Published online Jul 16, 2017. doi: 10.4253/wjge.v9.i7.319
Peer-review started: September 23, 2016
First decision: November 21, 2016
Revised: January 7, 2017
Accepted: April 18, 2017
Article in press: April 20, 2017
Published online: July 16, 2017
To determine specific volumetric laser endomicroscopy (VLE) imaging features associated with neoplasia at the gastroesophageal junction (GEJ) and gastric cardia.
During esophagogastroduodenoscopy for patients with known or suspected Barrett’s esophagus, VLE was performed before biopsies were taken at endoscopists’ discretion. The gastric cardia was examined on VLE scan from the GEJ (marked by top of gastric folds) to 1 cm distal from the GEJ. The NinePoints VLE console was used to analyze scan segments for characteristics previously found to correlate with normal or abnormal mucosa. Glands were counted individually. Imaging features identified on VLE scan were correlated with biopsy results from the GEJ and cardia region.
This study included 34 cases. Features characteristic of the gastric cardia (gastric rugae, gastric pit architecture, poor penetration) were observed in all (100%) scans. Loss of classic gastric pit architecture was common and there was no difference between those with neoplasia and without (100% vs 74%, P = NS). The abnormal VLE feature of irregular surface was more often seen in patients with neoplasia than those without (100% vs 18%, P < 0.0001), as was heterogeneous scattering (86% vs 41%, P < 0.005) and presence of anomalous glands (100% vs 59%, P < 0.05). The number of anomalous glands did not differ between individual histologic subgroups (ANOVA, P = NS).
The transition from esophagus to gastric cardia is reliably identified on VLE. Histologically abnormal cardia mucosa produces abnormal VLE features. Optical coherence tomography algorithms can be expanded for use at the GEJ/cardia.
Core tip: This is a retrospective study to explore volumetric laser endomicroscopy (VLE) imaging features associated with neoplasia at the gastroesophageal junction (GEJ) and gastric cardia. Histologically abnormal mucosa due to inflammation or neoplasia more often produces abnormal VLE imaging. Specifically, VLE imaging features of irregular surface, heterogeneous scattering and presence of anomalous glands were more often seen in cases of neoplasia than those without. The GEJ and gastric cardia can be difficult to assess endoscopically for dysplasia, and VLE imaging in this area can aid in a “red-flag” biopsy technique.
- Citation: Gupta N, Siddiqui U, Waxman I, Chapman C, Koons A, Valuckaite V, Xiao SY, Setia N, Hart J, Konda V. Use of volumetric laser endomicroscopy for dysplasia detection at the gastroesophageal junction and gastric cardia. World J Gastrointest Endosc 2017; 9(7): 319-326
- URL: https://www.wjgnet.com/1948-5190/full/v9/i7/319.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i7.319
Barrett’s esophagus (BE) has been well established as a precursor to esophageal adenocarcinoma (EAC)[1,2]. Management of patients diagnosed with BE includes surveillance endoscopy[3]. Using the Seattle protocol, targeted biopsies of visible lesions should be taken followed by 4-quadrant biopsies at 2 cm intervals along the length of the BE segment[4]. In cases of known dysplasia, the random 4-quadrant biopsies should be taken every 1 cm[3].
Though currently the standard of care, these techniques are subject to sampling error since random biopsies may miss areas of high-grade dysplasia (HGD) or intramucosal carcinoma (IMC)[5,6]. There has been increasing interest in evaluating advanced imaging modalities which may allow for better visualization of the entire upper GI mucosal surface and subsurface in order to increase diagnostic yield with targeted biopsies[7,8].
Optical coherence tomography (OCT) is an imaging technique that utilizes low-coherence interferometry to produce high resolution images of biologic tissue by measuring back-scatter light intensity from a near-infrared light source[9]. Recently, an OCT based technology called Fourier-domain OCT or volumetric laser endomicroscopy (VLE) is now commercially available and offers higher imaging speed and improved sensitivity as compared to traditional OCT[10]. The system allows for real time cross-sectional imaging of the esophagus and proximal stomach as an adjuvant to esophagogastroduodenoscopy (EGD). Surface and subsurface architecture such as mucosal layers, gastric pits, and gland morphology can be identified[11]. Several studies have analyzed the correlation between OCT images and histology from biopsy specimens. Specifically, a blinded prospective study found OCT to have an 81% specificity for diagnosing squamous intestinal metaplasia (SIM) at the squamocolumnar junction (SCJ)[12].
Due to the limited knowledge about VLE findings at the gastroesophageal junction (GEJ) and gastric cardia, the purpose of this study is to correlate VLE imaging characteristics with histology in this area in order to determine specific features associated with neoplasia.
This was a retrospective study conducted at a tertiary care center with a referral BE practice. Patients with known or suspected Barrett’s esophagus presented for EGD and VLE. During EGD, biopsies were taken with cold forceps in a targeted and random fashion. Using the NinePoints VLE console, a segment of the VLE scan was delineated from the GEJ (marked by top of gastric folds) to 1 cm distal from the GEJ in order to approximate the gastric cardia. These segments were analyzed frame-by-frame to determine the presence of various imaging features. This analysis was done by a trained reviewer who was initially blinded to the corresponding pathology. Once the image analysis was completed, biopsy results from the GEJ to 1 cm distal to the GEJ were reviewed in order to determine the highest level of pathology found in the segment. Patients were then grouped according to the highest level of pathology indicted on biopsy report: neoplasia, Barrett’s with no dysplasia, inflamed cardia, and normal mucosa. Within these histologic subgroups, the frequency of each imaging characteristic was calculated based on the VLE scan analysis that had been done.
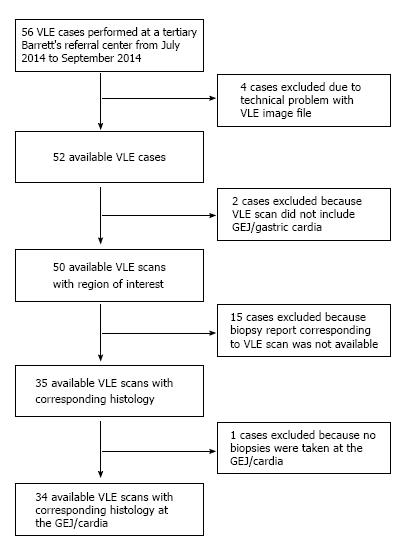
From July 2014 to September 2015, forty-six patients underwent a total of fifty-six procedures with VLE scan at a tertiary care center with referral Barrett’s practice. These patients were undergoing screening or had known BE and were undergoing endoscopic follow-up. Cases were included in the study of they had a VLE scan available for review and had biopsies taken specifically at the GEJ/gastric cardia.
Cases were excluded if the VLE scan did not include imaging of the GEJ/gastric cardia, biopsies were not taken in this region, or if there was a technical problem with the VLE scan.
Of the fifty-six cases, thirty-four met criteria for inclusion in the study. This study was approved by the Institutional Review Board.
All endoscopic procedures were performed by 3 expert endoscopists with experience in detection and management of BE. EGD procedures were performed using the high resolution Olympus GIF-HQ190 gastroscope. After insertion of the gastroscope, the esophagus, GEJ and gastric cardia were first examined by WLE for gross evidence of BE. Narrow band imaging features with near focus was also used. Following this, VLE ODFI imaging was performed using the NinePoints system described below. Lastly, biopsies were taken with cold forceps and/or endoscopic mucosal resection at the endoscopist’s discretion.
The NinePoints Medical VLE optical frequency domain imaging (ODFI) system was used in this study. Technical specifications of ODFI imaging are described in detail in previous publications[12]. Briefly, the NinePoints VLE system includes a balloon centered probe and user console with monitor. The probe consists of a transparent balloon surrounding a laser light source and optical system. Commercially available probe sizes range from 14-25 mm. These are compatible with endoscope channels 2.8 mm and lager. After the balloon is placed into the esophagus and inflated, the central component helically scans while simultaneously retracting throughout the length of the balloon (6 cm). A data set is generated using interferometry and measurement of optical reflection delay from the laser light source. The scan takes 90 s to complete and produces circumferential cross-sectional images of the tissue abutting the edge of the inflated balloon probe. In total, 1200 cross sectional images are obtained from the mucosa to a depth of 3 mm. These images have a resolution of 7 μm making it comparable to low-power microscopy.
VLE images were analyzed on a console that allows for simultaneous cross-sectional and longitudinal views. Additionally, a zoom view was available for both dimensions.
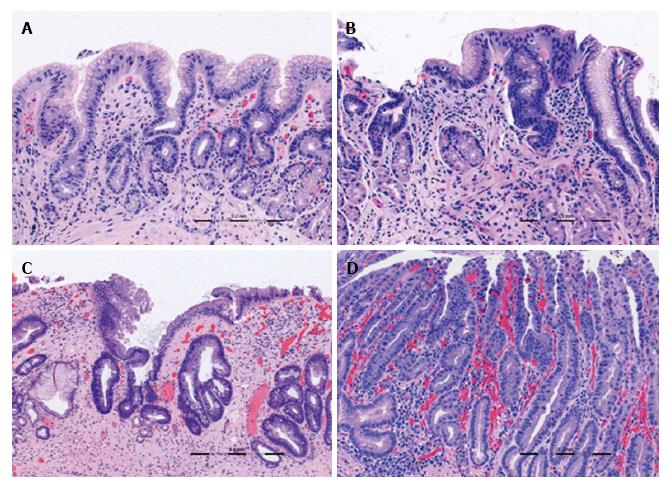
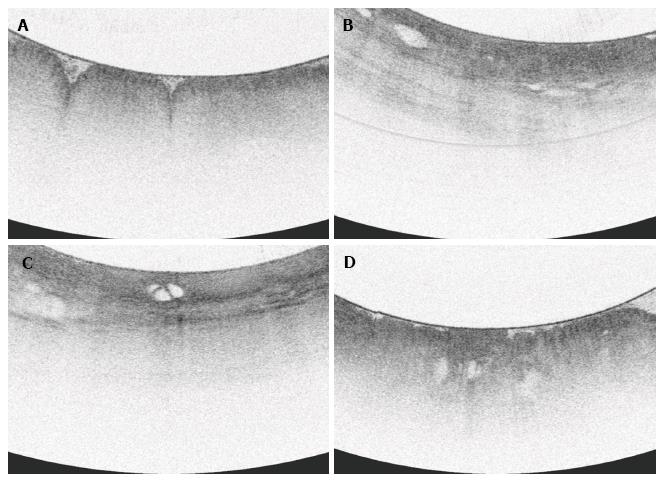
All VLE scans were viewed using the NinePoints VLE console. For each scan included in the study, the corresponding endoscopy report was reviewed to determine the centimeter marking at which the top of gastric folds was seen. The corresponding centimeter marking was found on the VLE scan and this was designated as the GEJ. Each scan was assessed frame by frame from the GEJ to 1 cm below the GEJ. Each frame was viewed circumferentially for presence of the specified features which have been found in previous studies to correlate with normal or abnormal mucosa (Table 1). The features or interest were based on OCT criteria set forth by Evans et al[12] and included gastric rugae, gastric pit architecture, image penetration, homogenous or heterogeneous scattering, surface to subsurface intensity, and surface irregularity. Following this, another review was done during which the number of typical, atypical, and septated glands were counted individually. Glands were deemed to be atypical if they were dilated or had irregular morphology, similar to the definition used in previous publications[13].
| Diagnosis | Imaging criteria on volumetric laser endomicroscopy |
| Squamous epithelium | Layered horizontal architecture |
| Absence of glands | |
| Gastric cardia | Vertical pit architecture |
| Regular glandular architecture | |
| Poor image penetration | |
| Homogeneous scattering | |
| Regular, broad gastric rugae | |
| Metaplasia | Lack of layered or vertical pit architecture |
| Heterogeneous scattering | |
| Irregular surface | |
| Atypical glandular structure |
Statistical analysis was performed using Microsoft Excel software for Windows. χ2 test was used when comparing the proportion of each group that exhibited a particular imaging feature. The t-test was used when determining if the number of typical and atypical glands differed between two groups. Analysis of variance (ANOVA) analysis was performed comparing the number of atypical glands between all histologic subgroups. A P-value of < 0.05 was considered statistically significant in this study. The statistical methods of this study were reviewed by a biostatistician through the University of Chicago Biostatistics Laboratory which is part of the Department of Public Health Sciences.
Thirty-four patients with VLE imaging and cardia level biopsies were included in the study and had an average age of 63 years (SD 9). Twenty-two patients had undergone prior therapy while twelve had not. Of the 22 patients who had undergone prior treatment, 4 patients had undergone endoscopic mucosal resection (EMR), 6 radiofrequency ablation (RFA), 12 hybrid therapy.
Hiatal hernias were present in 20 cases. In two cases, the patient was status post a fundoplication and in one case the patient was status post a duodenal switch surgery. Visible BE was seen during EGD in 22 cases of which 14 had short segment BE and 8 had long segment BE. Visible lesions at the GEJ/gastric cardia such as nodularity or abnormal vascularity were seen in 4 cases.
In patients who had no prior treatment for BE, the highest pathology identified from the GEJ and cardia region was intramucosal carcinoma (IMC)/high grade dysplasia (HGD) in one case, Barrett’s with low grade dysplasia (LGD) in four cases, Barrett’s without no dysplasia (NDBE) in four cases, and normal mucosa (NL) in three cases.
Among the 22 patients who had previously undergone treatment, the pre-treatment pathology was IMC in 1 case, HGD in 11 cases, LGD in 8 cases and NDBE in 2 cases and study procedure pathology was LGD in 2 cases, NDBE in 2 cases, inflamed gastric cardia (IGC) in 9 cases, and NL in 9 cases.
| IMC/HGD (n = 1) | LGD (n = 6) | NDBE (n = 6) | Inflamed cardia (n = 9) | No diagnostic abnormality (n = 12) | Neoplastic1 (n = 7) | Non-neoplastic2 (n = 27) | P-value3 | |
| Gastric rugae | 1 (100) | 6 (100) | 6 (100) | 9 (100) | 12 (100) | 7 (100) | 27 (100) | NS |
| Gastric pit architecture | 1 (100) | 6 (100) | 6 (100) | 9 (100) | 12 (100) | 7 (100) | 27 (100) | NS |
| Poor penetration | 1 (100) | 6 (100) | 6 (100) | 9 (100) | 12 (100) | 7 (100) | 27 (100) | NS |
| Loss of normal gastric pit architecture | 1 (100) | 6 (100) | 5 (83) | 8 (89) | 7 (58) | 7 (100) | 20 (74) | NS |
| Irregular surface | 1 (100) | 6 (100) | 2 (33) | 1 (11) | 2 (20) | 7 (100) | 5 (19) | < 0.0001 |
| Heterogeneous scattering | 1 (100) | 5 (83) | 5 (83) | 6 (67) | 3 (25) | 6 (86) | 14 (52) | < 0.005 |
| Epithelial glands | 1 (100) | 6 (100) | 6 (100) | 9 (100) | 12 (100) | 7 (100) | 27 (100) | NS |
| Anomalous glands | 1 (100) | 6 (100) | 5 (83) | 6 (67) | 5 (42) | 7 (100) | 16 (59) | < 0.05 |
Of the fifty-six VLE cases available for this study, twelve cases were excluded due to inadequate VLE imaging of the GEJ/cardia or because corresponding biopsies were not taken in that region.
Features characteristic of the gastric cardia (gastric rugae, gastric pit architecture, and poor penetration) were observed in all (100%) scans. A focal area with loss of normal gastric pit architecture was also a prevalent finding, seen in 79.4% of total patients. Heterogeneous scattering was found in exactly half (50%) of patients. Irregular surface was less common, seen in 35% of patients. Similarly, subsurface intensity greater than surface intensity was a rare finding, also occurring only in 6 of 34 patients (17.6%).
All 34 cases showed some typical epithelial glands with a range of 3-56 glands in the GE junction and cardia region examined. Anomalous glands were found in 25 of 34 scans (73.5%) and the number of anomalous glands ranged from 2 to 39.
Focal loss of normal gastric pit architecture was a prevalent finding, notably in patients with neoplasia, non-dysplastic Barrett’s, and inflamed cardia. There was no difference of this feature between those with neoplasia and those without (100% vs 74%, P = NS). However, patients with all types of abnormal cardia (IMC/HGD, LGD, NDBE, IGC) more frequently had loss of normal gastric pit architecture than patients with no mucosal abnormality (90.0% vs 58.3%, P < 0.05).
Irregular surface was more often seen in patients with neoplasia than those without neoplasia (100% vs 18.5%, P < 0.0001). Irregular surface was also more often seen in those patients with neoplasia compared to those with IGC (100% vs 11.1%, P < 0.001).
A greater proportion of patients with neoplasia had heterogeneous scattering as compared to patients without neoplasia (85.7% vs 40.7%, P < 0.005). Additionally, patients with neoplasia or non-dysplastic BE analyzed together more often had heterogeneous scattering compared to those with inflamed cardia or normal mucosa (84.6% vs 28.5%, P < 0.005).
Anomalous glands were more commonly found in patients with neoplasia as opposed to those without (100% vs 59.2%, P < 0.05). When analyzed together, patients with either neoplasia or non-dysplastic BE were found to have anomalous glands more often that those with inflammation or normal mucosa (92.3% vs 52.4%, P < 0.05).
In terms of the number of anomalous glands found, ANOVA analysis did not reveal a difference between individual histologic subgroups (P = NS). When grouped, patients with neoplasia did not have a significantly higher number of anomalous glands than all patients without neoplasia (t-test P = NS). However, patients with neoplasia did have significantly more anomalous glands than the subgroup of patients with no mucosal abnormality (t-test P < 0.05). Septated glands were seen in 8 patients across histology subtypes and did not appear to be associated with higher levels of pathology.
Notably, WLE or narrow band imaging (NBI) failed to detect suspicious lesions within BE at the GEJ or cardia in 3 cases of LGD.
This is the first study to analyze the correlation between imaging features and histology specifically in the GEJ and gastric cardia region. Our first main finding was that all scans exhibited features such as broad based rugae, gastric pits, and poor penetration. These features have been previously described in the gastric cardia, and our findings confirm that these are reliable markers for identifying the transition from tubular esophagus to gastric cardia.
We found that patients with neoplasia at the GEJ and gastric cardia more frequently have abnormal features on VLE imaging. In fact, the loss of normal gastric pit architecture was the only abnormal feature that did not differ significantly between those with neoplasia and those without. This is likely because this feature was loosely defined, and any deviation from the normal gastric pit architecture was counted. In the tubular esophagus, loss of layering is found in cases of NDBE. Thus, it was already known that NDBE can appear with an irregular architecture. In addition, gastric pit architecture appears as subtle alternation of vertical dark and light bands at the mucosal surface and this imaging feature could have been easily disturbed by artifact.
Other studies of VLE have similarly shown this imaging modality to be a safe and useful adjuvant to endoscopy. The safety and feasibility of VLE imaging was evaluated by Wolfson et al[14] who were able to successfully perform VLE imaging in 87% of a 100 patient cohort. Probe and console issues were the reason for unsuccessful VLE imaging in 13 patients. Two minor mucosal lacerations occurred in the study and neither required therapy. The diagnostic utility of VLE has been explored by several groups. Trindade et al[15] presented a small case series which found that targeted biopsy by VLE upstaged or diagnosed dysplasia in several patients who then became candidates for ablation or resection. VLE has even been found to detect dysplasia missed by other advanced imaging techniques such as NBI[16] and missed on random biopsy[17]. Currently, two validated OCT image assessment algorithms exist. The OCT-scoring index (OCT-SI) created in 2005 by Evans et al]13] focuses on signal intensity and glandular architecture whereas the newer VLE diagnostic algorithm (VLE-DA) from Leggett et al[18] is based on degree of mucosal effacement, surface intensity, and atypical glands. The OCT scoring index (score > 2) was found to have an 83% sensitivity and 75% specificity for dysplasia detection when tested in-vivo[13]. The VLE-DA performed slightly better with 86% sensitivity, 88% specificity, and 87% diagnostic accuracy, thought this is based on an ex-vivo study[18]. Based on our results, we propose adding “red-flag” features of irregular surface and heterogeneous scattering in the GEJ region to the current protocols in order to capture areas of dysplasia which may otherwise be missed.
These interpretation systems are focused on the tubular esophagus. However, there is a role for expanding the applicability of these criteria to the GEJ/cardia region since this is a difficult place to assess endoscopically and can harbor SIM or dysplasia[19]. Cardia tissue may be present at the anatomical region of the cardia but may also be present in a mosaic pattern in Barrett’s esophagus amidst intestinal type mucosa and fundic type mucosa. The appearance of cardia type tissue within this mosaic pattern in the tubular esophagus is a potential confounder and may be a cause for false positives in VLE interpretation.
One limitation of this study is that exact correlation of biopsy location to VLE scan location was unable to be performed in this retrospective study. Rather, a circumferential area scanning 1 cm in length was designated as the GEJ/gastric cardia region and biopsy and imaging features from this area were compared. Currently, general location correlation can be done by matching a registration line on the probe to one of the cross-sectional image. This allows for clock-face orientation. However, to allow for even more precise targeting Suter et al[20] validated the recently developed method for using a cautery marking laser coupled into the VLE balloon catheter’s optical fiber to mark areas of interest with simultaneously acquiring a VLE image.
The results of this study show a promising role for VLE as an adjuvant for endoscopic assessment of the GEJ and gastric cardia region. The current OCT-scoring algorithms may be expanded to include GEJ/cardia assessment in order to target areas that exhibit irregular surface, heterogeneous scattering, or anomalous glands. A prospective study validating these features utilizing 1:1 histologic correlation will be required. With in-vivo laser marking soon to be commercially available, we anticipate with this be a feasible study in the near future. Ultimately, the clinical comparison of yield of VLE targeted biopsy protocol compared with a standard Seattle protocol biopsy protocol will be needed to assess clinical impact.
Barrett’s esophagus can lead to the development of esophageal adenocarcinoma. Because of this, patients with known Barrett’s esophagus are recommended to have surveillance upper endoscopies. Biopsies are taken of visible lesions as well as in a random fashion according to the Seattle Protocol. However, this technique is suboptimal because occult dysplasia may still be missed. Several adjuvant modalities have been explored to help identify potentially dysplastic areas and allow for more targeted biopsies. Targeted biopsies improve dysplasia detection rates. volumetric laser endomicroscopy (VLE) is a type of OCT imaging modality that produces cross sectional images of the esophagus and proximal stomach. It is currently used for Barrett’s surveillance and biopsy targeting in the tubular esophagus. This study explores the use of VLE specifically at the gastroesophageal junction (GEJ) and gastric cardia to determine the correlation of imaging features with neoplasia in this region.
In Barrett’s esophagus, there is increasing interest in a moving toward targeted biopsies by identifying abnormal “red-flag” areas. The correlation of VLE imaging with histology is increasingly being studied, however many of these studies are ex-vivo and their applicability may be limited. Additionally, most studies of VLE are limited to the tubular esophagus even though VLE scans also image the GEJ and gastric cardia. The results of this in-vivo study contribute to understanding the applicability of VLE imaging at the GEJ/cardia, as well as contributing to knowledge of correct VLE image interpretation.
This is the only study known to date which focus on VLE imaging characteristics specifically at the GEJ and gastric cardia. This is important because dysplasia can occur in this region just as it can in the tubular esophagus. Similar to prior VLE studies, this study found that VLE features of abnormal surface architecture and atypical glands are associated with dysplasia. However, this study also found that the scattering pattern of the VLE image (heterogeneous vs homogeneous) was correlated with dysplasia and should be considered in the image assessment.
This study suggests that VLE imaging is useful for assessing the GEJ and gastric cardia. Current OCT scoring systems can be expanded for use in this region.
Barrett’s esophagus: A pre-cancerous cellular change in the esophagus that is often the result of longstanding acid reflux. Optical coherence tomography (OCT): An imaging technique that produces high resolution images of biologic tissue by measuring back-scatter light intensity from a near-infrared light source. Volumetric laser endomicroscopy (VLE): A specific type of OCT that is probe-based and produces cross sectional images of the esophagus and gastric cardia.
This retrospective study correlates VLE imaging characteristics with histology at the GEJ and gastric cardia, which is helpful to determine specific features associated with neoplasia. The authors investigated a new area of invasive gastroenterology field. Their findings have some novel findings and also lead new investigations as a prospective designed.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bilir C, Chen JQ S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212-215. [PubMed] [Cited in This Article: ] |
| 2. | Sharma P, Falk GW, Weston AP, Reker D, Johnston M, Sampliner RE. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 234] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 4. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 990] [Cited by in F6Publishing: 982] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 5. | Cameron AJ, Carpenter HA. Barrett’s esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. Am J Gastroenterol. 1997;92:586-591. [PubMed] [Cited in This Article: ] |
| 6. | Pohl J, Pech O, May A, Manner H, Fissler-Eckhoff A, Ell C. Incidence of macroscopically occult neoplasias in Barrett’s esophagus: are random biopsies dispensable in the era of advanced endoscopic imaging? Am J Gastroenterol. 2010;105:2350-2356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, Manfredi M, Navaneethan U, Maple JT, Pannala R. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc. 2016;83:684-98.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Muthusamy VR, Kim S, Wallace MB. Advanced Imaging in Barrett’s Esophagus. Gastroenterol Clin North Am. 2015;44:439-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Testoni PA. Optical coherence tomography. ScientificWorldJournal. 2007;7:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Vakoc BJ, Shishko M, Yun SH, Oh WY, Suter MJ, Desjardins AE, Evans JA, Nishioka NS, Tearney GJ, Bouma BE. Comprehensive esophageal microscopy by using optical frequency-domain imaging (with video). Gastrointest Endosc. 2007;65:898-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Sauk J, Coron E, Kava L, Suter M, Gora M, Gallagher K, Rosenberg M, Ananthakrishnan A, Nishioka N, Lauwers G. Interobserver agreement for the detection of Barrett’s esophagus with optical frequency domain imaging. Dig Dis Sci. 2013;58:2261-2265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Evans JA, Bouma BE, Bressner J, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest Endosc. 2007;65:50-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Evans JA, Poneros JM, Bouma BE, Bressner J, Halpern EF, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:38-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Wolfsen HC, Sharma P, Wallace MB, Leggett C, Tearney G, Wang KK. Safety and feasibility of volumetric laser endomicroscopy in patients with Barrett’s esophagus (with videos). Gastrointest Endosc. 2015;82:631-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Trindade AJ, George BJ, Berkowitz J, Sejpal DV, McKinley MJ. Volumetric laser endomicroscopy can target neoplasia not detected by conventional endoscopic measures in long segment Barrett’s esophagus. Endosc Int Open. 2016;4:E318-E322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Atkinson C, Singh S, Fisichella PM. Volumetric laser endomicroscopy in the detection of neoplastic lesions of the esophagus. Dig Liver Dis. 2016;48:692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Trindade AJ, Vamadevan AS, Sejpal DV. Finding a needle in a haystack: use of volumetric laser endomicroscopy in targeting focal dysplasia in long-segment Barrett’s esophagus. Gastrointest Endosc. 2015;82:756; discussion 757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Leggett CL, Gorospe EC, Chan DK, Muppa P, Owens V, Smyrk TC, Anderson M, Lutzke LS, Tearney G, Wang KK. Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett’s esophagus. Gastrointest Endosc. 2016;83:880-888.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Pascarenco OD, Boeriu A, Mocan S, Pascarenco G, Drasoveanu S, Galeanu M, Dobru D. Barrett’s esophagus and intestinal metaplasia of gastric cardia: prevalence, clinical, endoscopic and histological features. J Gastrointestin Liver Dis. 2014;23:19-25. [PubMed] [Cited in This Article: ] |
| 20. | Suter MJ, Gora MJ, Lauwers GY, Arnason T, Sauk J, Gallagher KA, Kava L, Tan KM, Soomro AR, Gallagher TP. Esophageal-guided biopsy with volumetric laser endomicroscopy and laser cautery marking: a pilot clinical study. Gastrointest Endosc. 2014;79:886-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |