Published online Feb 27, 2021. doi: 10.4254/wjh.v13.i2.218
Peer-review started: October 2, 2020
First decision: December 3, 2020
Revised: December 14, 2020
Accepted: December 28, 2020
Article in press: December 28, 2020
Published online: February 27, 2021
Matrix metalloproteinases (MMPs) participate in the degradation of extracellular matrix compounds, maintaining the homeostasis between fibrogenesis and fibrolytic processes in the liver. However, there are few studies on the regulation of liver MMPs in fibrosis progression in humans.
To assess the production activity and regulation of matrix metalloproteinases in liver fibrosis stages in chronic hepatitis C (CHC).
A prospective, cross-sectional, multicenter study was conducted. CHC patients were categorized in fibrosis grades through FibroTest®and/or FibroScan®. Serum MMP-2, -7, and -9 were determined by western blot and multiplex suspension array assays. Differences were validated by the Kruskal-Wallis and Mann-Whitney U tests. The Spearman correlation coefficient and area under the receiver operating characteristic curve were calculated. Collagenolytic and gelatinase activity was determined through the Azocoll substrate and zymogram test, whereas tissue inhibitor of metalloproteinase-1 production was determined by dot blot assays.
Serum concentrations of the MMPs evaluated were higher in CHC patients than in healthy subjects. MMP-7 distinguished early and advanced stages, with a correlation of 0.32 (P < 0.001), and the area under the receiver operating characteristic displayed moderate sensitivity and specificity for MMP-7 in F4 (area under the receiver operating characteristic, 0.705; 95% confidence interval: 0.605-0.805; P < 0.001). Collagenolytic activity was detected at F0 and F1, whereas gelatinase activity was not detected at any fibrosis stage. Tissue inhibitor of metalloproteinase-1 determination showed upregulation in F0 and F1 but downregulation in F2 (P < 0.001).
High concentrations of inactive MMPs were present in the serum of CHC patients, reflecting the impossibility to restrain liver fibrosis progression. MMPs could be good diagnostic candidates and therapeutic targets for improving novel strategies to reverse liver fibrosis in CHC.
Core Tip: The relevance of this prospective study was to evaluate the role of matrix metalloproteinases in the pathophysiology of liver fibrosis in chronic hepatitis C patients. Matrix metalloproteinases could be used as possible therapeutic targets and as a monitoring tool in treatment-experienced patients that continue to present with liver fibrosis and develop cirrhosis and/or hepatocellular carcinoma.
- Citation: Martinez-Castillo M, Hernandez-Barragan A, Flores-Vasconcelos I, Galicia-Moreno M, Rosique-Oramas D, Perez-Hernandez JL, Higuera-De la Tijera F, Montalvo-Jave EE, Torre-Delgadillo A, Cordero-Perez P, Muñoz-Espinosa L, Kershenobich D, Gutierrez-Reyes G. Production and activity of matrix metalloproteinases during liver fibrosis progression of chronic hepatitis C patients. World J Hepatol 2021; 13(2): 218-232
- URL: https://www.wjgnet.com/1948-5182/full/v13/i2/218.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i2.218
Liver fibrosis is a convergence of repair mechanisms for chronic cellular damage, which can be induced by several etiologies, including the hepatitis B and C viruses, alcoholic liver disease (ALD) and nonalcoholic fatty liver disease, among others[1]. The intricate mechanism of the tissue repair response of extracellular matrix (ECM) compounds has been associated with the balance between fibrogenesis and fibrolysis[2]. In a normal liver, ECM proteins, fibronectin, laminin, proteoglycans and collagen types I, III, IV and V comprise approximately 0.5% of the wet weight[2,3]. The unregulated accumulation of ECM can result in chronic liver damage, promoting cirrhosis and hepatocellular carcinoma (HCC), with the subsequent death of patients. The uncontrolled deposition of collagen in the liver parenchyma involves the unceasing activation of hepatic stellate cells (HSCs), which represent approximately 5% to 10% of resident liver cells[4].
Some of the representative features of activated or transdifferentiated HSCs to myofibroblast-like cells are the loss of vitamin A storage, proliferation, inflammation, chemotaxis and ECM production[5,6]. Transforming growth factor beta (TGF-β) is the most potent fibrogenic cytokine and promotes smad-3 protein activation, stimulating the active transcription of collagen type I and III[7]. TGF-β can also activate the MAPK/p38/c-JNK pathway[8], inducing a continuous proinflammatory milieu. Other proliferative HSC inductors have been well described, such as PDGF, CTGF and VEGF[1].
The control of collagen and other ECM elements is known to be regulated by the family of matrix metalloproteinases (MMPs)[9]. The MMPs have been classified into broad groups related to their activity: Collagenases, gelatinases, membrane-type MMPs, stromelysins and matrilysins[10]. MMPs also play important roles in the degradation and activation of immune mediators (e.g., cytokines and antimicrobial peptides)[2]; those biologic regulators are usually released as zymogens that require additional processing in the extracellular space by self-activation, the indirect action of plasminogen and the assistance of transmembrane MMP activity. Thus, some reports state that MMPs may display dual roles in liver fibrosis, depending on the timing of action. Proteolytic activity is mainly controlled by reversible tissue inhibitors of metalloproteinases (TIMPs, 1-4)[11,12]. In fact, activated HSCs have been reported to upregulate TIMP-1, enabling the accumulation of ECM proteins in the extracellular space[13,14].
Approximately 20 years ago, MMP-2 was described to be overexpressed in the liver parenchyma of human fibrotic and cirrhotic patients[15], and a direct association with collagen I expression was also reported in an animal model[14]. In addition, MMP-2 participated in the activation of TGF-β and the modulation of IL-1β, TNF-α and MCP-3 by proteolytic cleavage[16]. In 2000, Lichtinghagen et al[17] reported that peripheral blood cells revealed a correlation between the MMP-2/TIMP-1 ratio and the histologic grade of fibrosis in patients with chronic active cirrhosis due to hepatitis C. The authors concluded that said ratio could be used as a progression marker in patients with chronic liver disease[17]. MMP-2 (gelatinases A) and MMP-9 (gelatinases B) were recently suggested as serum biomarkers of ALD severity in a region in Poland[18]. The chronologic expression of MMP-2 and MMP-9 in hepatic fibrosis was proposed using different animal models of fibrosis. Those MMPs were relatively overexpressed and TIMP-1 was downregulated after the fibrosis inductor was eliminated[14].
A recent multi-analysis of serum proteins demonstrated that MMP-7 was directly associated with fibrosis. The authors suggested that MMP-7 could be a valuable indicator of advanced fibrosis and might play a role in liver fibrogenesis, due to its role as a matrix remodeling factor[19]. However, there is little information about MMP-7 and liver fibrosis progression in the liver as well as at the serum level.
The correct determination of liver fibrosis stages is imperative for making the diagnosis and implementing therapeutic decisions. At present, there is no evidence of the production and activity of MMP-2, MMP-7 or MMP-9 or their correlation with fibrosis progression in serum samples from patients. In the present work, we evaluated the serum concentration and proteolytic capacity of MMP-2, -7 and -9 in chronic hepatitis C (CHC) patients according to fibrosis progression.
A prospective, cross-sectional, observational study was conducted. Patients were carefully selected from the Hospital General de México, “Dr. Eduardo Liceaga,” the Universidad Autónoma de Nuevo Leon and the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán.” The patients included in the study were diagnosed with CHC (n = 119) and were treatment naïve. Fibrosis degrees were classified according to international guidelines by the FibroTest®and/or FibroScan®methods (F0, F1, F2, F3 or F4). The fibrosis stages of patients classified by FibroScan® and FibroTest® were grouped into similar intermediate classifications (F0-F1, F1-F2, F2-F3 and F3-F4). Patients whose tests were concordant or in close stages were included, whereas patients whose results were discrepant were discarded. Patients with clinical evidence of risk alcohol consumption (AUDIT > 8) and/or systemic infections (e.g., bacteria, flu, autoimmune diseases, etc.) and comorbidities (e.g., diabetes and hypertension) were excluded. The control group consisted of blood bank donors from the Hospital General de México with negative serology for HIV and hepatitis A, B and C viruses and classified as non-risk drinkers (AUDIT < 8) (n = 119).
The anthropometric variables collected for both sexes were age, height, weight and body mass index (kg/m2; weight/height2), and the biochemical parameters were hemoglobin, hematocrit, leukocyte count, platelets, total bilirubin, direct bilirubin, aspartate aminotransferase, alanine aminotransferase and gamma glutamyl transpeptidase.
A total of 30 mL of blood was drawn from all participants for the samples; 20 mL were used for the biochemical tests and 10 mL to obtain serum. The samples were centrifuged at 3500 rpm for 10 min, and the serum was recovered and stored at -80 °C until its use for evaluating MMP concentration and regulation.
Serum samples were randomly collected from CHC patients and healthy individuals and incubated at 65 °C for 7 min. Total protein concentration was evaluated by the Bradford method[20]. The sample buffer (Laemmli 2X) and 10% β-mercaptoethanol (Bio-Rad) were then added and the final protein concentration was adjusted to 10 µg/µL for all the samples evaluated.
The proteins were separated using 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Electrophoresis was performed at 100 V for 1 h, and the proteins were then transferred to PVDF membranes (Perkin-Elmer) at 400 mA for 60 min. The membranes were blocked with 7% skim milk dissolved in PBS and incubated for 2 h. Solid phase detection was carried out overnight at 4 °C in agitation, utilizing MMP-2, MMP-7, and MMP-9 mouse polyclonal antibodies (1:1000) (Santa Cruz Biotechnology). The membranes were washed three times with 0.05% Tween 20 in PBS and incubated with secondary goat-anti-mouse-IgG peroxidase-conjugated antibodies (1:2500) (Santa Cruz Biotechnology) for 1 h at 37 °C. The membranes were then washed with 0.05% Tween 20 in PBS and exposed to a luminol kit reagent (Santa Cruz Biotechnology) using Kodak film. Densitometry analysis was performed using the ImageJ program (http://rsb.info.nih.gov/nih-image), and the results were expressed as relative optical density.
MMP concentration was evaluated by multiplex suspension array technology (Millipore®). Nontreated serum samples from patients and controls (25 µL) were evaluated using the HMMP2MAG-55K kit, which allowed the simultaneous determination of MMP-2, -7 and -9 concentrations with no cross-reactivity and minimal intra- and interassay error (% CV < 10) (Merck, Millipore ®, United States). The data were acquired utilizing Luminex200 MAGPIX® Systems equipment, following the supplier’s specifications (series number 10294005; Merck, Millipore, United States). The data were validated with internal standards and controls, and minimum and maximum detection values for each protein were obtained using Luminex XPONENT software.
To determinate the collagenolytic activity of MMPs in serum, we used the chromogenic substrate, Azocoll. Two milligrams of Azocoll were incubated with 10 µg/µL of total serum protein and adjusted to 500 µL by the addition of an activation buffer at pH 9.0 (100 mmol/L glycine, 2 mmol/L CaCl2) (J.T. Baker, PA, United States). The serums were incubated overnight with shaking at 37 °C in duplicate. The reaction was stopped with 10% trichloroacetic acid (500 µL) (Sigma-Aldrich, MO, United States), and the samples were centrifuged at 4600 × g for 15 min. The supernatants were collected, and their absorbances were evaluate at 520 nm. A total of 2.5 µg/mL (4.5 U/mL) of collagenase from Clostridium histolyticum was used for the positive control. Protease activity was reported as units per milligram; that unit of measure is equivalent to the amount of substrate degraded in 1 min per milliliter[21].
MMP activity was analyzed in serum from CHC patients and control subjects by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, copolymerized with 0.1% porcine skin gelatin (type A) (Sigma-Aldrich) or collagen (Collagen Standard from SIRCOL kit assays; Biocolor, United Kingdom) as the substrate. Concentrations of 10 µg of protein were loaded per well. Electrophoresis was performed at 100 V at 4 ºC for 3 h. A total of 5 µg/mL of broad-spectrum collagenase from Clostridium histolyticum was used for the positive control (Collagenase P; Roche, United States). After electrophoresis, the gels were washed twice with a 2.5% Triton X-100 (Sigma-Aldrich) solution for 15 min with shaking. For MMP activation, the gels were incubated overnight with buffer solution at pH 9.0 (100 mmol/L glycine and 2 mmol/L CaCl2 with or without 2 mmol/L dithiothreitol). Finally, the gels were stained with 0.5% (w/v) Coomassie brilliant blue R-250 for 30 min. Protease activities were observed after the gels were decolored with methanol-acetic acid-water (%) (50:10:40) until clear bands on a blue background were obtained.
To determine TIMP-1 in the different grades of liver fibrosis, we performed dot blot assays. All samples (5 µg/mL) were placed by drops onto PVDF membranes (0.22 µm), and bound protein was determined with Ponceau S solution (Sigma-Aldrich). Dot blots were determined using an anti-TIMP-1 polyclonal antibody (1:500) overnight, followed by incubation with secondary anti-goat antibody (1:2500; Invitrogen, MA, United States). The blots were exposed to a luminol kit reagent (Santa Cruz Biotechnology, TX, United States) using Kodak photographic film. The densitometry analysis was evaluated with the ImageJ program (http://rsb.info.nih.gov/nih-image), and the data were expressed as relative optical density.
The continuous variables were described as mean ± standard error of the mean and the qualitative variables as absolute and relative frequencies (%). The qualitative variables were analyzed using the chi-square test, and the continuous parameters were analyzed using the Mann-Whitney U test. Relative optical density data and MMP and TIMP-1 activity from the western blot and Azocoll assays were plotted using GraphPad Prism Software V6 (CA, United States). The P values were calculated using two-way ANOVA and Bonferroni’s Multiple Comparisons Test. The Pearson correlation was calculated, and the area under the receiver operating characteristic curve for MMP-7 was determined in all the fibrosis stages to determine its relevance as a biomarker. Differences were considered statistically significant when the P value was less than 0.05. The statistical analysis was performed using the IBM SPSS Statistics for Windows, Version 22 program (IBM Corp, NY, United States).
A total of 119 patients with CHC were included. Unexpectedly, the CHC group mainly consisted of women. The demographics and biochemical information were contrasted with 119 healthy subjects that were predominantly male (Table 1). Only body mass index did not display differences in the biometric analysis. The data are summarized in Table 1.
| CHC, n = 119 | CT, n = 119 | P value | |
| Sex, n (%) | < 0.001 | ||
| Men | 53 (45) | 75 (63) | |
| Women | 66 (55) | 44 (37) | |
| Age in yr | 54 ± 13 | 37 ± 10 | < 0.001 |
| BMI in kg/m2 | 27 ± 1 | 28 ± 1 | 0.340 |
| Hb in g/dL | 14 ± 2 | 16 ± 1 | < 0.001 |
| Leu as 103/µL | 5.1 ± 1.0 | 7.4 ± 0.2 | < 0.001 |
| Platelets as × 103 | 177 ± 78 | 272 ± 62 | < 0.001 |
| Total bilirubin in mg/dL | 2.52 ± 1.70 | 0.75 ± 0.29 | 0.001 |
| Direct bilirubin in mg/dL | 0.18 ± 0.15 | 0.07 ± 0.06 | 0.001 |
| AST in UI/L | 60 ± 5 | 30 ± 11 | < 0.001 |
| ALT in UI/L | 63 ± 5 | 26 ± 18 | < 0.001 |
| GGT in UI/L | 92.50 ± 10.67 | 30.55 ± 2.43 | < 0.001 |
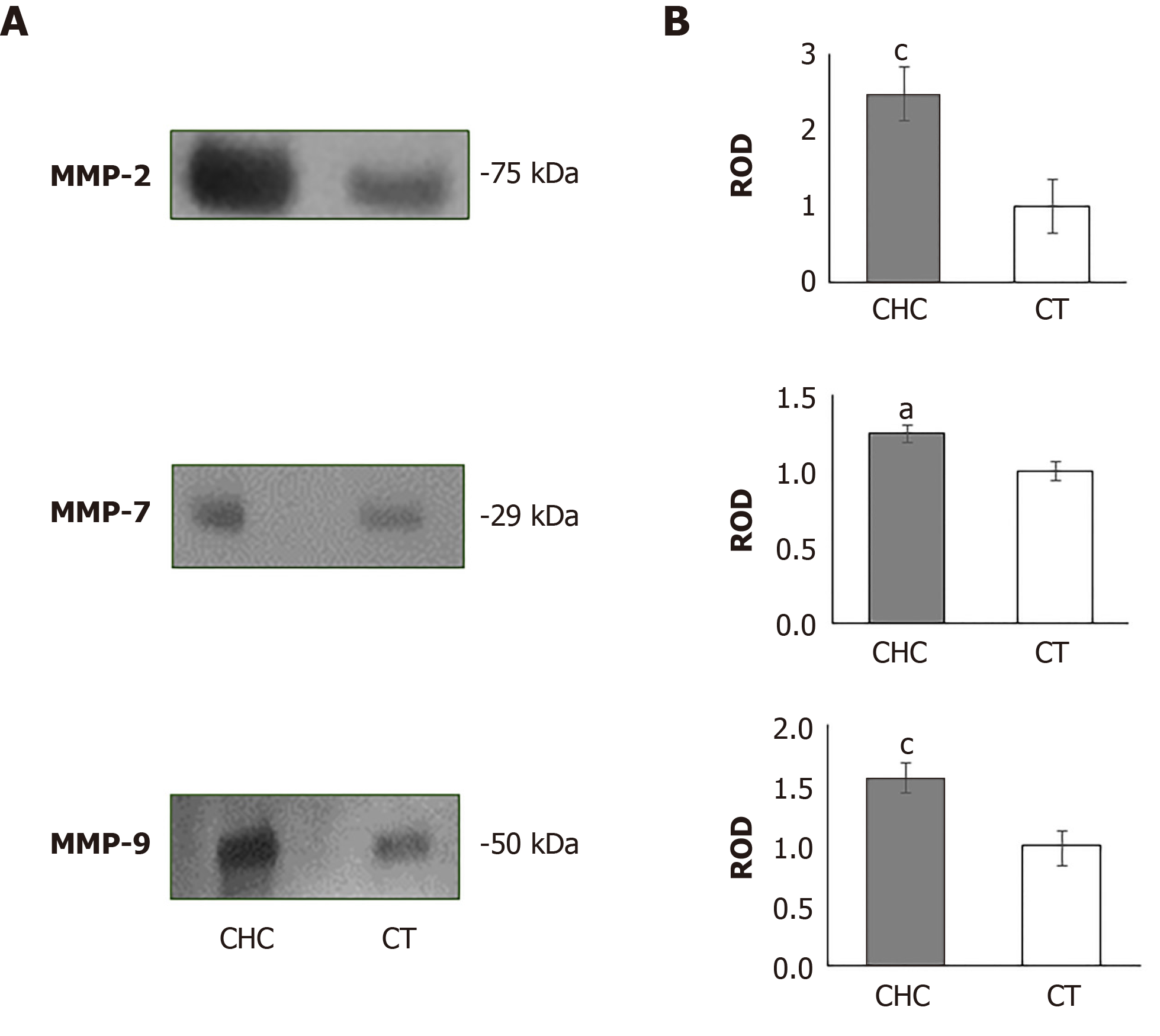
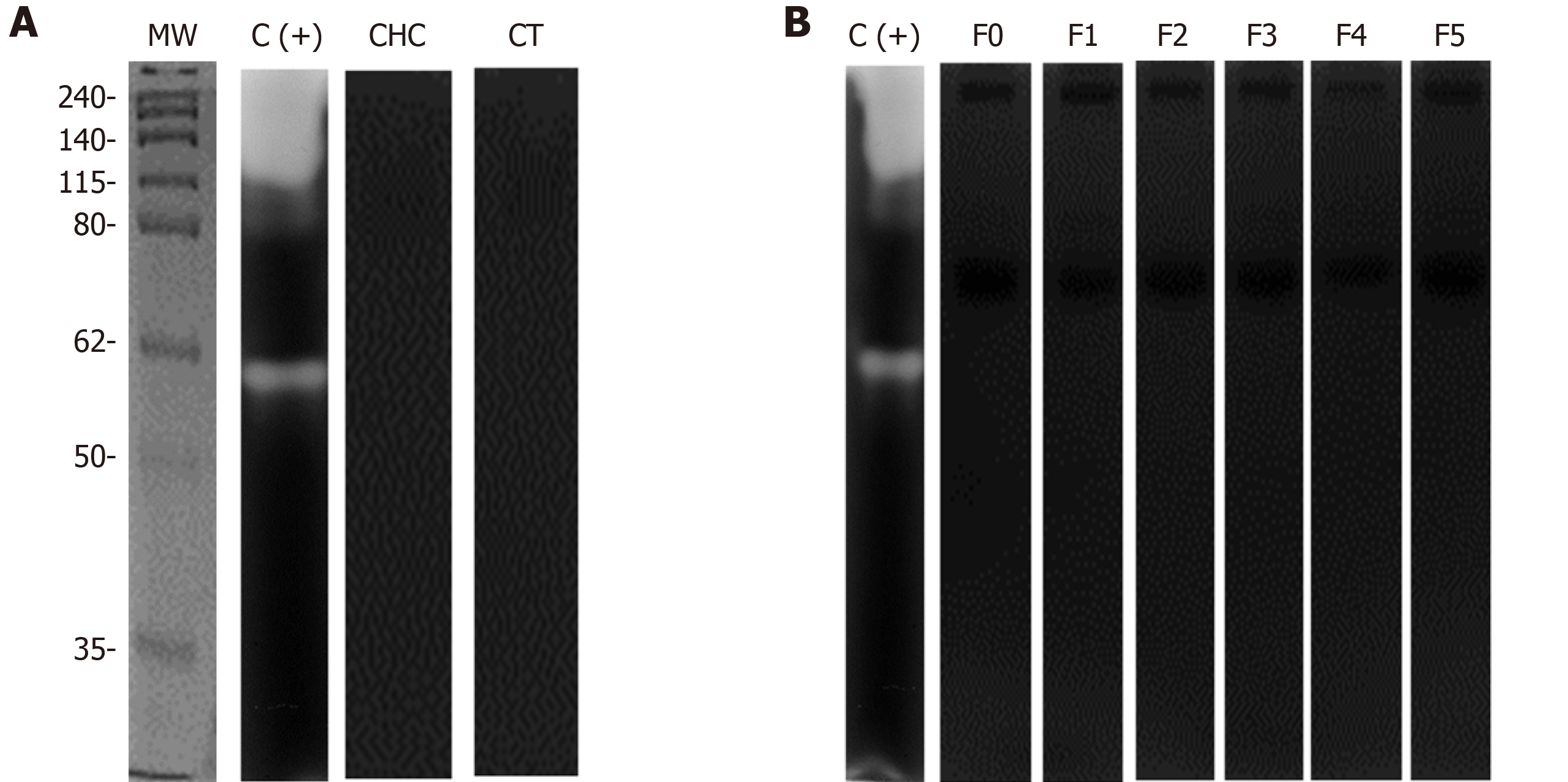
To evaluate the presence of MMPs in serum from patients and healthy individuals, we first determined the presence of each of the MMPs by western blot assays. The results showed the presence of bands with molecular weights of 75, 29 and 50 kDa, using specific antibodies against MMP-2, MMP-7, and MMP-9, respectively (Figure 1A). The densitometric analysis of all the MMPs evaluated showed an evident increase in the CHC patients (Figure 1B).
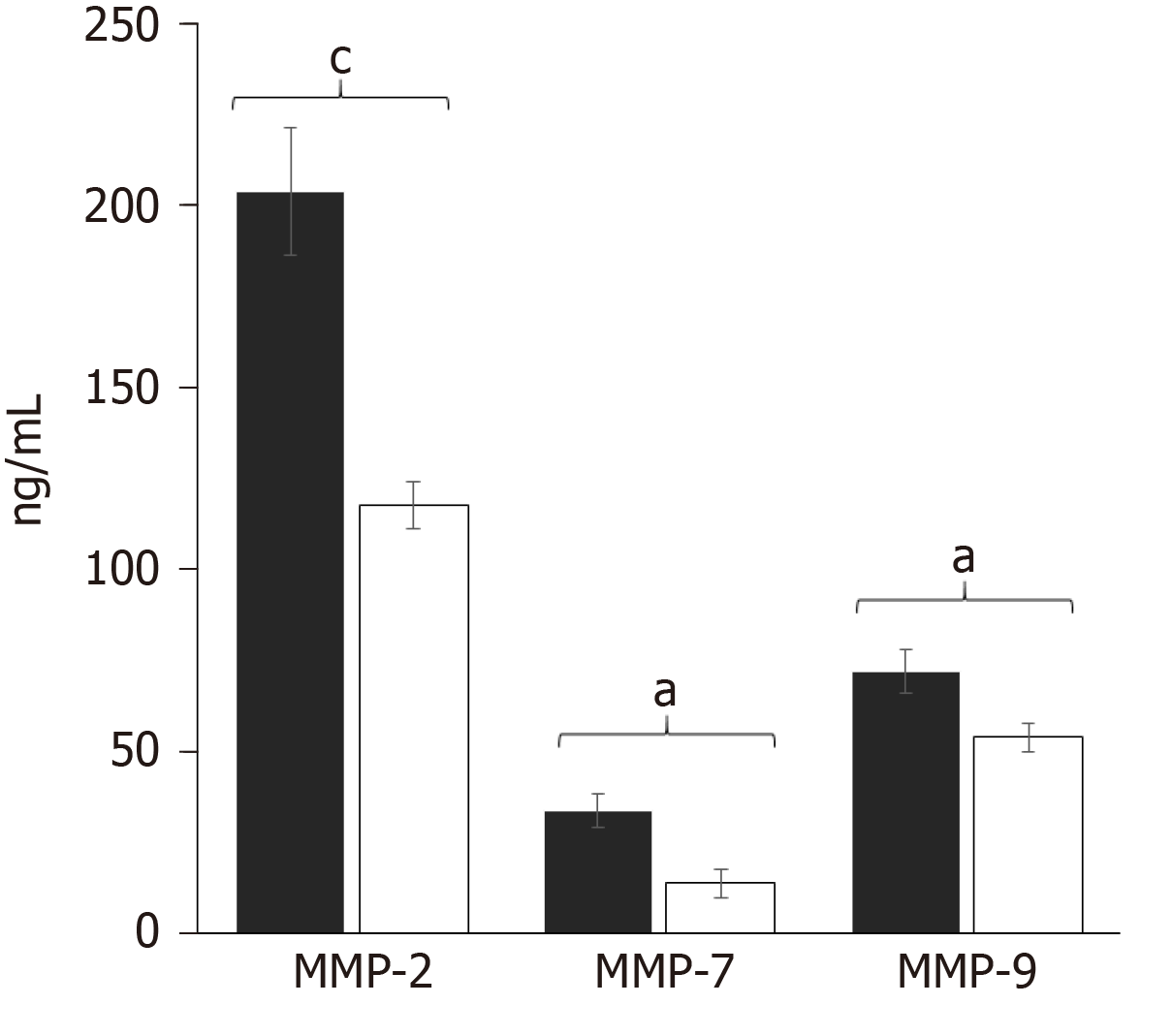
After observing those differences, we evaluated the specific concentration of each MMP by multiplex suspension array technology. Interestingly, the multiplexed determination of MMPs in the CHC patients (n = 119) and controls (n = 119) showed that MMP-2 had higher concentration values compared with MMP-7 and MMP-9 (Figure 2). The results correlated with the western blot assays.
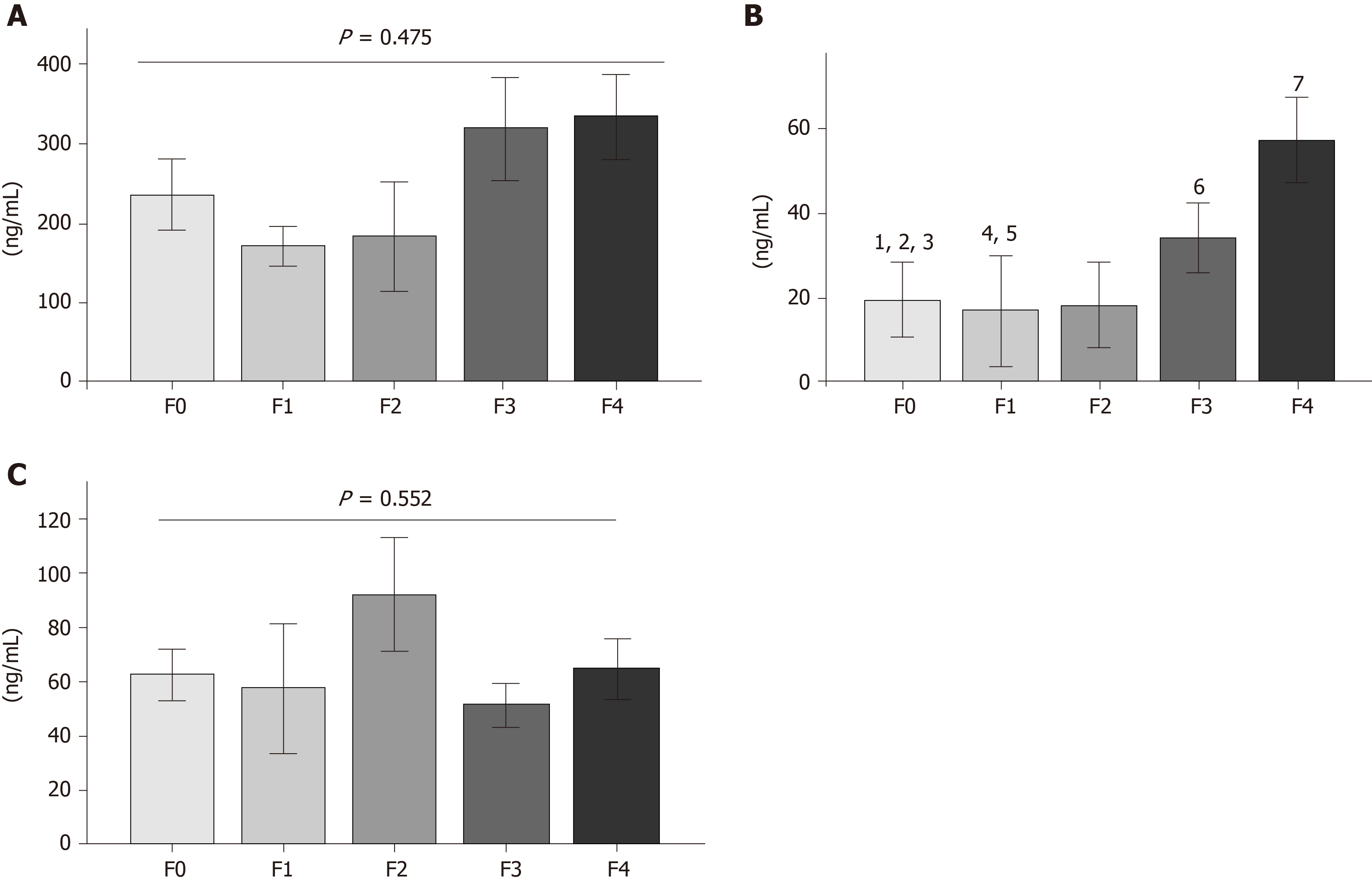
After determining MMP overproduction, the CHC patients were categorized into fibrosis stages (F0, F1, F2, F3 and F4) (Supplementary Table 1). Mean patient age was between 55 and 60 years, and body mass index was higher in F4 (Table 2). The comparative results of serum MMP-2 concentrations suggested decreases in stages F0, F1, and F2, but no differences were observed in any of the fibrosis grades (Figure 3A). In contrast, MMP-7 displayed a continuous increase according to fibrosis stage progression. Statistical differences were found in F0 vs F1, F0 vs F3, F0 vs F4, F1 vs F3, F1 vs F4, F2 vs F3 and F2 vs F4 (Figure 3B). Finally, the MMP-9 analysis showed no tendency or difference between each fibrosis stage.
| F0 (36) | F1 (11) | F2 (14) | F3 (20) | F4 (38) | Differences | |
| Sex, n (%) | ||||||
| Men | 10 (30) | 6 (60) | 7 (50) | 9 (45) | 13 (37) | N/A |
| Women | 26 (70) | 5 (40) | 7 (50) | 11 (55) | 25 (63) | N/A |
| Age in yr | 50 ± 12 | 39 ± 7 | 55 ± 13 | 59 ± 10 | 55 ± 13 | F0-F1a, F0-F3a, F1-F2b, F1-F3b, F1-F4b |
| BMI in kg/m2 | 25 ± 4 | 26 ± 4 | 26 ± 3 | 27 ± 3 | 27 ± 5 | F0-F4a |
| Hb in g/dL | 15.0 ± 1.3 | 14.0 ± 2.0 | 14.0 ± 2.0 | 14.0 ± 2.0 | 14.0 ± 2.0 | N/S |
| Leu in g/dL | 5.6 ± 1.3 | 6.5 ± 0.8 | 4.2 ± 1.4 | 4.7 ± 1.2 | 4.3 ± 2.2 | F0-F4b |
| Platelets as × 103 | 231 ± 56 | 233 ± 20 | 193 ± 74 | 163 ± 54 | 92 ± 39 | F0-F3b |
| F0-F4b | ||||||
| F1-F4b | ||||||
| F2-F4b | ||||||
| F3-F4b | ||||||
| Total bilirubin in mg/dL | 0.72 ± 0.31 | 0.44 ± 0.22 | 0.73 ± 0.28 | 1.75 ± 0.85 | 2.39 ± 2.7 | F0-F1b |
| F1-F4b | ||||||
| F2-F4a | ||||||
| F3-F4a | ||||||
| Direct bilirubin in mg/dL | 0.18 ± 0.10 | 0.07 ± 0.05 | 0.12 ± 0.10 | 0.23 ± 0.15 | 0.27 ± 0.3 | F0-F1a |
| AST in UI/L | 45 ± 7 | 38 ± 12 | 49 ± 11 | 74 ± 10 | 86 ± 16 | F0-F3b |
| F0-F4b | ||||||
| F1-F3a | ||||||
| F1-F4a | ||||||
| ALT in UI/L | 56 ± 11 | 56 ± 8 | 57 ± 10 | 69 ± 10 | 80 ± 13 | F0-F4b |
| GGT in UI/L | 70.49 ± 15.65 | 51.30 ± 19.43 | 52.62 ± 14.10 | 91.58 ± 18.74 | 144.00 ± 25.29 | F0-F4a |
| F1-F3a | ||||||
| F1-F4b | ||||||
| F2-F4a |
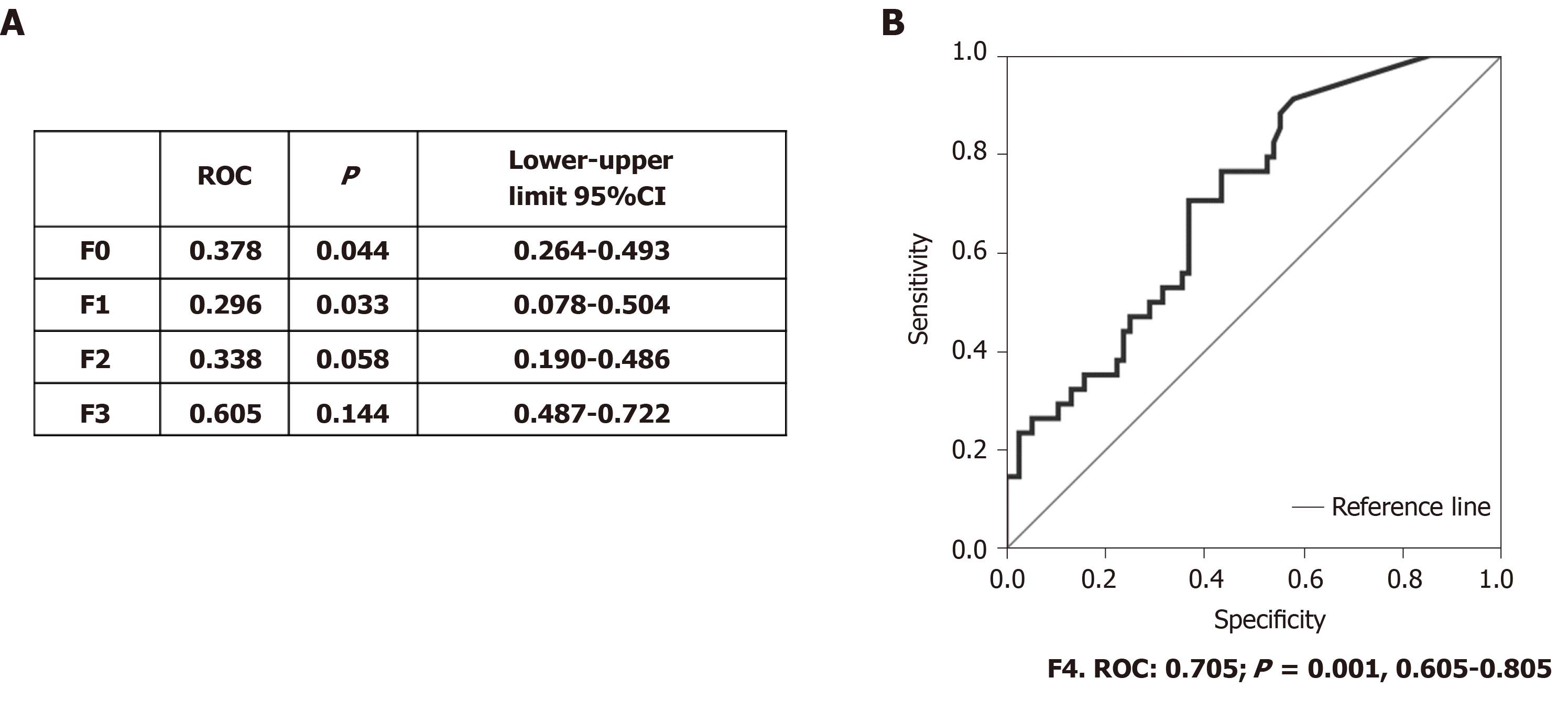
After determining that MMP-7 showed differences between fibrosis stages, we performed the Spearman correlation, and MMP-7 displayed moderate correlation (r = 0.32, P < 0.001) with CHC. We then determined the area under the receiver operating characteristic curve to evaluate MMP-7 as a candidate marker for fibrosis. Receiver operating characteristic (ROC) values > 0.7 were considered acceptable, whereas values below that point were discarded. The results showed that MMP-7 was not effective for distinguishing F0, F1, F2 or F3 (Figure 4A). However, the ROC values were acceptable in F4, the advanced fibrosis stage (Figure 4A and 4B). The use of MMP-7 as a complementary protein could improve the specificity and sensitivity of the available methods for determining that fibrosis stage.
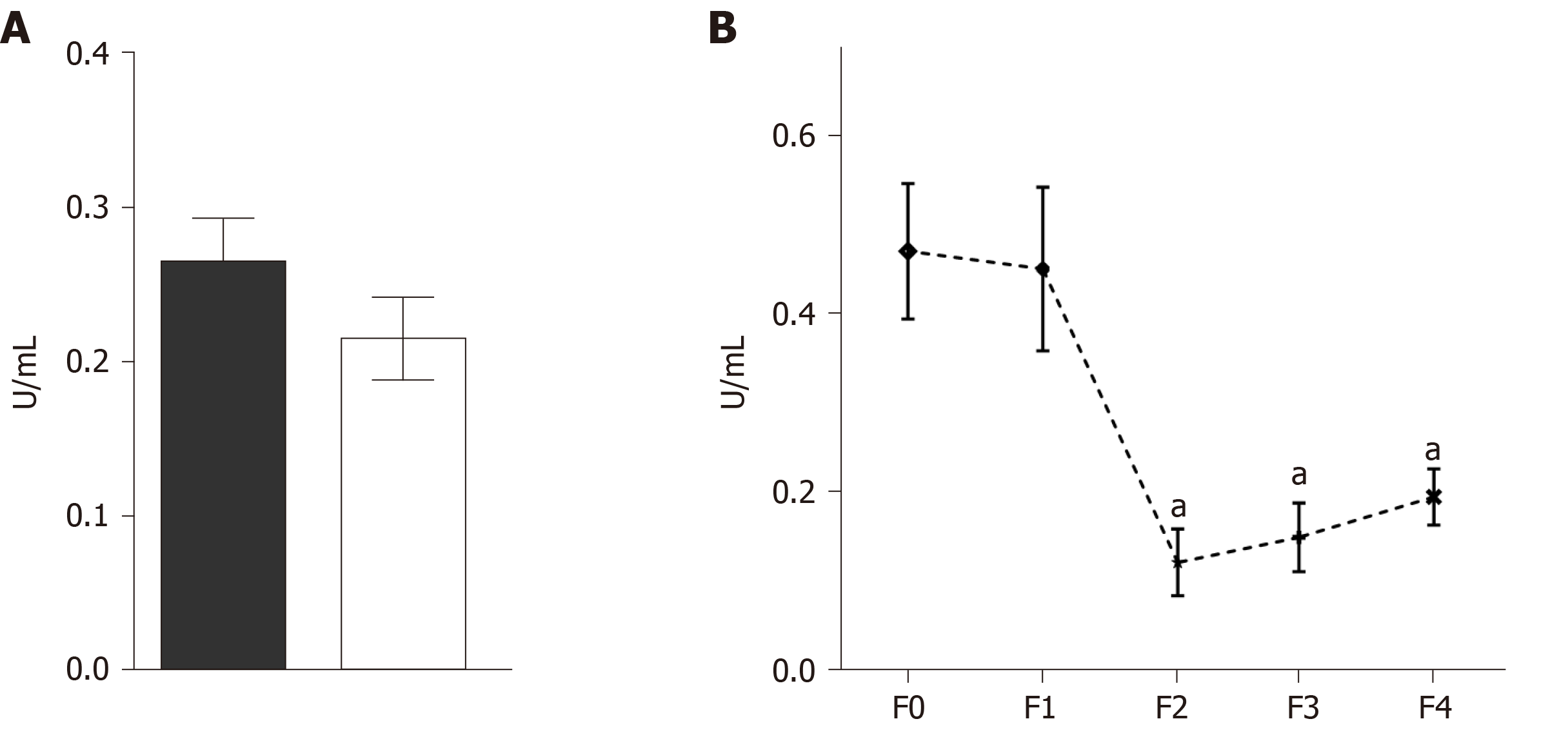
Zymograms and Azocoll assays were performed to evaluate whether serum MMPs had proteolytic activity. The quantitative analysis of the degradation of Azocoll demonstrated that activity in the CHC patients (n = 119) was slightly higher than in the controls (n = 119) (Figure 5A). The evaluation of activity in relation to fibrosis grades showed that F0 and F1 had collagenolytic activity and that F2, F3, and F4 values were lower than those of F1 (Figure 5B). Activity was adjusted to the positive collagenase activity of Clostridium histolyticum reaching absorbance at 4.5 U/mL. The zymography assays did not detect any apparent activity of gelatinase or collagenase in either the patients or the controls (Figure 6). In contrast, the positive controls showed activity in the range of 150 to 250 kDa and activity close to 62 kDa. Similar results were observed in collagen-zymogram (data not shown). Regarding the zymography assays of fibrosis stages in the CHC patients, no enzymatic activity was shown in the sample evaluated (Figure 6B).
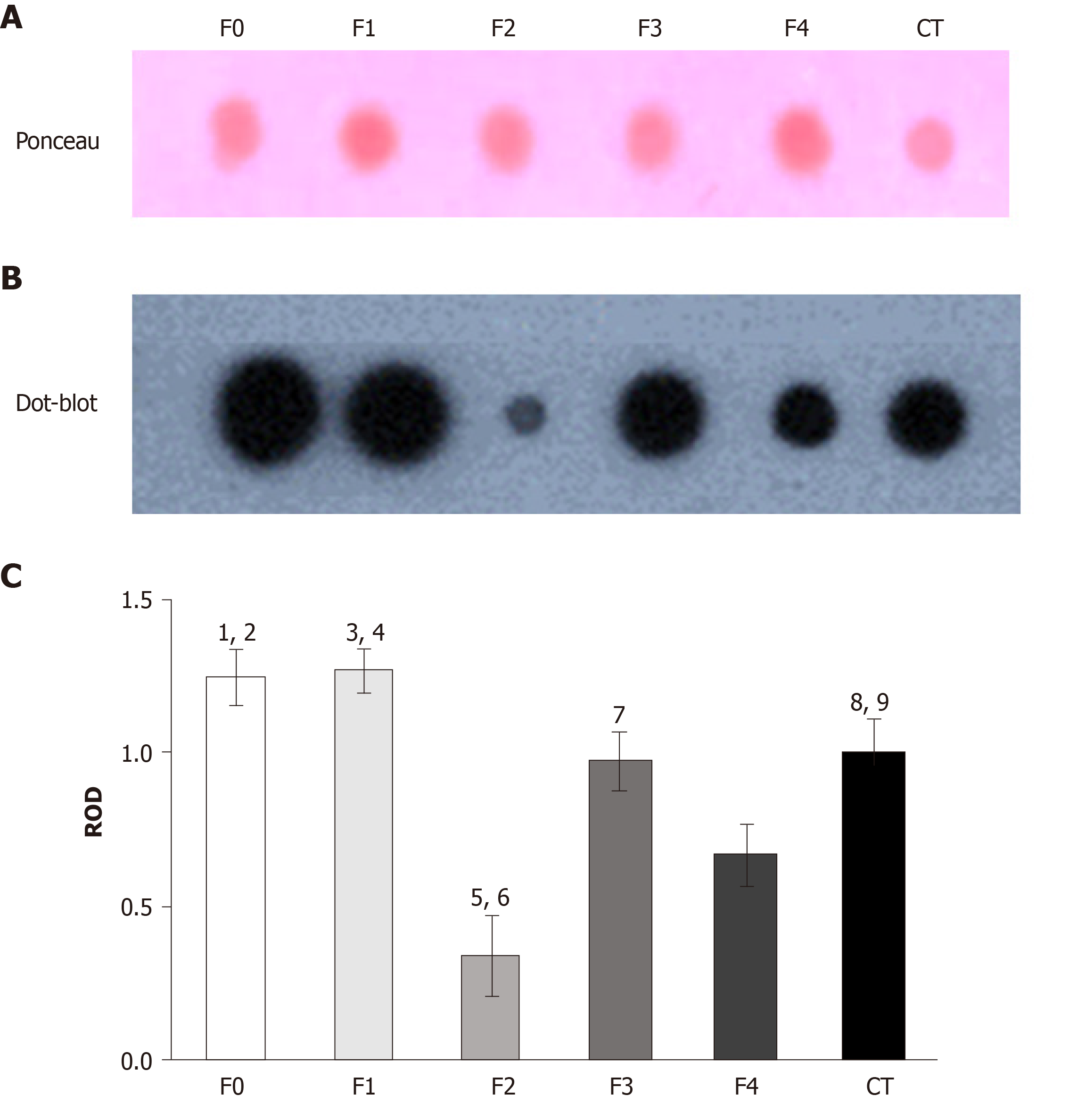
To explore TIMP-1 regulation in the serum from patients with different fibrosis grades, we performed dot blot assays. At the early stages of fibrosis (F0 and F1), the patients had high levels of TIMP-1. However, at F2, TIMP-1 diminished sharply, but the levels were recovered in F3 and F4 (Figure 7B). The data were compared with the control levels. The PVDF membrane was stained with Ponceau S solution as the protein control load (Figure 7A). Statistical differences were determined through the densitometric analysis of TIMP-1 in the different fibrosis grades and the control subjects (Figure 7C).
Up to the year 2010, approximately two million deaths worldwide (an estimated 4% of total deaths) were associated with liver diseases that included acute hepatitis, cirrhosis and liver cancer[22]. Novel antiviral treatments and changes in lifestyle have improved survival and quality of life, albeit treatment is not always successful in cases of chronic hepatitis C virus (HCV) infection. In addition, achieving alcohol abstinence and adherence to diet is no easy task[23-25]. Importantly, some patients that have been treated, and in whom viral infection is clinically eliminated continue to present with liver damage and develop cirrhosis and/or HCC[26]. Fibrosis is a common feature in the wound-healing response for most damage inductors and can be considered the key to adequate or inadequate liver parenchyma function. HSCs activated by TGF-β are thought to be the major source of collagens and TIMPs, but inactivation of that cell line is not enough to restore normal liver function. The degradation of excessive ECM is also necessary[14,27]. Collagens are degraded by MMPs, which are secreted by Kupffer cells and HSCs as proenzymes, and their activation occurs in the extracellular space. In the present work, we evaluated the serum concentration and proteolytic capacity of MMP-2, -7 and -9, in CHC patients according to fibrosis progression.
The demographic data revealed that the CHC population was older than the control group, correlating with progression and evolution time of hepatitis C, which is usually diagnosed in advanced-age patients. In contrast, the control individuals were young, which is the common age range of blood donors (35 to 45 years)[28]. Furthermore, both the CHC and control subjects presented with the obesity criteria with no apparent impact on biochemical values, including platelets and liver enzymes (aspartate aminotransferase, alanine aminotransferase and gamma glutamyl transpeptidase), which showed evident clinical alterations in CHC. Moreover, young patients were mainly identified as F1, which possibly was related to the fact that some of the study subjects then became blood donors (whose common age is from 35 to 45 years). Through the viral panel, these donors were found to be positive for HCV. Advanced stages of fibrosis were found in patients whose age ranged from 55 to 60 years.
Despite the fact that MMP-1, MMP-2, MMP-9 and MMP-13 are the most common MMPs related to liver fibrosis regulation[29], in 2015 MMP-7 was observed to be associated with liver fibrosis in biliary atresia[30]. In our study, the CHC patients had higher concentrations of MMP-7 compared with the healthy individuals, which correlated with the report of upregulation of MMP-7 in cirrhosis[19]. We provided evidence that absolute values of MMP-7 were able to distinguish mild, moderate and advanced fibrosis stages.
After evaluating the regulation of MMP-7 through fibrosis progression and observing the differences according to fibrosis stages, we performed ROC analyses in each of the stages of fibrosis (data not shown). MMP-7 showed acceptable ROC values for distinguishing F4 from the other stages of fibrosis. In previous reports, multiple analyses and multivariate logistic regression modeling of MMP-7 with hyaluronic acid, MMP-1, α-fetoprotein and APRI enhanced diagnostic accuracy to 0.938 in advanced fibrosis[19]. Those types of analyses could possibly improve area under the receiver operating characteristic values in the other fibrosis stages. MMP-7 and other liver proteins in serum (e.g, TIMP-1 and IGFBP-7, among others)[19] can potentially improve the available diagnostic methods by enabling the precise discrimination of mild, moderate and advanced fibrosis stages. Those results are promising, and MMP-7 has been considered a predictive biomarker in other fibrogenesis pathologies, such as kidney fibrosis and idiopathic pulmonary fibrosis[31,32]. Thus, the correct clinical evaluation is crucial before using serum markers as diagnostic tools. On the other hand, both MMP-2 and MMP-9 have been proposed as serum biomarkers in ALD, and their concentrations have increased according to Child-Pugh score progression[18]. However, their activity and regulation in fibrosis stages has not been previously evaluated in detail in CHC patients.
MMP-1 is known to degrade collagen I and III, which are typical indicators of liver fibrosis[27,33]. However, in human liver biopsy samples, active MMP-2 expression in the liver parenchyma has been observed[15]. In 2011, MMP-2 was reported to suppress collagen I expression in a murine toxin-induced liver fibrosis model[34]. In the present study, we identified higher concentrations of MMP-2 in the serum of CHC patients compared with healthy subjects. Similarly, HCV-infected patients were reported to have higher circulating levels of MMP-2 than healthy donors[35,36].
Additionally, TIMP-1 levels have been shown to be significantly higher in HCV vs healthy donors, suggesting the presence of the inactive form of MMP-2[17]. Interestingly, we found no proteolytic activity of that gelatinase type A in our results. Our findings correlate with the histopathologic events of human liver biopsies of patients with chronic hepatitis and cirrhosis of the liver, in which in situ hybridization showed a strong label of inactive MMP-2 in HSCs located in the lobules and periportal areas and in fibroblasts in the fibrous septa[15]. Our dot blot results showed that TIMP-1 had a pattern like that of MMP-2 production, demonstrating an apparent expression in mild fibrosis (F0-F1) and an important reduction in F2 suggesting that TIMP-1 acts on serum MMP-2 regulation in patients. Perhaps MMP-2 downregulation requires less regulation by its inhibitor. In addition, the analysis of fibrosis grades did not display differences in MMP-2 at any stage evaluated. A recent meta-analysis suggests that lower serum levels of MMP-2 can be found in F2 and F3. The production of MMP-2 has been reported in HSCs, monocytes, lymphocytes, dendritic cells and fibroblasts[37]. However, secretion into serum could be due to alterations in the cellular mechanism caused by HCV (e.g., methylation and acetylation)[38]. Those results support the evidence that inactive MMP-2 is overproduced in CHC, but its role in fibrosis progression is uncertain.
We also found that the behavior of gelatinase B, or MMP-9, was like that of MMP-2. Our results described high levels of MMP-9 in the CHC patients, and the zymography analysis showed no gelatinase activity in the general substrate under physiologic conditions in any of the fibrosis stages evaluated (pH 7.0 and 37 °C). MMP-9 is mainly produced by Kupffer cells, but it can also be produced by lymphocytes and endothelial cells. The inactive presence of MMP-9 in the serum of patients with CHC in our study could be explained by the inactive form of MMP-2, which is its natural activator[39]. Furthermore, denaturalized collagen (Azocoll) was used as the specific substrate for MMP-7[40] showing activity in F0 and F1. However, the collagenolytic activity was drastically reduced after F2, as occurs with TIMP-1 at those stages. TIMP-1 can act as an inhibitor of MMP-7[41], but our results showed strong collagenase activity and higher levels of TIMP-1 at the same stages of fibrosis, suggesting that TIMP-1 could be involved in the partial regulation of MMP-7[38]. Similarly, TIMP-1 has also been reported to directly inhibit MMP-9. In fact, MMP-9 has been suggested as a therapeutic target for fibrosis resolution because it is related to the transdifferentiation process of HSCs and apoptosis of that cell line[29]. A longitudinal study reported an approximate 40% reduction of MMP-9 levels in patients treated with dual and triple antiviral therapies but no changes in MMP-2, TIMP-1, or TIMP-2, which the authors suggested was related to the reduction of liver inflammation[35].
Taken together, our results strongly suggest that MMPs and their activity, when determined in serum, could be complementary indicators in the diagnosis of inflammation and fibrosis, especially MMP-7 in advanced stages. The inactive stage of MMPs could be due to alterations in synthesis and production (acetylation and deacetylation, translation or post-transduction modification) caused by the HCV[38]. The identification of novel strategies or therapeutic targets to induce the fibrolytic function of MMPs could be crucial for improving the recovery from liver damage, preventing patients from progressing to HCC, even after receiving direct-acting antiviral treatment. It is also important to be familiar with the fibrolytic process in other liver diseases (nonalcoholic fatty liver disease and ALD) to understand and distinguish molecular and cellular events so that strategies can be implemented to reduce the exacerbated production of ECM and the consequent development of cirrhosis. In short, our results strongly suggest that serum MMP-7 could be used as a complementary indicator in the diagnosis of advanced fibrosis in CHC. Collagenolytic activity occurred mainly at early fibrosis stages (F0 and F1), but gelatinase activity was not detected at any fibrosis stage. Our study provides novel evidence of the increasing production and downregulation of serum MMP activity in CHC patients during the fibrolytic process and CHC progression.
Serum concentrations of MMPs were upregulated in patients with CHC, but their collagenolytic activity was limited to early fibrosis stages, whereas gelatinase functions were inactive during fibrosis progression despite their higher circulating concentrations.
The concentration and activity of MMPs, especially MMP-7, could be complementary indicators in the diagnosis of advanced fibrosis. It is possible that the HCV modulates the cellular and molecular mechanisms of MMP production affecting their correct fibrolytic functions and potentially resulting in progression to HCC. Further studies are needed to determine the exact mechanisms by which the HCV maintains MMPs inactive.
Matrix metalloproteinases (MMPs) maintain the homeostasis between fibrogenesis and fibrolytic processes in the liver. Few studies on the production and activity of liver MMPs and fibrosis progression have been performed in humans.
The correct determination of liver fibrosis stages is imperative for making the diagnosis and implementing therapeutic decisions. At present, there is no evidence of the production and activity of MMP-2, MMP-7 or MMP-9 or their correlation with fibrosis progression in serum samples from patients with liver diseases.
In the present prospective, cross-sectional, multicenter study, we assessed the production, activity and regulation of matrix metalloproteinases in liver fibrosis stages in chronic hepatitis C (CHC).
We selected CHC patients from the Hospital General de México, “Dr. Eduardo Liceaga,” the Universidad Autónoma de Nuevo Leon and the Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán.” Patients were categorized in fibrosis grades through FibroTest®and/or FibroScan®(F0, F1, F2, F3 or F4). Serum concentrations of MMP-2, -7 and -9 were determined. Differences were validated by the Kruskal-Wallis and Mann-Whitney U tests. Area under the receiver operating characteristic curve was calculated in fibrosis degrees. Proteolytic activity was validated by chromogenic and enzymatic assays and serum concentration, and the regulation of tissue inhibitor of metalloproteinases-1 was tested in fibrosis progression.
We compared 119 CHC patients with 119 healthy subjects. MMP-2, -7 and -9 concentrations were higher in the patients with CHC than in the control subjects. No differences between the serum concentrations of MMP-2 and MMP-9 were found, but MMP-7 showed differential regulation in accordance with fibrosis stages as well as an acceptable receiver operating characteristic (0.705), in advanced fibrosis (F4). Collagenolytic MMP activity was maintained in F0 and F1 but decreased significantly in F2, F3 and F4. Gelatin activity was not observed in any stage of fibrosis. The concentration of tissue inhibitor of metalloproteinases-1 was lower in F2 and F4 compared with F0, F1 and healthy subjects. Inactive MMPs were found in the serum of the CHC patients.
Elevated concentrations of inactive MMPs were present in the serum of CHC patients, reflecting the impossibility to restrain liver fibrosis progression. MMPs could be used in the diagnosis of liver fibrosis and the treatment for its reversal in CHC.
Given that MMP-2, -7 and -9 have not been simultaneously evaluated in the serum from liver fibrosis patients, MMPs could be used to improve the currently available diagnostic methods and as therapeutic targets. They could also be used as a monitoring tool in treatment-experienced patients that continue to present with liver fibrosis and develop cirrhosis and/or hepatocellular carcinoma.
We are grateful to Daniel Santana-Vargas for his assistance in the statistical revision of the present study. Thanks to Postdoctoral Fellowship program at National Autonomous University of Mexico (to M-MC).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding L, Feng QS S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX
| 1. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1221] [Cited by in F6Publishing: 1570] [Article Influence: 224.3] [Reference Citation Analysis (0)] |
| 2. | Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44-46:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 309] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 3. | Martinez-Hernandez A, Delgado FM, Amenta PS. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest. 1991;64:157-166. [PubMed] [Cited in This Article: ] |
| 4. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1567] [Cited by in F6Publishing: 1567] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 5. | Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2013;3:1473-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 6. | Foglia B, Cannito S, Bocca C, Parola M, Novo E. ERK Pathway in Activated, Myofibroblast-Like, Hepatic Stellate Cells: A Critical Signaling Crossroad Sustaining Liver Fibrosis. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol. 2006;44:57-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Hanafusa H, Ninomiya-Tsuji J, Masuyama N, Nishita M, Fujisawa J, Shibuya H, Matsumoto K, Nishida E. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161-27167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3192] [Cited by in F6Publishing: 3499] [Article Influence: 249.9] [Reference Citation Analysis (0)] |
| 10. | Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 11. | Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21 Suppl 3:S88-S91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3186] [Cited by in F6Publishing: 3146] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 13. | Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 365] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 14. | Roderfeld M, Hemmann S, Roeb E. Mechanisms of fibrinolysis in chronic liver injury (with special emphasis on MMPs and TIMPs). Z Gastroenterol. 2007;45:25-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Takahara T, Furui K, Yata Y, Jin B, Zhang LP, Nambu S, Sato H, Seiki M, Watanabe A. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology. 1997;26:1521-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160-1167. [PubMed] [Cited in This Article: ] |
| 17. | Lichtinghagen R, Huegel O, Seifert T, Haberkorn CI, Michels D, Flemming P, Bahr M, Boeker KH. Expression of matrix metalloproteinase-2 and -9 and their inhibitors in peripheral blood cells of patients with chronic hepatitis C. Clin Chem. 2000;46:183-192. [PubMed] [Cited in This Article: ] |
| 18. | Prystupa A, Boguszewska-Czubara A, Bojarska-Junak A, Toruń-Jurkowska A, Roliński J, Załuska W. Activity of MMP-2, MMP-8 and MMP-9 in serum as a marker of progression of alcoholic liver disease in people from Lublin Region, eastern Poland. Ann Agric Environ Med. 2015;22:325-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Irvine KM, Wockner LF, Hoffmann I, Horsfall LU, Fagan KJ, Bijin V, Lee B, Clouston AD, Lampe G, Connolly JE, Powell EE. Multiplex Serum Protein Analysis Identifies Novel Biomarkers of Advanced Fibrosis in Patients with Chronic Liver Disease with the Potential to Improve Diagnostic Accuracy of Established Biomarkers. PLoS One. 2016;11:e0167001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8015] [Cited by in F6Publishing: 39803] [Article Influence: 829.2] [Reference Citation Analysis (0)] |
| 21. | Mitro K, Bhagavathiammai A, Zhou OM, Bobbett G, McKerrow JH, Chokshi R, Chokshi B, James ER. Partial characterization of the proteolytic secretions of Acanthamoeba polyphaga. Exp Parasitol. 1994;78:377-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC Med. 2014;12:159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 2017;4:e000139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Childs K, Merritt E, Considine A, Sanchez-Fueyo A, Agarwal K, Martinez-Llordella M, Carey I. Immunological Predictors of Nonresponse to Directly Acting Antiviral Therapy in Patients With Chronic Hepatitis C and Decompensated Cirrhosis. Open Forum Infect Dis. 2017;4:ofx067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Hartl J, Scherer MN, Loss M, Schnitzbauer A, Farkas S, Baier L, Szecsey A, Schoelmerich J, Schlitt HJ, Kirchner GI. Strong predictors for alcohol recidivism after liver transplantation: non-acceptance of the alcohol problem and abstinence of < 3 months. Scand J Gastroenterol. 2011;46:1257-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Zeng QL, Li ZQ, Liang HX, Xu GH, Li CX, Zhang DW, Li W, Sun CY, Wang FS, Yu ZJ. Unexpected high incidence of hepatocellular carcinoma in patients with hepatitis C in the era of DAAs: Too alarming? J Hepatol. 2016;65:1068-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 684] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 28. | Zucoloto ML, Gonçalez T, Custer B, McFarland W, Martinez EZ. Comparison of the demographic and social profile of blood donors and nondonors in Brazil. Health Soc Care Community. 2019;27:330-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Roeb E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018;68-69:463-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 30. | Huang CC, Chuang JH, Chou MH, Wu CL, Chen CM, Wang CC, Chen YS, Chen CL, Tai MH. Matrilysin (MMP-7) is a major matrix metalloproteinase upregulated in biliary atresia-associated liver fibrosis. Mod Pathol. 2005;18:941-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Ke B, Fan C, Yang L, Fang X. Matrix Metalloproteinases-7 and Kidney Fibrosis. Front Physiol. 2017;8:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Bauer Y, White ES, de Bernard S, Cornelisse P, Leconte I, Morganti A, Roux S, Nayler O. MMP-7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2017;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Iimuro Y, Nishio T, Morimoto T, Nitta T, Stefanovic B, Choi SK, Brenner DA, Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Radbill BD, Gupta R, Ramirez MC, DiFeo A, Martignetti JA, Alvarez CE, Friedman SL, Narla G, Vrabie R, Bowles R, Saiman Y, Bansal MB. Loss of matrix metalloproteinase-2 amplifies murine toxin-induced liver fibrosis by upregulating collagen I expression. Dig Dis Sci. 2011;56:406-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Latronico T, Mascia C, Pati I, Zuccala P, Mengoni F, Marocco R, Tieghi T, Belvisi V, Lichtner M, Vullo V, Mastroianni CM, Liuzzi GM. Liver Fibrosis in HCV Monoinfected and HIV/HCV Coinfected Patients: Dysregulation of Matrix Metalloproteinases (MMPs) and Their Tissue Inhibitors TIMPs and Effect of HCV Protease Inhibitors. Int J Mol Sci. 2016;17:455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Tseng GC, Peng YC, Wang HH, Lee FH, Huang LR. Matrix metalloproteinases 2 and 9 correlate with alanine aminotransferase and hepatitis C virus in blood donors. Hepatogastroenterology. 2008;55:535-538. [PubMed] [Cited in This Article: ] |
| 37. | Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69: 851-859. [PubMed] [Cited in This Article: ] |
| 38. | Asselah T, Bièche I, Sabbagh A, Bedossa P, Moreau R, Valla D, Vidaud M, Marcellin P. Gene expression and hepatitis C virus infection. Gut. 2009;58:846-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Nguyen M, Arkell J, Jackson CJ. Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 40. | Kihira Y, Mori K, Miyazaki K, Matuo Y. Production of recombinant human matrix metalloproteinase 7 (Matrilysin) with potential role in tumor invasion by refolding from Escherichia coli inclusion bodies and development of sandwich ELISA of MMP-7. Urol Oncol. 1996;2:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Le AP, Friedman WJ. Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. J Neurosci. 2012;32:703-712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |