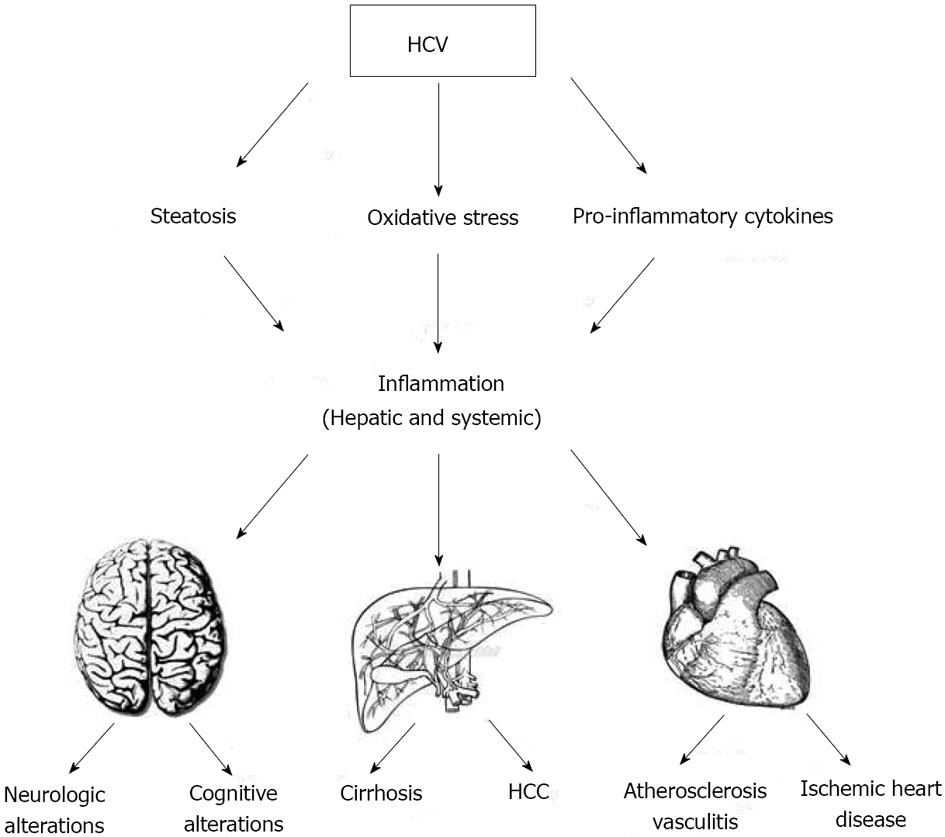
The aim of the present study was to review the current knowledge on the hepatic and systemic clinical impact of chronic HCV infection as a consequence of local and systemic inflammation. Figure 1 schematically depicts HCV-related factors which give rise to inflammation and its associated clinical conditions.
HCV consists of a single-stranded RNA genome encoding a single polyprotein, which is post-translationally processed into single known proteins, 4 structural (C, E1, E2 and p7) and 6 non-structural (NS2, NS3, NS4A, NS4B, NS5A and NS5B)[3]. Some of these proteins have a role in starting and maintaining chronic inflammation. NS5A, for instance, promotes inappropriate upregulation of cyclooxygenase-2 (COX-2)[4], which is an inducible COX isozyme able to contribute to chronic inflammation and fibrosis through production of various prostaglandins. Suppression of COX-2 protein levels has been reported to be accompanied by suppression of HCV replication[5].
Chronic HCV infection is characterized by the presence and activation of inflammatory cells in the liver, which are responsible for the persistent inflammatory state contributing to liver fibrosis and damage[6,7]. In addition to local inflammation in the liver, a concomitant low-grade systemic inflammation has been supposed in several studies, as suggested by increased pro-inflammatory cytokine serum levels and activation of blood monocytes in individuals with chronic HCV infection[8,9]. Moreover, chronic HCV infection has been associated with oxidative stress (OXS) activation, which may play a role in development of local and systemic inflammation.
A number of proinflammatory cytokines appears to be activated in chronic HCV infection. The important role of interleukin-1β (IL-1β) has recently been emphasized[10]. Specifically, HCV has been shown to induce IL-1β production and secretion; hepatic macrophages have been found to produce high concentrations of IL-1β within HCV-infected liver; IL-1β has been demonstrated to play a critical role in inducing liver inflammation and disease progression. HCV-activated Nod-like receptor P3 (NLRP3) inflammasome has been found to be able to induce production of IL-1β, which, in turn, stimulates synthesis of pro-inflammatory cytokines and chemokines, other than gene expression linked to HCV disease severity[11,12].
A cross-talk between HSCs and HCV-infected hepatocytes has been described, which appears to be a key point in HCV-related inflammation. HCV-infected hepatocytes seem to be able to ignite inflammation in response to HSCs. Indeed, in in vitro co-cultures of HCV-infected hepatocytes and HSCs, IL-1β secreted by HSCs was shown to induce production of several pro-inflammatory cytokines and chemokines, such as IL-6, IL-8, and macrophages inflammatory proteins (MIP-1α and MIP-1β), by hepatocytes[13]. Moreover, HCV-related proteins (NS3, NS4, NS5) have been reported to trigger human KCs to produce inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-1β[14].
Hyperproduction of certain cytokines may cause unbalance leading to specific consequences in the short and long term period. For instance, a higher TNF-α/IL-10 ratio has been found in patients with severe liver disease and hepatocellular carcinoma (HCC)[15]. Furthermore, a significant correlation between TNF-α and the degree of hepatic inflammation, expressed as histologic activity index (HAI), has been reported; likewise, TGF-β levels have been found to be significantly correlated with histologic fibrosis score[16]. TNF-α and TGF-β levels have been found to be simultaneously increased according to the severity of inflammation and fibrosis[17].
HCV has also been shown to activate toll-like receptors (TLRs), molecules implicated in the production of proinflammatory cytokines in the cells of innate immunity. Specifically, HCV core protein and NS3 protein have been demonstrated to activate TLR2[18,19]. TLRs activate, in turn, NF-κB with subsequent transcription of inflammatory genes. Recent data have revealed increased expression of microRNA-155 and TNF-α production in monocytes, following TLR4 and TLR8 stimulation by HCV core, NS3, and NS5 proteins in chronic HCV infection[20].
In patients with chronic hepatitis C (CHC), intestinal bacterial overgrowth has been reported, which is usually followed by bacterial translocation and elevated blood concentrations of endotoxin (LPS)[21]. LPS induces local and systemic inflammation and is associated with progression to end-stage liver disease. Indeed, HCV-infected patients have been shown to harbor high plasma levels of LPS, intestinal fatty acid binding protein (a marker of enterocyte death), sCD14 (produced by LPS-activated monocytes) and IL-6. Markers of inflammation are remarkably elevated in individuals with severe disease than in those with minimal fibrosis[21]. TNF-α, one of the main cytokines produced following LPS stimulation, seems to be able to induce liver damage by TNF-receptor 1-mediated apoptosis[22]. Moreover, HSCs, which also express functional receptors for both bacterial endotoxin, including TLRs (particularly TLR4), and peptidoglycan recognition proteins, are able to develop a pro-inflammatory phenotype following LPS exposure[23].
CHC infection has been found to be associated with both hepatic and systemic OXS. Increased OXS in hepatitis C has been observed to be significantly linked to chronic inflammation[24,25], although iron overload, liver damage, and proteins encoded by HCV may also play a part. In CHC, OXS results from loss of equilibrium between reactive oxygen species (ROS) and antioxidant defense. ROS and reactive nitrogen species (RNS) are critically involved in the creation of oxidative stimuli required for physiologic hepatocyte homeostasis[26]. OXS-induced damage affects hepatocytes, endothelial cells, KCs, and HSCs through inflammation, ischemia, apoptosis, necrosis and regeneration[27,28]. ROS play an important role in fibrogenesis through increased proliferation of hepatic stellate cells as well as TGF-β and collagen synthesis[29]. Moreover, ROS interfere with repair of damaged DNA, thus making cells more susceptible to spontaneous or mutagen-induced alterations[30]. Increased ROS/RNS levels are associated with decreased antioxidant levels. Therefore, increased production of reactive oxygen and nitrogen species, along with decreased antioxidant defense, favor development and progression of hepatic and extrahepatic complications of HCV infection.
A meaningful role for liver steatosis as “contact point” between OXS and liver damage in CHC patients infected by HCV genotype non-3 has been demonstrated[31]. Hepatic steatosis is a striking feature of HCV infection, having been reported in more than 50% of HCV-infected patients[32]. Both HCV and metabolic conditions, e.g., visceral obesity and insulin resistance (IR), play a role in the development of steatosis[32]. Recently, we have demonstrated that genetic and antropometric host factors may also be implicated in the development of steatosis in CHC patients. Indeed, the patatin-like phospholipase domain-containing 3 gene (PNPLA3) p.I148M polymorphism has been reported to influence the development of liver steatosis[33,34]; on the other hand, visceral obesity can increase the association between PNPLA3 p.I148M with liver steatosis[34].
Obesity and liver steatosis are commonly observed among patients with CHC and are risk factors for both inflammation and increased hepatic fibrosis. Obesity is associated with a low-grade, chronic inflammatory response that may contribute to pathogenesis of obesity-related comorbidities. Obesity and steatosis are associated with increased expression of typical inflammatory markers, such as IL-6 and TNF-α[35]. Hepatic TNF-α has been correlated with increased inflammatory activity, hepatic fibrosis, and liver injury in chronic HCV[35]. In obese-HCV subjects, an enhanced T helper-1 cytokine profile has been associated with hepatocellular injury; accordingly, an increased expression of T cell chemoattractants (IP-10 and MCP-1) in the liver of obese HCV subjects, along with increased inflammatory cell recruitment and CD3 expression, have been reported[36].
Visceral obesity plays an important role in the regulation of glucose and lipid metabolism in CHC patients. In obese HCV-infected patients, elevated levels of proinflammatory cytokines (namely, TNF-α and IL-6), able to inhibit insulin signalling, and reduced adiponectin levels[37], with consequent development of liver steatosis and IR, have been reported. Obesity has also been associated with increased liver fibrosis and poor response to antiviral treatment[38]. HCV also works by promoting the development of steatosis. Experimental models have demonstrated that expression levels of HCV core protein have profound effects on liver inflammation, steatosis and fibrosis[39]. Generally, IR precedes and has a key role in the development of hepatic steatosis in HCV infection. IR induces steatosis by overflow of substrates to the liver, increased de novo lipogenesis, and decreased fatty acid oxidation.
Steatosis has been associated with increased production of reactive oxygen species, which promotes lipid peroxidation and resulting hepatic stellate cell activation. Moreover, steatosis-induced hepatic inflammation has been shown to increase production of several proinflammatory and profibrotic cytokines. In addition, steatosic liver is more sensitive to TNF-α-mediated inflammation, liver injury, and apoptosis[40].
The mechanisms by which HCV-associated steatosis induces fibrosis are complex and not fully understood. OXS, proinflammatory cytokines, IR, and apoptosis seem to play an important role in the development of fibrosis associated with steatosis. Several reports, including a meta-analysis, have demonstrated that increased liver inflammation was associated with accelerated fibrosis in HCV patients with steatosis[41].
HCV infection portends an increased risk to develop type 2 diabetes (DM). HCV-infected patients with DM generally harbor a more severe chronic hepatitis and have a higher risk of developing hepatic cirrhosis and its complications (i.e., hepatic encephalopathy, ascites), a shorter life expectancy, and a higher risk of HCC[42]. During IR, several inflammatory cytokines and lipid metabolites, such as free fatty acids, interfere with normal insulin signaling and promote DM[43]. TNF-α has been identified as the key molecule promoting development of IR and diabetes during HCV infection[44]. In addition, TNF-α is known to increase ROS production; hyperglycemia has also been recognized as a factor leading to OXS; eventually, increased generation of reactive species triggers a signaling cascade capable to alter the activity of insulin receptor substrates, leading to IR[45].
Cannabinoid receptors (CB) are found in high concentration in many organs, including the liver. There are two G protein-coupled CB, CB1 and CB2[46]. CB1 is found in high concentration in the brain, but is also present in many peripheral tissues such as the liver, adipose tissue, and gut. CB2 is found primarily in the immune system, but is also expressed in peripheral tissues including the liver[47]. Recent studies have suggested involvement of the endocannabinoid system in liver diseases. Specifically, CB1 seems to be upregulated in patients with CHC[48], while CB2 seems to have hepatoprotective properties in alcoholic liver disease[49] and in obese children[50]. Recently, we have evaluated the role of a functional polymorphism of the cannabinoid receptor type 2 in a cohort of CHC patients[51]. Our data demonstrated that HCV patients carrying the CB2-63 QQ variant polymorphism had advanced liver disease, higher serum ALT values, and higher necroinflammatory activity than those with the CB2-63 RR and QR variants. Moreover, the CB2-63 QQ variant and fibrosis score were identified as independent factors associated with higher scores of necro-inflammatory activity (HAI > 8). Thus, the data suggest that the CB2-63 QQ variant is associated with more severe hepatic necroinflammation in anti-HCV-positive patients, thus confirming a role for the CB2 receptor in HCV-associated inflammation and cellular proliferation as well[51].
When inflammation fails to resolve an acute infection and chronic inflammation ensues, this process can lead to accumulation of fibrotic tissue. Fibrosis can be considered the result of unbalanced extracellular matrix production and degradation; its generation involves complex mechanisms including fibrogenesis, proliferation, contractility, chemotaxis, matrix degradation, and cytokine release[52].
The majority of the studies carried out in CHC patients have identified hepatic inflammation as the key pathological substrate driving fibrosis development[53]. On the other hand, therapeutic amelioration of hepatic inflammation in CHC has been associated with decreased fibrosis progression[54].
Mechanisms involved in the interrelationship between inflammation and fibrosis are complex and not completely understood. Cytokines play an important role in inflammation, regeneration, and fibrosis during chronic HCV infection; availability of cytokine patterns reflecting different stages of liver disease would be extremely useful in the clinic. In this regard, hepatocyte growth factor is a specific marker of liver cirrhosis[55]. Other proinflammatory small molecules, like ROS and other insoluble mediators, such as the hepatic neomatrix during wound healing, appear to play a role in the development of liver fibrosis[29].
Hepatic inflammation mediates fibrogenesis also in patients with liver steatosis and CHC. In a meta-analysis including large and geographically different groups of CHC patients, steatosis was confirmed to be significantly and independently associated with progression of hepatic fibrosis through liver inflammation[41].
The role of miR-122 in CHC progression has been recently described. Circulating miR-122 serum levels appeared to be elevated at early stages of disease with high inflammatory activity and low fibrosis levels, while they decreased in the presence of severe fibrosis, probably due to loss of liver cells[56].
Genetic factors have been hypothesized to favor development and progression of fibrosis. In chronic HCV infection, a higher frequency of gene polymorphisms for key inflammatory mediators has been found in association with advanced disease[57]; specifically, definite polymorphisms in CCR5, RANTES, and MCP-1 alleles may predispose CHC patients to a higher degree of liver inflammation and advanced fibrosis[58,59]. COX-2 has too been found involved in inflammation; its -1195GG genotype has been recognized as a genetic marker for liver disease progression in Japanese patients with CHC[60].
Chronic HCV infection is a major risk factor for HCC development worldwide. CHC can progress to HCC through fibrosis and cirrhosis. HCC is the 3rd leading cause of cancer death worldwide; it typically arises in patients with chronic inflammation and cirrhosis in 90% of cases[61].
At present, a large body of data links HCV infection, inflammation, free radical production, and carcinogenesis in CHC patients[62-66].
Although HCC pathogenesis in the setting of HCV infection has been subjected to extensive investigations, no conclusive data are available. However, both HCV-induced chronic inflammation and cytokines, involved in fibrosis development and liver cell proliferation, are considered as major pathogenic mechanisms. In contrast to HBV, HCV does not integrate into the host genome and does not have a reverse transcriptase. In infected subjects, both viruses are known to trigger an immune-mediated inflammatory response which either clears infection or slowly destroys the liver[67]. Thus, HCV can be carcinogenic by damaging liver tissue through chronic inflammation. This latter condition predisposes susceptible cells to neoplastic transformation.
During inflammation, a variety of proinflammatory cytokines, chemokines, growth factors and inflammatory enzymes are released[68]. Inflammation may induce cellular DNA mutations through oxidative/nitrosative stress[30]. Free radical production and oxidative genomic injury trigger the cascade of epigenetic (altered DNA methylation), genomic (mutations), and post-genomic (protein oxidation and cytokine synthesis) events leading to HCC[69]. Initially, ROS interact directly with DNA, damaging specific genes controlling cell growth and differentiation, cell cycle, apoptosis, lipid peroxidation and DNA damage repair[70,71]. Moreover, HCV-infected patients have been shown to display increased lipid peroxidation levels[72]. An increased oxidative stress gene response in patients with HCV-related fibrosis and cirrhosis has also been demonstrated by microarray and proteomics studies[73]. Thus, during chronic HCV infection, increased ROS, in part due to inflammation, may impair repair of damaged DNA, resulting in higher cell susceptibility to genetic alterations; this promotes the development and progression of HCC[69,74]. Inflammatory cells have been reported to release cytokines, chemokines, nitric oxide, particularly, an inducible isoform of nitric oxide synthase (iNOS), and NO-derived RNS, which too can cause DNA damage and cellular proliferation[75-77].
Chief elements linking inflammation to cancer through oxidative/nitrosative stress appear to be prostaglandins and cytokines. Capone et al [78] evaluated the serum levels of 50 different cytokines, chemokines, and growth factors in 26 patients affected by HCC superimposed on chronic HCV hepatitis and liver cirrhosis. A number of proinflammatory molecules (IL-1α, IL-6, IL-8, IL-12p40, GM-CSF, CCL27, CXCL1, CXCL9, CXCL10, CXCL12, β-NGF) were found to be significantly increased in HCC patients compared to healthy controls. Interestingly, IL-8 and IL-6 concentrations were demonstrated to significantly correlate with a larger tumor burden[78].
HCV tropism for lymphatic tissue has been conclusively demonstrated. Since infected lymphocytes constitute the main reservoir of the virus, this could explain the development of several lymphoproliferative disorders. Mixed cryoglobulinemia (MC), a B-cell lymphoproliferative disease, has been commonly reported in association with chronic HCV infection; it may progress to overt lymphoma in some subjects.
MC is featured by the presence of serum cryoglobulins, i.e., immunoglobulins (Igs) capable to reversibly precipitate at low temperatures and to form immune complexes consisting of complement, monoclonal and polyclonal Igs (type II MC), or polyclonal Igs (type III MC), with specificity against HCV antigens. Monoclonal IgM with rheumatoid factor (RF) activity is typically detected in type II MC. Viral antigens and HCV-RNA have been consistently isolated from immune complexes, thus confirming the role of the virus in MC pathogenesis[79-81].
Cryoglobulinemia may cause vasculitis due to precipitation of cryoglobulin-containing immune complexes in small- and medium-sized blood vessels. Plugging and thrombosis of small vessels and a systemic inflammatory syndrome are responsible for the many clinical signs and symptoms of MC, including livedo, purpura (particularly on dependent areas), ulcers, arthralgia, arthritis, etc. Vasculitis may also involve the kidneys, the gastrointestinal tract, the peripheral nervous system, and/or other body compartments.
Cryoglobulins are detectable in about 50% of CHC patients; symptomatic disease is usually limited to about 15% of cases. However, symptomatic MC has been found to be associated with a poor prognosis[82]; about 10% of patients are prone to develop B-cell malignancies, especially B-cell non-Hodgkin lymphoma[83-85].
Mechanisms responsible for progression of HCV infection to cryoglobulinemia and other lymphoproliferative disorders are still unknown. Cryoglobulins result from a complex interaction between the virus and the host. In this regard, HCV core protein has been hypothesized to play an important role, having been found within the cryoprecipitate[79,86]. Indeed, HCV core protein has been reported to interact with the globular domain of C1q complement receptor, suggesting a role in complement activation[87,88]. Specifically, HCV core protein may be hypothesized to boost inflammation by enhancing complement activation. Finally, certain motifs on HCV/E2 glycoprotein and HCV/NS3 region have been proposed to act as molecular mimickers of specific Ig portions[89,90].
An association between chronic HCV infection and atherosclerosis has been frequently reported in the literature[91-94]. Chronic HCV infection has also been reported in association with coronary artery disease[94-96] and stroke[97].
As previously stated, HCV infection is known to promote immune stimulation, cytokine production, and chronic inflammation[98,99]. HCV has been described to be able to create an inbalance between Th1 and Th2 cytokines, thus altering the equilibrium between cellular immunity, promoted and maintained by IL-2, TNF-α and interferon γ (IFN-γ), and humoral immunity, sustained by IL-4, IL-5, IL-6 and IL-10[100]. HCV-driven prevalence of inflammatory cytokines can be supposed to contribute to development of cardiovascular disease due to several effector mechanisms, including enhancement of intracellular adhesion molecules, expression of anti-endothelium antibodies, and generation of OXS and IR[91].
Recently, the interrelationships between HCV infection, atherosclerosis, and immune response have been carefully examined. In the Heart and Soul Study[101], patients with HCV infection were demonstrated to have higher TNF-α levels and a higher risk of cardiac failure and death than patients without HCV infection. Mostafa et al[102] found a more pronounced intima-media thickness in HCV-positive patients than controls; Olivera et al[103] reported an intermediate cardiovascular risk, as measured by the Framingham score, a higher levels of proinflammatory cytokines (IL-6 and TNF-α), and a higher ratio of proinflammatory/anti-inflammatory cytokines (TNF-α/IL10 and IL-6/IL-10) in non-obese, non diabetic, HCV-infected patients with respect to controls[103].
Cytokine imbalance has also been associated with development of IR in HCV-infected patients. A high TNF-α/adiponectin ratio has been found correlated with IR and atherosclerosis development[104]. Moreover, elevated levels of inflammatory biomarkers, such as matrix metalloproteinase-9, intercellular adhesion molecule-1, and oxidized low-density lipoproteins, have been observed in patients with CHC. Besides the role of HCV in the development of chronic inflammation involving the arteries, HCV RNA sequences and intermediate replicative forms have been detected within carotid plaques, thus suggesting in situ viral replication and consequent local pro-atherogenic action[105,106].
A histological feature of CHC is liver steatosis[32]. As for nonalcoholic fatty liver disease (NAFLD)[107-109], HCV-related steatosis can be considered a cardiometabolic risk factor[94]. Patients with chronic HCV infection have been shown to have a higher prevalence of carotid atherosclerosis than both healthy controls and NAFLD patients; moreover, it may detect at a younger age, particularly in the presence of liver steatosis. Indeed, like steatosis, HCV viral load has been found to be independently associated with carotid atherosclerosis[94]. In HCV patients, atherosclerosis was shown to be independently associated with elevated systemic inflammatory markers, such as C reactive protein and fibrinogen[94]. HCV and HCV-related steatosis have been hypothesized to favor the development of atherosclerosis independently of known risk factors such as hypercholesterolemia, smoking and hypertension, through multiple cardiometabolic risk factors, including inflammatory cytokines, hyperhomocysteinemia, hypoadiponectinemia, IR, and features of the metabolic syndrome[94]. Indeed, HCV patients have been reported to have high levels of hepatic and systemic markers of inflammation, particularly ESR, CRP, fibrinogen, and N-terminal pro-brain natriuretic peptide[110]. Overall, available data point to chronic HCV infection as a factor capable to increase the risk of atherosclerosis and vascular disease through systemic inflammation[110-113].
About 50% of patients with HCV infection suffer from neuropsychiatric symptoms, “brain fog”, weakness, fatigue, along with some degree of quality of life impairment, regardless of liver disease severity[114] and HCV replication[115]. A direct effect of HCV on the brain or neurotoxic effects of HCV-related systemic inflammation have been hypothesized.
HCV neuroinvasion has been described[116,117], which could be the cause of neurological disturbances in HCV patients. Evidence suggests that the brain, but not the peripheral nerves or skeletal muscles, is a permissive site for viral replication, as inferred by the detection of replicative intermediate forms of HCV RNA and viral proteins within the central nervous system[118]. Additional mechanisms, contributing to neurological dysfunction, are possibly related to the effects of circulating inflammatory cytokines and chemokines reaching the brain through an altered blood-brain barrier[119]. Evidence for a direct role for peripheral proinflammatory cytokines in determining impairment of neurocognitive function has been obtained from animal models. Specifically, increased peripheral IL-1 and IL-6 have been found to correlate with increased levels of the same cytokines in the prefrontal cortex and hyppocampus[120]. HCV within the brain seems to be able to also induce a local inflammatory response, since macrophages infected in vitro with HCV are able to produce TNF-α and IL-8[121-123].
Neurologic alterations during HCV infection include a broad spectrum of manifestations, ranging from cerebrovascular events to autoimmune syndromes. In HCV infected patients, acute cerebrovascular events, such as ischemic stroke, transient ischemic attacks, lacunar syndromes have been reported, particularly in cryoglobulinemic patients[124-127].
A consistent proportion of HCV-infected patients complain of chronic depression and anxiety. Moreover, about 15% of patients suffer from recurrent depression[128]. At present, definitive conclusions regarding the pathogenesis of cognitive dysfunction, fatigue, and depression in chronic HCV infection cannot be drawn. Many studies seem to suggest a possible direct role for HCV, due to its ability to replicate within the brain[118]. In addition, brain microvascular endothelial cells have been recently demonstrated to support HCV tropism and replication. HCV has been shown to induce apoptosis in these cells, with consequent alterations in the blood-brain barrier, microglia activation, and, eventually, diffusion of inflammatory cytokines and chemokines into the brain[129].
Because of the role of inflammation in the progression of CHC and its systemic manifestations, an anti-inflammatory approach to control and eventually arrest disease would result theoretically useful. For instance, interruption of COX-2 signaling seems to be a possible approach for controlling HCV replication and associated diseases[6,130]. In this regard, non-toxic concentrations of aqueous extracts of the edible seaweed Gracilaria tenuistipitata have been successfully tried in vitro[130].
Considering the effects of LPS on circulating monocytes and resident KCs, attenuation of microbial translocation and its inflammatory consequences may be deemed advantageous to improve clinical outcome in HCV infection[21].
Therapy for cryoglobulinemia should encompass three objectives: elimination of HCV infection, control of B lymphocyte proliferation, and symptomatic treatment of immune-complex disease. Standard of care for HCV treatment with pegylated interferon plus ribavirin should be considered as the first-line therapeutic option in patients with mild to moderate HCV-related MC; a prolonged treatment (up to 72 wk) may be considered in the case of virological non-responders showing clinical and laboratory improvements. Rituximab (RTX), a chimeric moAb specific for CD20 antigen used for treatment of autoimmune and lymphoproliferative disorders, should be considered in patients with severe vasculitis and/or skin ulcers, peripheral neuropathy, or glomerulonephritis[131]. In a recent study carried out in patients with HCV-related cryoglobulinemia, triple therapy with pegylated IFN-α, ribavirin, and RTX was shown to yield a a complete response in 54.5% of patients, as opposed to 33.3% in those who received only pegylated IFN-α and ribavirin (P < 0.05)[132].
Several studies investigating the effects of selenium, glycyrrhizin, polyprenoic acid, vitamin E, and vitamin C have demonstrated that comsumption of higher amounts of antioxidants was not associated with a decreased risk of developing HCC. At present, the best strategy to prevent HCC still lies in antiviral treatment of chronic HCV infection[133]. In the future, novel therapeutic strategies targeting molecules involved in HCV-induced OXS may be expected to enter clinical practice as soon as these mechanisms will be progressively unraveled.
IL-1β is strictly involved in inflammation[134]. Drugs blocking IL-1β seem to be able to control inflammation irrespective of the molecular pathways activated by this cytokine. Canakinumab, a human monoclonal antibody selectively specific for IL-1β, has been shown to reduce the levels of many inflammatory biomarkers. Canakinumab is generally well tolerated and could be theoretically conceived for use in the secondary prevention of cardiovascular disease[134,135].
Pentoxyfilline (PTX), a nonspecific phosphodiesterase inhibitor with anti-inflammatory and anti-fibrogenic properties, has been successfully used in different models of liver disease, including non-alcoholic steatohepatitis[136], inflammation[137], fibrosis/cirrhosis[138], alcoholic liver disease[139] and endotoxinemia[140]. Its beneficial effects have been associated with downregulation of TNF-α, IL-1, IL-6, TGF-β, IFN-γ along with downregulation of stellate cell activation, procollagen I mRNA expression[141], cell proliferation, and extracellular matrix synthesis[142]. PTX has been shown to affect the expression of several pro-inflammatory cytokines in both liver and peripheral blood mononuclear cell of patients with hepatitis C, particularly TNF-α and IL-1β production; thus, PXT could also be considered for inhibition of inflammation in patients with CHC[143].
The endocannabinoid system can be considered a promising target for treatment of CHC, for the reasons stated above[51]. Indeed, the efficacy of peripherally restricted CB1 antagonists on fibrosis has been already validated in preclinical models of NAFLD. Similarly, CB2 receptor is currently considered as a promising anti-inflammatory and antifibrogenic target, although clinical development of CB2 agonists is still awaited[144].
A growing body of evidence supports the essential role of CHC-related inflammation in the pathogenesis of hepatic and extrahepatic HCV-related disease. Defining the mechanisms of HCV-induced hepatic inflammation is paramount for devising attractive approaches to avert HCV-related liver disease. The way to go is still long and difficult, however, understanding the pathogenesis of HCV-related inflammation may help discover novel elements to be targeted for more effective treatment of CHC and its complications. In this regard, current knowledge suggests exploring therapeutic options targeting “inflammation” in CHC in future clinical trials, particularly in nonresponders to standard antiviral treatment.
P- Reviewer Dang SS S- Editor Song XX L- Editor A E- Editor Wu HL
| 1. | Kasprzak A, Zabel M, Biczysko W, Wysocki J, Adamek A, Spachacz R, Surdyk-Zasada J. Expression of cytokines (TNF-alpha, IL-1alpha, and IL-2) in chronic hepatitis C: comparative hybridocytochemical and immunocytochemical study in children and adult patients. J Histochem Cytochem. 2004;52:29-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 583] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 3. | Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 490] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Núñez O, Fernández-Martínez A, Majano PL, Apolinario A, Gómez-Gonzalo M, Benedicto I, López-Cabrera M, Boscá L, Clemente G, García-Monzón C. Increased intrahepatic cyclooxygenase 2, matrix metalloproteinase 2, and matrix metalloproteinase 9 expression is associated with progressive liver disease in chronic hepatitis C virus infection: role of viral core and NS5A proteins. Gut. 2004;53:1665-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Gretton S, Hughes M, Harris M. Hepatitis C virus RNA replication is regulated by Ras-Erk signalling. J Gen Virol. 2010;91:671-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Matsuzaki K, Murata M, Yoshida K, Sekimoto G, Uemura Y, Sakaida N, Kaibori M, Kamiyama Y, Nishizawa M, Fujisawa J. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Dalagiorgou G, Vassilaki N, Foka P, Boumlic A, Kakkanas A, Kochlios E, Khalili S, Aslanoglou E, Veletza S, Orfanoudakis G. High levels of HCV core+1 antibodies in HCV patients with hepatocellular carcinoma. J Gen Virol. 2011;92:1343-1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Heydtmann M. Macrophages in hepatitis B and hepatitis C virus infections. J Virol. 2009;83:2796-2802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 10. | Lapiński TW. The levels of IL-1beta, IL-4 and IL-6 in the serum and the liver tissue of chronic HCV-infected patients. Arch Immunol Ther Exp (Warsz). 2001;49:311-316. [PubMed] [Cited in This Article: ] |
| 11. | Burdette D, Haskett A, Presser L, McRae S, Iqbal J, Waris G. Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J Gen Virol. 2012;93:235-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 13. | Nishitsuji H, Funami K, Shimizu Y, Ujino S, Sugiyama K, Seya T, Takaku H, Shimotohno K. HCV infection induces inflammatory cytokines and chemokines mediated by the cross-talk between hepatocytes and stellate cells. J Virol. 2013;87:8169-8178. [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Hosomura N, Kono H, Tsuchiya M, Ishii K, Ogiku M, Matsuda M, Fujii H. HCV-related proteins activate Kupffer cells isolated from human liver tissues. Dig Dis Sci. 2011;56:1057-1064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Aroucha DC, do Carmo RF, Moura P, Silva JL, Vasconcelos LR, Cavalcanti MS, Muniz MT, Aroucha ML, Siqueira ER, Cahú GG. High tumor necrosis factor-α/interleukin-10 ratio is associated with hepatocellular carcinoma in patients with chronic hepatitis C. Cytokine. 2013;62:421-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Neuman MG. Cytokines--central factors in alcoholic liver disease. Alcohol Res Health. 2003;27:307-316. [PubMed] [Cited in This Article: ] |
| 17. | Neuman MG, Schmilovitz-Weiss H, Hilzenrat N, Bourliere M, Marcellin P, Trepo C, Mazulli T, Moussa G, Patel A, Baig AA. Markers of inflammation and fibrosis in alcoholic hepatitis and viral hepatitis C. Int J Hepatol. 2012;2012:231210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Bala S, Tilahun Y, Taha O, Alao H, Kodys K, Catalano D, Szabo G. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J Transl Med. 2012;10:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, Kleiner DE, Deeks SG, Liang TJ, Heller T. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220-1230, 1230.e1-e3. [PubMed] [Cited in This Article: ] |
| 22. | Shimizu S, Yamada Y, Okuno M, Ohnishi H, Osawa Y, Seishima M, Moriwaki H. Liver injury induced by lipopolysaccharide is mediated by TNFR-1 but not by TNFR-2 or Fas in mice. Hepatol Res. 2005;31:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Brun P, Castagliuolo I, Pinzani M, Palù G, Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571-G578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Yadav D, Hertan HI, Schweitzer P, Norkus EP, Pitchumoni CS. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2634-2639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Choi J, Forman HJ, Ou JH, Lai MM, Seronello S, Nandipati A. Redox modulation of the hepatitis C virus replication complex is calcium dependent. Free Radic Biol Med. 2006;41:1488-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Sies H. Oxidative stress: introductory remarks. London: Academic Press 1985; . [Cited in This Article: ] |
| 27. | Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 539] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 28. | Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: Part I. General considerations and redox biology in hepatitis. J Surg Res. 2010;162:95-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 457] [Cited by in F6Publishing: 438] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Farinati F, Cardin R, Bortolami M, Burra P, Russo FP, Rugge M, Guido M, Sergio A, Naccarato R. Hepatitis C virus: from oxygen free radicals to hepatocellular carcinoma. J Viral Hepat. 2007;14:821-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Vidali M, Tripodi MF, Ivaldi A, Zampino R, Occhino G, Restivo L, Sutti S, Marrone A, Ruggiero G, Albano E. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J Hepatol. 2008;48:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 813] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 33. | Valenti L, Rumi M, Galmozzi E, Aghemo A, Del Menico B, De Nicola S, Dongiovanni P, Maggioni M, Fracanzani AL, Rametta R. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2011;53:791-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 34. | Zampino R, Coppola N, Cirillo G, Boemio A, Pisaturo M, Marrone A, Macera M, Sagnelli E, Perrone L, Adinolfi LE. Abdominal fat interacts with PNPLA3 I148M, but not with the APOC3 variant in the pathogenesis of liver steatosis in chronic hepatitis C. J Viral Hepat. 2013;20:517-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Jonsson JR, Barrie HD, O’Rourke P, Clouston AD, Powell EE. Obesity and steatosis influence serum and hepatic inflammatory markers in chronic hepatitis C. Hepatology. 2008;48:80-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Palmer C, Corpuz T, Guirguis M, O’Toole S, Yan K, Bu Y, Jorgenson J, Talbot M, Loi K, Lloyd A. The effect of obesity on intrahepatic cytokine and chemokine expression in chronic hepatitis C infection. Gut. 2010;59:397-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 843] [Cited by in F6Publishing: 726] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 38. | Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003;38:639-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 39. | Chang ML, Yeh CT, Lin DY, Ho YP, Hsu CM, Bissell DM. Hepatic inflammation mediated by hepatitis C virus core protein is ameliorated by blocking complement activation. BMC Med Genomics. 2009;2:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Walsh MJ, Vanags DM, Clouston AD, Richardson MM, Purdie DM, Jonsson JR, Powell EE. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology. 2004;39:1230-1238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 42. | Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:665-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Knobler H, Schattner A. TNF-α, chronic hepatitis C and diabetes: a novel triad. QJM. 2005;98:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 45. | Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 400] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 46. | Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1519] [Cited by in F6Publishing: 1453] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 47. | Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Bátkai S. Endocannabinoids and the control of energy homeostasis. J Biol Chem. 2008;283:33021–33025. [PubMed] [Cited in This Article: ] |
| 48. | van der Poorten D, Shahidi M, Tay E, Sesha J, Tran K, McLeod D, Milliken JS, Ho V, Hebbard LW, Douglas MW. Hepatitis C virus induces the cannabinoid receptor 1. PLoS One. 2010;5:pii: e12841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, Pecker F, Mallat A, Lotersztajn S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 50. | Carrasquer A, Nebane NM, Williams WM, Song ZH. Functional consequences of nonsynonymous single nucleotide polymorphisms in the CB2 cannabinoid receptor. Pharmacogenet Genomics. 2010;20:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Coppola N, Zampino R, Bellini G, Macera M, Marrone A, Pisaturo M, Boemio A, Nobili B, Pasquale G, Maione S. Association Between a Polymorphism in Cannabinoid Receptor 2 and Severe Necroinflammation in Patients With Chronic Hepatitis C. Clin Gastroenterol Hepatol. 2013;May 22; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Ahmad W, Ijaz B, Gull S, Asad S, Khaliq S, Jahan S, Sarwar MT, Kausar H, Sumrin A, Shahid I. A brief review on molecular, genetic and imaging techniques for HCV fibrosis evaluation. Virol J. 2011;8:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53:318-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Morishima C, Shiffman ML, Dienstag JL, Lindsay KL, Szabo G, Everson GT, Lok AS, Di Bisceglie AM, Ghany MG, Naishadham D. Reduction in Hepatic Inflammation Is Associated With Less Fibrosis Progression and Fewer Clinical Outcomes in Advanced Hepatitis C. Am J Gastroenterol. 2012;Jun 12; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Costantini S, Capone F, Guerriero E, Maio P, Colonna G, Castello G. Serum cytokine levels as putative prognostic markers in the progression of chronic HCV hepatitis to cirrhosis. Eur Cytokine Netw. 2010;21:251-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 56. | Trebicka J, Anadol E, Elfimova N, Strack I, Roggendorf M, Viazov S, Wedemeyer I, Drebber U, Rockstroh J, Sauerbruch T. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol. 2013;58:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 57. | Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol. 2002;160:641-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Promrat K, McDermott DH, Gonzalez CM, Kleiner DE, Koziol DE, Lessie M, Merrell M, Soza A, Heller T, Ghany M. Associations of chemokine system polymorphisms with clinical outcomes and treatment responses of chronic hepatitis C. Gastroenterology. 2003;124:352-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Mühlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Schölmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Miyashita M, Ito T, Sakaki M, Kajiwara A, Nozawa H, Hiroishi K, Kobayashi M, Kumada H, Imawari M. Genetic polymorphism in cyclooxygenase-2 promoter affects hepatic inflammation and fibrosis in patients with chronic hepatitis C. J Viral Hepat. 2012;19:608-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 61. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1196] [Cited by in F6Publishing: 1285] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 62. | Huang YS, Hwang SJ, Chan CY, Wu JC, Chao Y, Chang FY, Lee SD. Serum levels of cytokines in hepatitis C-related liver disease: a longitudinal study. Zhonghua Yixue Zazhi (Taipei). 1999;62:327-333. [PubMed] [Cited in This Article: ] |
| 63. | Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 226] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 64. | Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer. 2009;125:2264-2269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 65. | Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 66. | Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381-2386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 627] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 67. | Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 607] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 68. | Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 69. | Pal S, Polyak SJ, Bano N, Qiu WC, Carithers RL, Shuhart M, Gretch DR, Das A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J Gastroenterol Hepatol. 2010;25:627-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, Fujii H, Rusyn I. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1182-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Adelman R, Saul RL, Ames BN. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci USA. 1988;85:2706-2708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 408] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 72. | Konishi M, Iwasa M, Araki J, Kobayashi Y, Katsuki A, Sumida Y, Nakagawa N, Kojima Y, Watanabe S, Adachi Y. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J Gastroenterol Hepatol. 2006;21:1821-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Diamond DL, Jacobs JM, Paeper B, Proll SC, Gritsenko MA, Carithers RL, Larson AM, Yeh MM, Camp DG, Smith RD. Proteomic profiling of human liver biopsies: hepatitis C virus-induced fibrosis and mitochondrial dysfunction. Hepatology. 2007;46:649-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 74. | Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1242] [Cited by in F6Publishing: 1149] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 75. | Hofseth LJ, Hussain SP, Wogan GN, Harris CC. Nitric oxide in cancer and chemoprevention. Free Radic Biol Med. 2003;34:955-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Lirk P, Hoffmann G, Rieder J. Inducible nitric oxide synthase--time for reappraisal. Curr Drug Targets Inflamm Allergy. 2002;1:89-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Szabó C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 344] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 78. | Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, Scala S, Izzo F, Castello G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw. 2010;21:99-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 30] [Reference Citation Analysis (0)] |
| 79. | Sansonno D, Lauletta G, Nisi L, Gatti P, Pesola F, Pansini N, Dammacco F. Non-enveloped HCV core protein as constitutive antigen of cold-precipitable immune complexes in type II mixed cryoglobulinaemia. Clin Exp Immunol. 2003;133:275-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 925] [Cited by in F6Publishing: 952] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 81. | Agnello V. Hepatitis C virus infection and type II cryoglobulinemia: an immunological perspective. Hepatology. 1997;26:1375-1379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Adinolfi LE, Utili R, Attanasio V, Zampino R, Ragone E, Tripodi MF, Ruggiero G. Epidemiology, clinical spectrum and prognostic value of mixed cryoglobulinaemia in hepatitis C virus patients: a prospective study. Ital J Gastroenterol. 1996;28:1-9. [PubMed] [Cited in This Article: ] |
| 83. | Vassilopoulos D, Calabrese LH. Hepatitis C virus infection and vasculitis: implications of antiviral and immunosuppressive therapies. Arthritis Rheum. 2002;46:585-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Donada C, Crucitti A, Donadon V, Tommasi L, Zanette G, Crovatto M, Santini GF, Chemello L, Alberti A. Systemic manifestations and liver disease in patients with chronic hepatitis C and type II or III mixed cryoglobulinaemia. J Viral Hepat. 1998;5:179-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Gabrielli A, Zhang ZX, Cherubini G, Candela M, Savoldi S, Manzin A, Clementi M, Amoroso A, Sallberg M. Differential humoral immune response against hepatitis C virus antigenic synthetic peptides in infected patients with and without mixed cryoglobulinaemia. Clin Exp Immunol. 1996;105:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 87. | Ghebrehiwet B, Peerschke EI. Structure and function of gC1q-R: a multiligand binding cellular protein. Immunobiology. 1998;199:225-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Sansonno D, Tucci FA, Ghebrehiwet B, Lauletta G, Peerschke EI, Conteduca V, Russi S, Gatti P, Sansonno L, Dammacco F. Role of the receptor for the globular domain of C1q protein in the pathogenesis of hepatitis C virus-related cryoglobulin vascular damage. J Immunol. 2009;183:6013-6020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Hu YW, Rocheleau L, Larke B, Chui L, Lee B, Ma M, Liu S, Omlin T, Pelchat M, Brown EG. Immunoglobulin mimicry by Hepatitis C Virus envelope protein E2. Virology. 2005;332:538-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Chandra PK, Hazari S, Poat B, Gunduz F, Prabhu R, Liu G, Burioni R, Clementi M, Garry RF, Dash S. Intracytoplasmic stable expression of IgG1 antibody targeting NS3 helicase inhibits replication of highly efficient hepatitis C Virus 2a clone. Virol J. 2010;7:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 91. | Ishizaka N, Ishizaka Y, Takahashi E, Tooda Ei, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 92. | Ishizaka Y, Ishizaka N, Takahashi E, Unuma T, Tooda E, Hashimoto H, Nagai R, Yamakado M. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 93. | Völzke H, Schwahn C, Wolff B, Mentel R, Robinson DM, Kleine V, Felix SB, John U. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 2004;174:99-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Adinolfi LE, Restivo L, Zampino R, Guerrera B, Lonardo A, Ruggiero L, Riello F, Loria P, Florio A. Chronic HCV infection is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 95. | Vassalle C, Masini S, Bianchi F, Zucchelli GC. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 96. | Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 97. | Liao CC, Su TC, Sung FC, Chou WH, Chen TL. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One. 2012;7:e31527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 98. | Jacobson Brown PM, Neuman MG. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin Biochem. 2001;34:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876-1890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 535] [Cited by in F6Publishing: 524] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 100. | Abbas Z, Moatter T. Interleukin (IL) 1beta and IL-10 gene polymorphism in chronic hepatitis C patients with normal or elevated alanine aminotransferase levels. J Pak Med Assoc. 2003;53:59-62. [PubMed] [Cited in This Article: ] |
| 101. | Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail. 2009;15:451-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Mostafa A, Mohamed MK, Saeed M, Hasan A, Fontanet A, Godsland I, Coady E, Esmat G, El-Hoseiny M, Abdul-Hamid M. Hepatitis C infection and clearance: impact on atherosclerosis and cardiometabolic risk factors. Gut. 2010;59:1135-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 103. | Oliveira CP, Kappel CR, Siqueira ER, Lima VM, Stefano JT, Michalczuk MT, Marini SS, Barbeiro HV, Soriano FG, Carrilho FJ. Effects of hepatitis C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol. 2013;164:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Durante-Mangoni E, Zampino R, Marrone A, Tripodi MF, Rinaldi L, Restivo L, Cioffi M, Ruggiero G, Adinolfi LE. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 105. | Boddi M, Abbate R, Chellini B, Giusti B, Solazzo V, Soft F, Pratesi G, Pratesi C, Gensini G, Zignego AL. HCV infection facilitates asymptomatic carotid atherosclerosis: preliminary report of HCV RNA localization in human carotid plaques. Dig Liver Dis. 2007;39 Suppl 1:S55-S60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol. 2010;47:72-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 107. | Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J, Schminke U, Kessler C, John U. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastroenterol. 2005;11:1848-1853. [PubMed] [Cited in This Article: ] |
| 108. | Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, Valenti L, Maraschi A, Catapano A, Fargion S. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121:72-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 109. | Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20:481-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 110. | Lonardo A, Lombardini S, Scaglioni F, Carulli L, Ricchi M, Ganazzi D, Adinolfi LE, Ruggiero G, Carulli N, Loria P. Hepatic steatosis and insulin resistance: does etiology make a difference? J Hepatol. 2006;44:190-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 111. | Vivona N, Bivona G, Noto D, Sasso BL, Cefalù AB, Chiarello G, Falletta A, Ciaccio M, Averna MR. C-reactive protein but not soluble CD40 ligand and homocysteine is associated to common atherosclerotic risk factors in a cohort of coronary artery disease patients. Clin Biochem. 2009;42:1713-1718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 112. | Antonelli A, Ferri C, Ferrari SM, Galetta F, Franzoni F, Santoro G, De Marco S, Ghiri E, Fallahi P. High circulating N-terminal pro-brain natriuretic peptide and tumor necrosis factor-alpha in mixed cryoglobulinemia. World J Gastroenterol. 2009;15:5074-5079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 113. | Giannakoulas G, Hatzitolios A, Karvounis H, Koliakos G, Charitandi A, Dimitroulas T, Savopoulos C, Tsirogianni E, Louridas G. N-terminal pro-brain natriuretic peptide levels are elevated in patients with acute ischemic stroke. Angiology. 2005;56:723-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 114. | Tillmann HL. Hepatitis C virus infection and the brain. Metab Brain Dis. 2004;19:351-356. [PubMed] [Cited in This Article: ] |
| 115. | Goh J, Coughlan B, Quinn J, O’Keane JC, Crowe J. Fatigue does not correlate with the degree of hepatitis or the presence of autoimmune disorders in chronic hepatitis C infection. Eur J Gastroenterol Hepatol. 1999;11:833-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Seifert F, Struffert T, Hildebrandt M, Blümcke I, Brück W, Staykov D, Huttner HB, Hilz MJ, Schwab S, Bardutzky J. In vivo detection of hepatitis C virus (HCV) RNA in the brain in a case of encephalitis: evidence for HCV neuroinvasion. Eur J Neurol. 2008;15:214-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Bokemeyer M, Ding XQ, Goldbecker A, Raab P, Heeren M, Arvanitis D, Tillmann HL, Lanfermann H, Weissenborn K. Evidence for neuroinflammation and neuroprotection in HCV infection-associated encephalopathy. Gut. 2011;60:370-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 118. | Fletcher NF, McKeating JA. Hepatitis C virus and the brain. J Viral Hepat. 2012;19:301-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 119. | Monaco S, Ferrari S, Gajofatto A, Zanusso G, Mariotto S. HCV-related nervous system disorders. Clin Dev Immunol. 2012;2012:236148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Palin K, Bluthé RM, McCusker RH, Moos F, Dantzer R, Kelley KW. TNFalpha-induced sickness behavior in mice with functional 55 kD TNF receptors is blocked by central IGF-I. J Neuroimmunol. 2007;187:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 121. | McAndrews MP, Farcnik K, Carlen P, Damyanovich A, Mrkonjic M, Jones S, Heathcote EJ. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 122. | Hilsabeck RC, Hassanein TI, Carlson MD, Ziegler EA, Perry W. Cognitive functioning and psychiatric symptomatology in patients with chronic hepatitis C. J Int Neuropsychol Soc. 2003;9:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Senzolo M, Schiff S, D’Aloiso CM, Crivellin C, Cholongitas E, Burra P, Montagnese S. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17:3369-3374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 124. | Origgi L, Vanoli M, Carbone A, Grasso M, Scorza R. Central nervous system involvement in patients with HCV-related cryoglobulinemia. Am J Med Sci. 1998;315:208-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 125. | Ramos-Casals M, Robles A, Brito-Zerón P, Nardi N, Nicolás JM, Forns X, Plaza J, Yagüe J, Sánchez-Tapias JM, Font J. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin Arthritis Rheum. 2006;36:189-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Petty GW, Duffy J, Houston J. Cerebral ischemia in patients with hepatitis C virus infection and mixed cryoglobulinemia. Mayo Clin Proc. 1996;71:671-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 127. | Cacoub P, Sbaï A, Hausfater P, Papo T, Gatel A, Piette JC. [Central nervous system involvement in hepatitis C virus infection]. Gastroenterol Clin Biol. 1998;22:631-633. [PubMed] [Cited in This Article: ] |
| 128. | Carta MG, Angst J, Moro MF, Mura G, Hardoy MC, Balestrieri C, Chessa L, Serra G, Lai ME, Farci P. Association of chronic hepatitis C with recurrent brief depression. J Affect Disord. 2012;141:361-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Fletcher NF, Wilson GK, Murray J, Hu K, Lewis A, Reynolds GM, Stamataki Z, Meredith LW, Rowe IA, Luo G. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634-643.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 130. | Chen KJ, Tseng CK, Chang FR, Yang JI, Yeh CC, Chen WC, Wu SF, Chang HW, Lee JC. Aqueous extract of the edible Gracilaria tenuistipitata inhibits hepatitis C viral replication via cyclooxygenase-2 suppression and reduces virus-induced inflammation. PLoS One. 2013;8:e57704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 131. | Pietrogrande M, De Vita S, Zignego AL, Pioltelli P, Sansonno D, Sollima S, Atzeni F, Saccardo F, Quartuccio L, Bruno S. Recommendations for the management of mixed cryoglobulinemia syndrome in hepatitis C virus-infected patients. Autoimmun Rev. 2011;10:444-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 132. | Dammacco F, Tucci FA, Lauletta G, Gatti P, De Re V, Conteduca V, Sansonno S, Russi S, Mariggiò MA, Chironna M. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: a long-term study. Blood. 2010;116:343-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 133. | Castello G, Costantini S, Scala S. Targeting the inflammation in HCV-associated hepatocellular carcinoma: a role in the prevention and treatment. J Transl Med. 2010;8:109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 507] [Cited by in F6Publishing: 559] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 135. | Lonardo A, Ballestri R, Restivo L, Adinolfi LE, Loria P. Hepatitis C and cardiovascular risk: facts and controversies. 3rd ed. Hot Topic in Viral Hepatitis. Modena: FBCommunication 2012; 27-35 Available from: http//www.hottopicsin.com.. [Cited in This Article: ] |
| 136. | Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946-1952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 137. | Whitehouse MW. Anti-TNF-alpha therapy for chronic inflammation: reconsidering pentoxifylline as an alternative to therapeutic protein drugs. Inflammopharmacology. 2004;12:223-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 138. | Austin AS, Mahida YR, Clarke D, Ryder SD, Freeman JG. A pilot study to investigate the use of oxpentifylline (pentoxifylline) and thalidomide in portal hypertension secondary to alcoholic cirrhosis. Aliment Pharmacol Ther. 2004;19:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 139. | Hernández E, Correa A, Bucio L, Souza V, Kershenobich D, Gutiérrez-Ruiz MC. Pentoxifylline diminished acetaldehyde-induced collagen production in hepatic stellate cells by decreasing interleukin-6 expression. Pharmacol Res. 2002;46:435-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 140. | Coimbra R, Porcides RD, Melbostad H, Loomis W, Tobar M, Hoyt DB, Wolf P. Nonspecific phosphodiesterase inhibition attenuates liver injury in acute endotoxemia. Surg Infect (Larchmt). 2005;6:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 141. | Raetsch C, Jia JD, Boigk G, Bauer M, Hahn EG, Riecken EO, Schuppan D. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut. 2002;50:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 142. | Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29:181-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 143. | Gutierrez-Reyes G, Lopez-Ortal P, Sixtos S, Cruz S, Ramirez-Iglesias MT, Gutierrez-Ruiz MC, Sanchez-Avila F, Roldan E, Vargas-Vorackova F, Kershenobich D. Effect of pentoxifylline on levels of pro-inflammatory cytokines during chronic hepatitis C. Scand J Immunol. 2006;63:461-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 144. | Mallat A, Teixeira-Clerc F, Lotersztajn S. Cannabinoid signaling and liver therapeutics. J Hepatol. 2013;59:891-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |