Published online Jan 16, 2018. doi: 10.4253/wjge.v10.i1.16
Peer-review started: July 6, 2017
First decision: August 7, 2017
Revised: August 30, 2017
Accepted: November 3, 2017
Article in press: November 3, 2017
Published online: January 16, 2018
To investigate whether an uncovered self-expandable metal stent (UCSEMS) with a large diameter could prevent recurrent biliary obstruction (RBO).
Thirty-eight patients with malignant biliary obstruction underwent treatment with an UCSEMS with a 14-mm diameter (Niti-S 14). Retrospectively, we evaluated technical and functional success rate, RBO rate, time to RBO, survival time, and adverse events in these patients.
Stent placement success and functional success were achieved in all patients. Two patients (5.3%) had RBO due to tumor ingrowth or overgrowth. The median time to RBO was 190 (range, 164-215) d. The median survival time was 120 (range, 18-502) d. The 6-mo non-RBO rate was 91%. Other adverse events other than RBO occurred as follows: Acute cholecystitis, post-ERCP pancreatitis, hemobilia, and fever without exacerbation of liver injury, and liver abscess in 4 (10.3%), 3 (7.9%), 2 (5.3%), 1 (2.6%), and 1 (2.6%), respectively. Migration of the stents was not observed.
Niti-S 14 is considered to be a preferable metal stent because of a low rate of RBO with no migration.
Core tip: Our manuscript reports on 38 patients with unresectable distal malignant biliary obstruction (MBO) treated with a newly developed 14-mm diameter Niti-S biliary uncovered metal stent. The results could show the stent is preferable for the palliate treatment of unresectable distal MBO because of a low rate of recurrent biliary obstruction, no migration, a low rate of other complications, and a high success rate of placement.
- Citation: Kikuyama M, Shirane N, Kawaguchi S, Terada S, Mukai T, Sugimoto K. New 14-mm diameter Niti-S biliary uncovered metal stent for unresectable distal biliary malignant obstruction. World J Gastrointest Endosc 2018; 10(1): 16-22
- URL: https://www.wjgnet.com/1948-5190/full/v10/i1/16.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i1.16
Endoscopic transpapillary biliary stent placement is an established procedure for relieving jaundice and treating cholangitis in patients with malignant biliary obstruction (MBO). The treatment can contribute to the improvement of quality of life and prognosis of patients with unresectable MBO. A plastic tube stent had been widely used as the first generation of stent treatment for MBO[1], although it had the issue of being easily occluded due to its small diameter of 7 to 11 Fr.
In the last decade of the 20th century, a self-expandable metal stent (SEMS) with a wider diameter of 8 to 10 mm without being covered, i.e., an uncovered SEMS (UCSEMS), was developed with recognition for its efficacy in relieving jaundice with long term patency[2-5]. However, stent occlusion due to tumor ingrowth and food impaction was frequently experienced and thus requires a solution.
A covered SEMS (CSEMS) was produced to prevent tumor in growth through the stent mesh. The advantage of the CSEMS was long-term patency because the membrane could prevent tumor in growth[6]; however, this stent type could not perfectly avoid occlusion as sludge or food impaction was encountered, or stent migration easily occurred[7-9]. It was hypothesized that the larger stent diameter could contribute to maintaining a longer patency with supportive evidence by some reports[10-12]. Recently, a CSEMS with a 12-mm diameter, SUPREMO 12, was developed and verified this hypothesis[13]. However, easy migration of CSEMS remained an issue despite the larger diameter[13].
To prevent migration, an UCSEMS is preferable[6,14,15] to a CEMS, because the uncovered mesh of the stent is embedded in the bile duct wall and makes the stent keep still. However, occlusion due to tumor in growth remains unresolved for treatment by an UCSEMS. If an UCSEMS stent had a larger diameter, it could be expected to keep the bile flow despite tumor ingrowth and maintain a longer patency and a UCSEMS with a large diameter of 14 mm, Niti-S 14 (Taewoong Medical CO., Ltd., Seoul, South Korea), was developed. Herein, the efficacy and safety of the Niti-S 14 for MBO was evaluated.
We retrospectively evaluated the efficacy and safety of Niti-S 14, placed transpapillarily for consecutive and unresectable MBO from April 2014 to May 2016 in the following 3 institutions; Shizuoka General Hospital, Gifu Municipal Hospital, and Hamamatsu University Hospital. The outcome measures were rate of technical and functional achievement, rate of recurrent biliary obstruction (RBO)[16], time to RBO (TRBO)[16], survival time, and stent-related adverse events. Diagnosis of MBO was established by laboratory data, imaging findings, and histopathological examinations. Stage of the disease was determined by the findings of computed tomography or endoscopic ultrasonography.
Thirty-eight patients with MBO of the middle to lower part of the extrahepatic bile duct and expectance of survival for longer than 2 mo underwent treatment for MBO by Niti-S 14 placement (Table 1). Twenty-one males and 17 females were included with median age of 70 (range, 52-90) years. All patients had fair activity of daily living (ECOG-PS grade 0-2). Those with post-gastrectomy state (Billroth II or Roux-en-Y reconstruction) were excluded from candidates for this treatment. Causes of obstruction of the extrahepatic bile duct were pancreatic cancer, bile duct cancer, and metastatic lymphadenopathy in 36, 1, and 1 patients, respectively. Thirty-seven patients belonged to the clinical stage IV of the UICC TNM classification, and the remaining one patient was stage III. The median tumor size was 33 (range, 13-70) mm and the median length of the biliary stricture was 27 (range, 10-60) mm. The median diameter of the proximal bile duct was 13.5 (range, 7-20) mm.
| n = 38 | |
| Men/women | 21/17 |
| Age (yr) | 70 (52-90) |
| PS (0/1/2) | 8/21/9 |
| Diagnosis | |
| Pancreatic cancer | 36 |
| Bile duct cancer | 1 |
| Metastatic nodes | 1 |
| Clinical stage III/IV | 1/37 |
| Tumor size (mm) | 33 (13-70) |
| Length of the biliary stricture (mm) | 27 (10-60) |
| Maximum diameter of the proximal bile duct (mm) | 13.5 (7-20) |
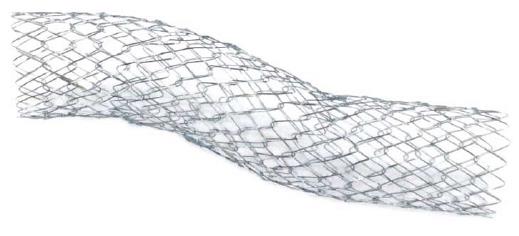
Niti-S 14 is a newly developed UCSEMS with braided structure made from nitinol, and a large diameter of 14 mm with a length of 60 or 80 mm (Figure 1). The outer diameter of the delivery sheath was 9 Fr. A 0.035-inch guide-wire can be used for introducing the stent into the bile duct.
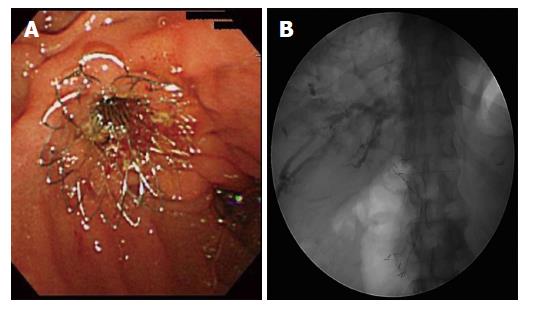
In all patients, Niti-S 14 was placed through the duodenum major papilla during endoscopic retrograde cholangiopancreatography. A 60- or 80-mm stent length was selected according to the length of the stricture. The distal end of the stent was placed in the duodenum (Figure 2). Endoscopic sphincterotomy (EST) was performed at the discretion of the operator, mainly to avoid post-ERCP pancreatitis. The stricture was not dilated by a balloon before stent placement. Niti-S 14 was used as the primary treatment for MBO in principal.
Clinical signs and symptoms and biochemical parameters of liver function and inflammation (aspartate transaminase, alanine transaminase, alkaline phosphatase, gamma glutamyl transpeptidase, total and direct bilirubin, and C-reactive protein levels) were evaluated at least monthly. Complications were defined according to the Tokyo Criteria 2014[14]. According to these criteria, RBO was defined as occlusion or symptomatic migration, and TRBO was the interval between stent placement and RBO, which was calculated instead of patency. The definition of post-ERCP pancreatitis (PEP) was new or worsened abdominal pain with serum amylase ≥ threefold the upper limit of normal, measured > 24 h after the procedure. Acute cholecystitis was diagnosed when a fever > 38 °C or right upper abdominal pain occurred with supportive imaging studies.
Stent patency duration and survival time were estimated by the Kaplan-Meier method. Continuous variables were analyzed using one-way analysis of variance, and categorical and binary variables were analyzed using Fisher’s exact test. All statistical tests were two-tailed and assessed at a 0.05 probability level. All analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, Illinois, United States).
In all patients, stent placement was successful (technical success rate = 100%) (Table 2). Stents with a length of 60 mm and 80 mm were selected for and placed in 14 and 24 patients, respectively. EST was performed before placement in 20 patients (52.6%) because the orifice of the major papilla was small with incomplete obstruction of the main pancreatic duct by pancreatic head cancer in 18 and without pancreatic head cancer in 2. In all patients, total bilirubin level deceased and normalized within 14 d and functional success (defined as 50% decrease in or normalization of the bilirubin level within 14 d of stent placement[14]) was achieved (functional success rate = 100%). Stent placement was performed after relieving jaundice by retrograde biliary drainage and naso-biliary drainage (NBD) in 9 (23.7%) and 2 (5.3%) patients, respectively, and for replacing a previously placed CSEMS with smaller diameter due to cholangitis in 5 (13.2%).
| n (%) | |
| Technical success | 38 (100) |
| Functional success | 38 (100) |
| Selected stent length (60/80 mm) | 14/24 (36.8/63.2) |
| Endoscopic sphincterotomy | 20 (52.6) |
| Previous drainage (RBD/NBD) | 9/2 (23.7/5.3) |
| Replacement for CSEMS | 5 (13.2) |
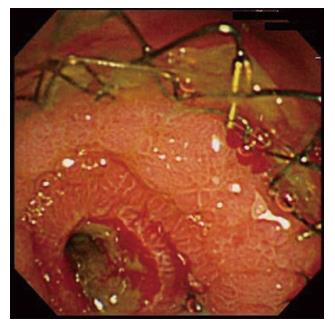
Two patients (5.3%) experienced RBO due to tumor ingrowth and overgrowth just above the upper end of the stent (Table 3). Jaundice with liver injury was recognized on 164 d and 215 d in two patients. The median TRBO was 190 (range, 164-215) d. RBO was treated by placing a CSEMS endoscopically across the obstructed biliary portion through the previously placed Niti-S 14. In the patient with tumor overgrowth, the Niti-S 14 was patent on endoscopic retrograde cholangiography, and endoscopic observation revealed coverage of the inside wall of the stent by a hyperplastic mucosal tissue (Figure 3).
| n (%) | |
| RBO | 2 (5.3) |
| Tumor ingrowth | 1 (2.6) |
| Tumor overgrowth | 1 (2.6) |
| Median TRBO (d) | 190 (164-215) |
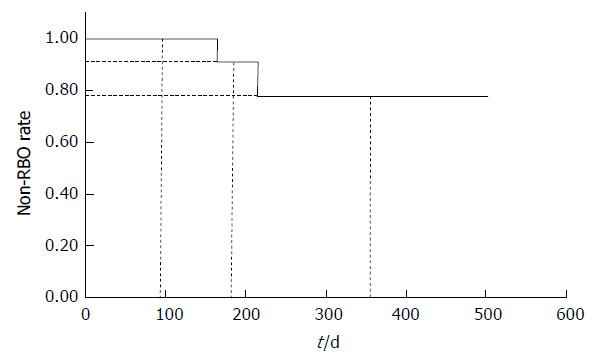
| Non-obstruction rates of 3, 6, 12 mo (%) | 100, 91, 78 |
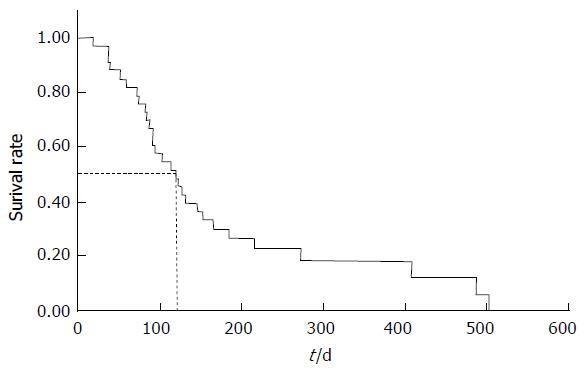
| Median survival time (d) | 120 (18-502) |
The non-obstruction rates of 3, 6 and 12 mo were 100%, 91% and 78%, respectively (Figure 4). The median survival time was 120 (range, 18-502) d (Figure 5).
Adverse events occurred in 11 patients (28.9%). RBO was recognized in two patients (5.3%) in the manner of tumor ingrowth and tumor overgrowth as described above. Adverse events other than RBO occurred as follows (Table 4): Acute cholecystitis, PEP, hemobilia, fever without exacerbation of liver injury, and liver abscess in 4 (10.3%), 3 (7.9%), 2 (5.3%), 1 (2.6%) and 1 (2.6%), respectively. Stent migration was not observed. Bile duct perforation was not experienced despite of the large diameter of 14 mm. Acute cholecystitis occurred on day 3, 32, 217 and 487 after stent placement in four respective patients and the inflamed and swollen gallbladder was punctured percutaneously without placing a percutaneous drainage tube with the infected bile aspirated from the gallbladder. PEP was diagnosed within the day after placement but was mild and treated by conservative ways. In 2 patients, hemobilia was recognized by examining the cause of hematemesis on day 92 in one and 119 in the other, and a fully covered EMS (WallFlex stent, 10 mm × 60 mm, Boston Scientific Corp, Natick, Mass, United States) was placed inside the 14-mm Niti-S with achievement of hemostat. In patients with cholangitis due to migration of a previously placed CSEMS, we swapped the previously placed stent to the Niti-S 14 in 5 patients. Among them, one patient had persistent high fever after replacement without cholecystitis despite the relief of hepatobiliary dysfunction; the patient was treated by antibiotic administration for 10 d. One patient experienced liver abscess, which was diagnosed on day 17 because of high fever, and was treated by percutaneous puncture with drainage tube placement. However, the patient died the next day due to septic shock with abscess rupture toward the peritoneal cavity.
| Complications | 11/38 (28.9%) | Time to event (d) |
| Acute cholecystitis | 4 (10.3) | 3, 32, 217, 487 |
| PEP | 3 (7.9) | 1 (each) |
| Hemorrhage | 2 (5.3) | 92, 119 |
| Fever without exacerbation of liver injury | 1 (2.6) | 1 |
| Liver abscess | 1 (2.6) | 17 |
The ideal stent is free from occlusion, migration, and other adverse events. Especially, occlusion and migration are major problems for treating MBO by SEMS. To resolve these complications, the 14-mm Niti-STM biliary uncovered-stent (Niti-S 14) was developed, which was characterized by an uncovered feature and a large diameter of 14 mm. On development, the diameter of 14 mm was expected to be large enough to prevent occlusion despite tumor ingrowth. In this study, the results support the superiority of the Niti-S 14 with a low RBO rate, lack of migration, low rates of other complications, and a high technical success rate.
Stent occlusion was recognized in just 2 patients (5.3%) with Niti-S 14, and the 6-mo stent patency was 91%. Previous reports described stent occlusion rates of 18%-38% using conventional types of UCSEMS with a diameter of 10 mm[5,6,15,16]. If our result of 5.3% in Niti-S 14 is comparable with that of previous reports, it is because of low incidence of tumor ingrowth. In patients with Niti-S 14, endoscopic observation of the stent showed mucosa or tumor tissue growth into the inside of the stent, which is the same finding observed with the conventional type of UCSEMS, while the stent was not occluded because the large 14-mm diameter could maintain the stent cavity. On the other hand, tumor overgrowth was recognized in one patient. The length of the stent might be insufficient to prevent bile duct obstruction due to overgrowth in patients with a large tumor, and tumor overgrowth resulting from RBO could be resolved by a longer Niti-S 14.
In CSEMS, stent occlusion by tumor ingrowth is rarely experienced, while tumor overgrowth, food impaction, and migration were relatively common causes of stent occlusion, with reported occlusion rates of 14%-23% in a fully-covered SEMS[6,15], 5.8%-29% in a partially covered SEMS[16,17], and 26% in SUPREMO 12[13]. In comparing our result with those of previous reports on CSEMS, an RBO rate of 5.3% was preferable.
Six-month stent patency was also evaluated previously, and reported to be 78%-90%, 70%-94%, 63%-91%, and 50% in a conventional type of UCSEMS[5,6,15,16], fully-covered SEMSs[6,18,19], partially-covered SEMS[16,17], and SUPREMO 12[13], respectively. Our result of 91% using Niti-S 14 was comparable or superior to that of these previous studies.
Niti-S 14 is an uncovered, which is a characteristic that prevents migration. A lack of migration also contributes to low RBO rate. RBO in patients with CSEMS placement was frequently due to stent migration in previous reports[13,16,20]; this complication was also observed if a partially-covered SEMS was used[20]. To prevent migration, selecting a UCSEMS may be desirable, and the other issue of tumor ingrowth should be resolved. As mentioned above, the large diameter of 14 mm could provide a solution for this problem.
Acute cholecystitis and PEP are relatively common adverse events after placing an SEMS with rates of 0%-10% and 0%-8%, respectively, in previous reports[6,15-23], and were experienced in 10.3% and 7.9% of patients with Niti-S 14, respectively. Despite the large diameter of the stent, the incidences were almost equal to those of previous reports. After placing Niti-S 14, EST was performed in 18 patients with pancreatic head cancer or without pancreatic head cancer in 2 for the purpose of preventing PEP, because the main pancreatic duct was not completely obstructed by the tumor and the orifice of the major papilla was small. As a result, PEP occurred in 5% of patients with EST and 11% of those without EST. In patients with EST, the incidence of PEP tended to be low, but it was not statistically significant. Those results suggest that the large diameter of a stent is not responsible for PEP and EST does not contribute to preventing PEP. Our result of performing EST to prevent PEP does not contradict the previous report describing that EST does not effectively act to prevent PEP in patients undergoing stent placement[24]. It is suggested that several factors besides obstructing a pancreatic duct orifice by a stent are responsible for PEP.
We succeeded placement of SEMS in all patients using Niti-S 14. Despite the large diameter of the stent, the delivery system of Niti-S 14 is thin (9 Fr) and soft. The characteristics of the Niti-S 14 delivery system could provide an optimal effect for endoscopic introduction of the delivery system into the bile duct through the duodenal papilla.
Although these preferable results were obtained in placing Niti-S 14, our study showed that patients undergoing Niti-S 14 placement had a shorter survival time of 113 (range, 18-502) d compared with those of previous reports[14,15,20]. In Niti-S 14, almost all patients had pancreatic cancer and the levels of CA19-9 tended to be higher. This tendency might lead to shorter survival, because, as it is widely known, pancreatic cancer has a poor prognosis and high CA19-9 levels indicate advanced tumor progression[25]. On the other hand, it cannot be denied that the larger diameter were responsible for shorter survival time. The problem of the shorter survival time should be resolved by further randomized control studies comparing Niti-S 14 with other types of stent. Another problem regarding the shorter survival time is this shorter observation time might lead to an apparent low rate of RBO.
In our study, persistent high fever was observed after replacing the CSEMS for Niti-S 14 because of cholangitis due to RBO from migration. Acute cholecystitis was not recognized in the patient. We speculate that this complication might be induced by an enwrapped infected bile duct epithelium, probably with micro-abscess. Moreover, we experienced one patient die on day 18 due to liver abscess in Niti-S 14. The abscess was large at diagnosis, and the possibility that the abscess had already developed by the time of stent placement was presumed.
In conclusion, Niti-S 14 is considered to be a preferable SEMS because of a low rate of RBO, no migration, a low rate of other complications, and a high success rate. However, this study is limited because of the small number of patients and the retrospective evaluation. Further prospective, multicenter, international double-blind controlled studies, comparing different type of stents (e.g., UCSEMS vs partially covered SEMS) are necessary, in order to standardize the best drainage policy.
Recurrent biliary obstruction (RBO) due to tumor ingrowth or migration remains to be resolved in endoscopic transpapillary biliary stent placement for malignant biliary obstruction (MBO).
It was expected that an uncovered self-expandable metal stent with a large diameter could prevent RBO.
Niti-S 14 is a large bore and uncovered metal stent, but is safe for treatment for MBO and considered to be a preferable SEMS because of a low rate of RBO with no migration.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Eleftheriadis NP, Fiori E, Slomiany BL S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Kozarek RA. Endoscopically placed biliary drains and stents. Am Fam Physician. 1982;26:189-192. [PubMed] [Cited in This Article: ] |
| 2. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25:207-212. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Prat F, Chapat O, Ducot B, Ponchon T, Pelletier G, Fritsch J, Choury AD, Buffet C. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47:1-7. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Kaassis M, Boyer J, Dumas R, Ponchon T, Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF, Burtin P. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57:178-182. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Saleem A, Leggett CL, Murad MH, Baron TH. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321-327.e1-e3. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Almadi MA, Barkun AN, Martel M. No benefit of covered vs uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:27-37.e1. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Yang Z, Wu Q, Wang F, Ye X, Qi X, Fan D. A systematic review and meta-analysis of randomized trials and prospective studies comparing covered and bare self-expandable metal stents for the treatment of malignant obstruction in the digestive tract. Int J Med Sci. 2013;10:825-835. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;34:412-417. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Siegel JH, Pullano W, Kodsi B, Cooperman A, Ramsey W. Optimal palliation of malignant bile duct obstruction: experience with endoscopic 12 French prostheses. Endoscopy. 1988;20:137-141. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Loew BJ, Howell DA, Sanders MK, Desilets DJ, Kortan PP, May GR, Shah RJ, Chen YK, Parsons WG, Hawes RH. Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc. 2009;70:445-453. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Mukai T, Yasuda I, Isayama H, Iwashita T, Itoi T, Kawakami H, Kogure H, Nakai Y. Pilot study of a novel, large-bore, fully covered self-expandable metallic stent for unresectable distal biliary malignancies. Dig Endosc. 2016;28:671-679. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kullman E, Frozanpor F, Söderlund C, Linder S, Sandström P, Lindhoff-Larsson A, Toth E, Lindell G, Jonas E, Freedman J. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915-923. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Telford JJ, Carr-Locke DL, Baron TH, Poneros JM, Bounds BC, Kelsey PB, Schapiro RH, Huang CS, Lichtenstein DR, Jacobson BC. A randomized trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72:907-914. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Isayama H, Hamada T, Yasuda I, Itoi T, Ryozawa S, Nakai Y, Kogure H, Koike K. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015;27:259-264. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Costamagna G, Tringali A, Reddy DN, Devière J, Bruno M, Ponchon T, Neuhaus H, Mutignani M, Rao GV, Lakhtakia S. A new partially covered nitinol stent for palliative treatment of malignant bile duct obstruction: a multicenter single-arm prospective study. Endoscopy. 2011;43:317-324. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Kahaleh M, Talreja JP, Loren DE, Kowalski TE, Poneros JM, Degaetani M, Raijman I, Sejpal DV, Patel S, Rosenkranz L. Evaluation of a fully covered self-expanding metal stent with flared ends in malignant biliary obstruction: a multicenter study. J Clin Gastroenterol. 2013;47:e96-100. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Petersen BT, Kahaleh M, Kozarek RA, Loren D, Gupta K, Kowalski T, Freeman M, Chen YK, Branch MS, Edmundowicz S. A multicenter, prospective study of a new fully covered expandable metal biliary stent for the palliative treatment of malignant bile duct obstruction. Gastroenterol Res Pract. 2013;2013:642428. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Isayama H, Mukai T, Itoi T, Maetani I, Nakai Y, Kawakami H, Yasuda I, Maguchi H, Ryozawa S, Hanada K. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointest Endosc. 2012;76:84-92. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Kawakubo K, Isayama H, Nakai Y, Togawa O, Sasahira N, Kogure H, Sasaki T, Matsubara S, Yamamoto N, Hirano K. Risk factors for pancreatitis following transpapillary self-expandable metal stent placement. Surg Endosc. 2012;26:771-776. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Shimizu S, Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Kondo H, Yoshida M, Yamashita H, Umemura S, Hori Y. Predictive factors for pancreatitis and cholecystitis in endoscopic covered metal stenting for distal malignant biliary obstruction. J Gastroenterol Hepatol. 2013;28:68-72. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Shimizu E, Kikuyama M, Hirai R, Matsumura K, Kin H, Nagasawa M, Ogawa K. Acute cholecystitis after expandable metal stent placement for malignant biliary obstruction (in Japanese). J Jp Bil Assoc. 2006;20:142-146. [Cited in This Article: ] |
| 24. | Sofi AA, Nawras A, Alaradi OH, Alastal Y, Khan MA, Lee WM. Does endoscopic sphincterotomy reduce the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis after biliary stenting? A systematic review and meta-analysis. Dig Endosc. 2016;28:394-404. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Dong Q, Yang XH, Zhang Y, Jing W, Zheng LQ, Liu YP, Qu XJ. Elevated serum CA19-9 level is a promising predictor for poor prognosis in patients with resectable pancreatic ductal adenocarcinoma: a pilot study. World J Surg Oncol. 2014;12:171. [PubMed] [DOI] [Cited in This Article: ] |