Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.311
Peer-review started: September 27, 2019
First decision: October 18, 2019
Revised: December 8, 2019
Accepted: December 23, 2019
Article in press: December 23, 2019
Published online: March 15, 2020
Preoperative chemoradiotherapy regimens using a second drug for locally advanced rectal cancer are still under clinical investigation.
To investigate the clinical outcomes of patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy using tegafur/gimeracil/oteracil (S-1) plus irinotecan (CPT-11).
This was a single-center retrospective study of 82 patients who underwent radical surgery for rectal cancer after chemoradiotherapy with S-1 (80 mg/m2/d), CPT-11 (60 mg/m2/d), and radiation (total 45 Gy) between 2009 and 2016. The median follow-up was 51 mo (range: 17–116 mo).
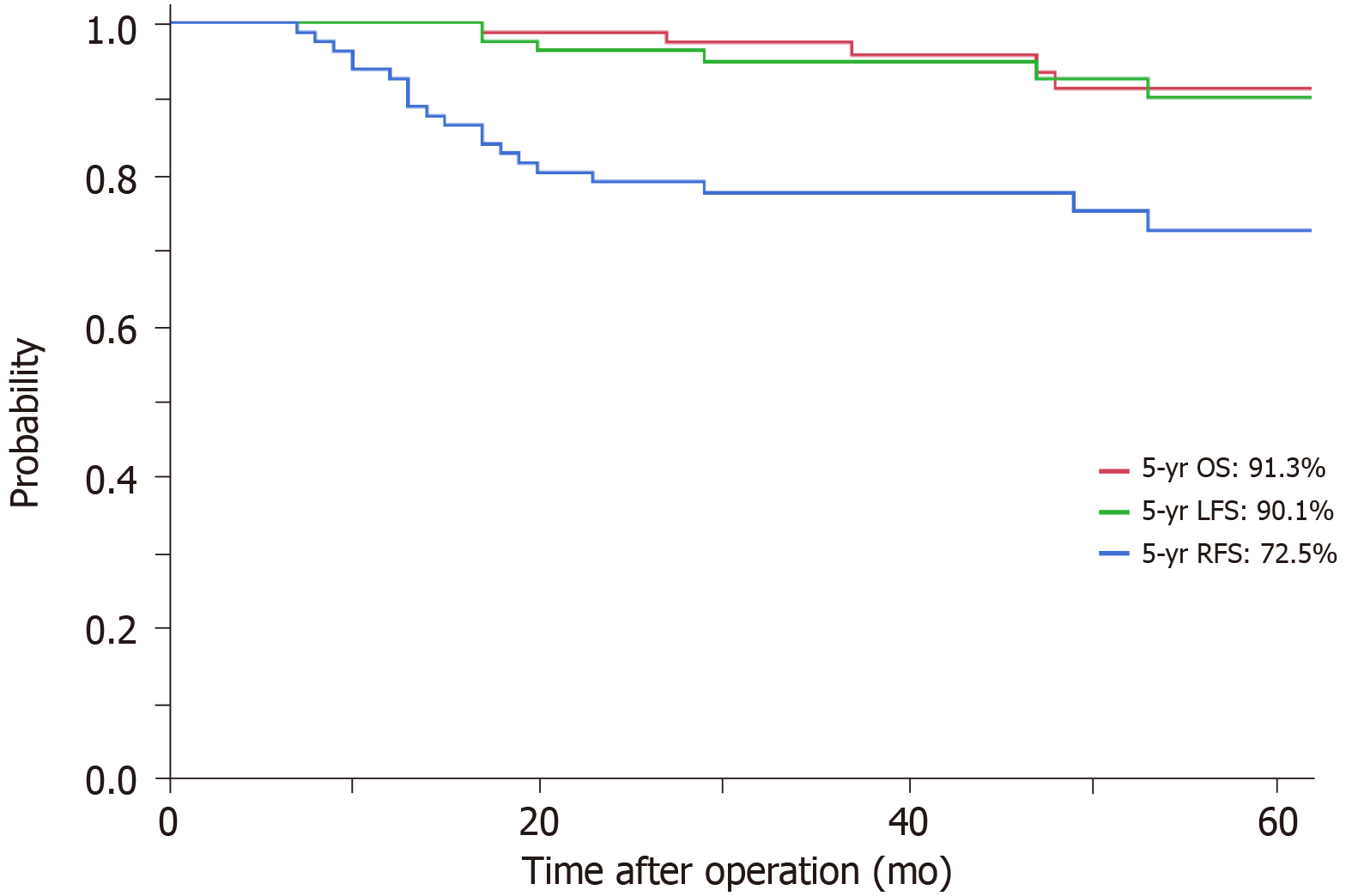
Twenty-nine patients (35.4%) had T3 or T4 rectal cancer with mesorectal fascia invasion, 36 (43.9%) had extramural vascular invasion, 24 (29.8%) had N2 rectal cancer and eight (9.8%) had lateral lymph node swelling. The relative dose intensity was 90.1% for S-1 and 92.9% for CPT-11. Seventy-nine patients (96.3%) underwent R0 resection. With regard to pathological response, 13 patients (15.9%) had a pathological complete response and 52 (63.4%) a good response (tumor regression grade 2/3). The 5-year local recurrence-free survival, relapse-free survival and overall survival rates were 90.1%, 72.5% and 91.3%, respectively. We analyzed the risk factors for local recurrence-free survival by Cox regression analysis and none were detected. Previously described risk factors such as T4 stage, mesorectal fascia invasion or lateral lymph node swelling were not detected as negative factors for local recurrence-free survival.
We demonstrated good compliance and favorable tumor regression in patients with locally advanced rectal cancer treated with preoperative S-1 and CPT-11.
Core tip: Lower advanced rectal cancer located within 8 cm of the anal verge carries a higher risk of local recurrence. The aim of this single-center retrospective study was to assess the clinical outcomes of patients with locally advanced rectal cancer treated preoperative chemoradiotherapy using tegafur/gimeracil/oteracil plus irinotecan. Grade 3 or 4 toxicity was mild and led to good relative dose intensity with on-schedule treatment. Also, we investigated the risk factors for local recurrence-free survival and relapse-free survival. Multivariate analysis detected no factors for local recurrence-free survival. Our study confirmed good compliance and favorable tumor regression.
- Citation: Kimura K, Beppu N, Doi H, Kataoka K, Yamano T, Uchino M, Ikeda M, Ikeuchi H, Tomita N. Impact of preoperative chemoradiotherapy using concurrent S-1 and CPT-11 on long-term clinical outcomes in locally advanced rectal cancer. World J Gastrointest Oncol 2020; 12(3): 311-322
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/311.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.311
In the 2000s, numerous studies were planned to investigate the optimal preoperative treatment strategies for advanced rectal cancer. The National Comprehensive Cancer Network and European Society for Medical Oncology consensus guidelines consider preoperative 5-fluorouracil (5-FU)-based chemoradiotherapy (CRT), 45–50.4 Gy, as standard treatment[1,2]. However, the local recurrence rate remains about 10%, and risk factors for local recurrence include T4 stage, mesorectal fascia invasion (MFI), extramural vascular invasion (EMVI) and lateral lymph node (LLN) swelling[3-6]. Multidisciplinary treatments were planned to overcome this issue, such as extended surgery, higher radiation doses, and concurrent use of second drugs, such as oxaliplatin or irinotecan (CPT-11)[7-11]. With regard to the concurrent use of second drugs, six prospective studies failed to confirm any additional benefit of oxaliplatin, and there was a significant increase in severe toxicity and an insufficient response rate[12-17]. However, several Phase II trials have demonstrated the feasibility, safety and effectiveness of CPT-11 as a second drug, with higher pathological complete response (pCR) rates[7,18-25]. UGT1A1 polymorphisms that can be used to predict the probability of severe toxicity would be of interest for proper therapeutic management using CPT-11[26]. Therefore, the purpose of this study was to investigate the clinical outcomes of 82 patients with locally advanced rectal cancer, located 8 cm from the anal verge, treated with preoperative CRT using tegafur/gimeracil/oteracil (S-1) plus CPT-11.
We included 82 patients with T3-4, N0-2, M0 rectal cancer located within 8 cm of the anal verge who were treated with preoperative CRT using S-1 plus CPT-11 between 2009 and 2016. Prior to preoperative therapy, all patients underwent staging work-ups that included digital rectal examination, measurement of tumor marker levels (carcinoembryonic antigen and carbohydrate antigen 19-9), chest X-ray, abdominal and pelvic computed tomography (CT) and magnetic resonance imaging (MRI). MRI was performed on two occasions, as part of initial staging and following preoperative therapy. Testing for UGT1A1*6 and *28 polymorphisms under national insurance was finally given approval in November 2008 in Japan, and it became measurable at our institution in March 2009. UGT1A1 polymorphisms are assessed only in cases in which consent is obtained after consultation with a specialist in hereditary diseases[27]. The protocol for the present study was based on the SAMRAI-1 trial[28].
The patients were divided into two groups in accordance with the European Society for Medical Oncology guidelines to confirm the outcomes for these subgroups[29]: (1) “bad” rectal cancer [T3(b)c/T4 with peritoneal or vaginal involvement only, N1–2, MFI negative]; and (2) “ugly” rectal cancer (T4 with overgrowth to adjacent organs, pelvic side walls or sacrum, LLN positive, MFI positive).
Preoperative CRT consisted of S-1 (Days 1-5, 8-12, 22-26 and 29-33; 80 mg/m2/d), CPT-11 (Days 1, 8, 22 and 29; 60 mg/m2/d), and radiation (total 45 Gy, 1.8 Gy/d, 5 d per week for 5 wk). Six to eight weeks after completion of preoperative CRT, the patients were scheduled to undergo radical surgery.
All patients underwent total mesorectal excision or extended total mesorectal excision (total mesorectal excision with adjacent visceral resection) to achieve R0 resection. The surgical procedure included low anterior resection, intersphincteric resection and abdominoperineal resection. Intersphincteric resection was recommended in accordance with tumor stage and location, patient age, and preoperative anal function, and patients who did not meet those criteria were selected for abdominoperineal resection. Diverting ileostomy was routinely constructed for all patients with intestinal continuity. LLN dissection was performed when pretreatment MRI showed that the LLNs had a short-axis diameter > 7 mm. Postoperative complications were assessed according to the Clavien-Dindo classification[30]. Pathological response to CRT was evaluated according to the Japanese Classification of Colorectal Carcinoma of the Japanese Society for Cancer of the Colon and Rectum (8th edition). Grade 0 was defined as no evidence of a therapeutic effect and Grade 3 was pCR[31]. We defined a good response as Grade 2 or 3 and poor response as Grade 0 or 1a/1b.
Hematological and nonhematological toxicity caused by preoperative CRT was evaluated according to the Common Terminology Criteria for Adverse Events, version 4.0[32]. Relative dose intensity was calculated as the ratio of the actual dose to the scheduled dose; S-1 (1600 mg/m2), CPT-11 (240 mg/m2) and full irradiation dose (45 Gy). Dose reductions of CPT-11 were not applied to the group of patients with UGT1A1 mutation.
Median follow-up was 51 mo (range, 17-116 mo). Postoperative adjuvant chemotherapy using 5-FU-based chemotherapy was recommended for all patients except those with ypT0/1 stage, high age, comorbidity, postoperative complications, and social factors. Patient surveillance was subsequently performed as follows: chest–abdominal CT every 6 mo, colonoscopy annually, and blood tests (including measurement of carcinoembryonic antigen and carbohydrate antigen 19-9 levels) at 3-mo intervals. Local recurrence was defined as the detection of a recurrent tumor within the pelvis, and recurrence was defined as the presence of recurrent disease outside the pelvis.
Local recurrence-free survival (LFS), relapse-free survival (RFS) and overall survival (OS) were estimated using the Kaplan–Meier method and compared using the log-rank test. The χ2 test was also used to evaluate associations between UGT1A1 polymorphisms and toxicity and feasibility of treatment. We further evaluated clinical factors associated with LFS and RFS to determine the optimal clinical criteria of this regimen by Cox proportional hazard regression model. Independent variables with P < 0.1 in univariate analysis were entered into a multivariate analysis and P < 0.05 was considered statistically significant. Statistical analyses were performed using JMP version 12.0 software (SAS Japan Inc., Tokyo, Japan).
The patients’ clinical characteristics are shown in Table 1. Clinical T4 stage was diagnosed in 10 patients (12.2%). Clinical N stage was deemed positive in 46 patients (56.1%). MRI revealed tumor involvement of the MF in 29 patients (35.4%). EMVI was observed in 36 patients (43.9%). According to the risk category of rectal cancer, 50 patients (61.0%) were divided into the bad group and 32 (39.0%) into the ugly group.
| Characteristic | n = 82 |
| Age (yr) | |
| Median (range) | 64 (34–79) |
| Sex | |
| Male | 60 (73.2) |
| Female | 22 (26.8) |
| Distance from anal verge (cm) | |
| Median (range) | 5.0 (0–8) |
| Size of tumor (cm) | |
| Median (range) | 4.5 (2–9) |
| Clinical T stage (before chemoradiotherapy) | |
| 3 | 72 (87.8) |
| 4 | 10 (12.2) |
| Mesorectal fascia invasion | |
| − | 53 (64.6) |
| + | 29 (35.4) |
| Extramural vascular invasion | |
| − | 46 (56.1) |
| + | 36 (43.9) |
| Clinical N stage (before chemoradiotherapy) | |
| − | 36 (43.9) |
| + | 46 (56.1) |
| Subgroup of locally advanced rectal cancer | |
| Bad | 50 (61.0) |
| Ugly | 32 (39.0) |
| UGT1A1 polymorphism (in 48 patients) | |
| Wild type | 25 (52.1) |
| Mutant type | 23 (47.9) |
The relative dose intensity was 90.1% for S-1, 92.9% for CPT-11 and 97.6% for RT. Toxicity data are shown in Table 2. Grade 3 or 4 hematological toxicity consisted of leukopenia (n = 15; 18.3%), neutropenia (n = 16; 19.5%) and febrile neutropenia (n = 3; 3.6%). Grade 3 or 4 nonhematological toxicity consisted of diarrhea (n = 22; 26.8%). For Grade 3 or 4 hematological toxicity, four of 16 neutropenia patients (25.0%) whose neutrophil count was reduced to < 500 cells/μL received granulocyte colony-stimulating factor. For Grade 3 or 4 nonhematological toxicity, four of 22 diarrhea patients (18.2%) were prescribed loperamide. All patients recovered after these conservative treatments.
| Toxicity | n = 82 | |
| Any Grade | Grade 3 or 4 | |
| Hematological toxicity | ||
| Neutropenia | 56 (68.3) | 16 (19.5) |
| Leukopenia | 59 (72.0) | 15 (18.3) |
| Febrile neutropenia | 3 (3.6) | 3 (3.6) |
| Thrombocytopenia | 11 (13.4) | 0 |
| Nonhematological toxicity | ||
| Diarrhea | 53 (64.6) | 22 (26.8) |
| Anorexia | 20 (24.4) | 1 (1.2) |
| Fatigue | 15 (18.3) | 0 |
| Nausea | 13 (15.9) | 0 |
Associations between toxicity/feasibility and UGT1A1 polymorphisms were investigated (Table 3). Forty-eight of 82 patients (58.5%) were assessed for UGT1A1 polymorphism, and 25 (52.1%) were wild type and 23 (47.9%) were mutant type. Patients with the mutant type had more Grade 3 or 4 hematological toxicity than those with the wild type had (P < 0.05). However, there was no significant difference in the incidence of nonhematological toxicity, including diarrhea, in either genotype (P = 0.65). There was no significant difference in CPT-11 dose intensity according to UGT1A1 polymorphisms despite the significant differences observed in hematological toxicity (P = 0.26).
| Wild type (n = 25) | Mutant type (n = 23) | P value | |
| Toxicity | |||
| Hematological toxicity (Grade 3 or 4) | 0 | 11 (47.8) | < 0.01 |
| Nonhematological toxicity (Grade 3 or 4) | 8 (32.0) | 6 (26.1) | 0.65 |
| Feasibility (%) | |||
| S-1 dose intensity (mean ± SD) | 90.9 ± 0.2 | 89.0 ± 0.2 | 0.38 |
| CPT-11 dose intensity (mean ± SD) | 93.0 ± 0.3 | 88.2 ± 0.2 | 0.26 |
| Treatment effect | |||
| Good response | 18 (72.0) | 22 (95.7) | < 0.01 |
| Poor response | 7 (28.0) | 1 (4.3) | |
| Pathological complete response | 5 (20.0) | 6 (26.1) | 0.61 |
Thirty-one patients (37.8%) underwent low anterior resection, 43 (52.4%) intersphincteric resection and eight (9.8%) abdominoperineal resection. Five patients (6.1%) underwent combined adjacent organ resection and eight (9.8%) LLN dissection.
The postoperative complications are shown in Table 4. Grade 3 pelvic infection was confirmed in nine patients (11.0%) and five (6.1%) developed Grade 3 ileus. Among the patients undergoing sphincter-preserving surgery, seven (9.5%) had Grade 3 anastomosis leakage. During follow-up, six patients could not undergo stoma takedown because of pelvic infection with anastomotic leakage (n = 4) and local recurrence (n = 2).
| Complication | n = 82 | |
| Any grade | Grade 3 | |
| Pelvic infection | 15 (18.3) | 9 (11.0) |
| Anastomosis leakage1 | 9 (12.2) | 7 (9.5) |
| Ileus | 11 (13.4) | 5 (6.1) |
| Bleeding | 1 (1.2) | 1 (1.2) |
| Surgical site infection | 2 (2.4) | 0 |
| Urinary dysfunction | 8 (9.8) | 0 |
| Venous thromboembolic event | 1 (1.2) | 0 |
| Re-operation | 0 | 0 |
Pathological findings are listed in Table 5. Thirteen patients (15.9%) achieved complete tumor regression with tumor regression grade 3 (pCR). T downstaging was seen in 41 patients (50.0%) and N downstaging in 36 (43.9%). R0 resection was performed in 79 of 82 patients (96.3%) and R1 resection in three (3.7%), with microscopic residual tumor in the anus levator muscle (n = 2) and pelvic plexus on the pelvic sidewall (n = 1). No patient had R2 resection. Patients with UGT1A1 mutations showed a significantly better response to CRT (including CPT-11) than those without mutations (Table 3).
| Pathological tumor characteristics | n = 82 |
| ypT stage | |
| 0 | 13 (15.9) |
| 1 | 5 (6.1) |
| 2 | 21 (25.6) |
| 3 | 25 (42.7) |
| 4 | 8 (9.8) |
| ypN stage | |
| − | 65 (79.3) |
| + | 17 (20.7) |
| yp TNM stage1 | |
| 0 | 13 (15.9) |
| I | 20 (24.4) |
| II | 32 (39.0) |
| III | 17 (20.7) |
| Residual tumor classification | |
| R0 | 79 (96.3) |
| R1 | 3 (3.7) |
| R2 | 0 |
| Histology | |
| well/moderately differentiated | 66 (80.5) |
| Poorly differentiated/mucinous/signet | 16 (19.5) |
| T downstaging | |
| − | 41 (50.0) |
| + | 41 (50.0) |
| N downstaging | |
| − | 46 (56.1) |
| + | 36 (43.9) |
| Tumor regression grade | |
| 1a | 18 (22.0) |
| 1b | 12 (14.6) |
| 2 | 39 (47.6) |
| 3 | 13 (15.9) |
| Adjuvant chemotherapy | |
| – | 56 (68.3) |
| + | 26 (31.7) |
Twenty-six patients (31.7%) received 5-FU-based adjuvant chemotherapy: UFT plus leucovorin (n = 19), mFOLFOX6 (n = 4), S-1 (n = 2) and capecitabine (n = 1). The reasons for not receiving adjuvant chemotherapy were: ypT0/1 stage (n = 18), high age (n = 11), comorbidity (n = 2), postoperative complications (n = 12), social factors (n = 6), and others (n = 7).
After a median follow-up of 51 mo, 5-year LFS, 5-year RFS and 5-year OS rates were 90.1%, 72.5% and 91.3%, respectively (Figure 1). Local recurrence was seen in six patients: LLNs (n = 4) and other sites (n = 2). Distant recurrence was detected in 20 patients: lung (n = 15), liver (n = 6), para-aortic region (n = 2), inguinal region (n = 1) and bone (n = 1). Some patients had overlapping metastases. LFS did not differ significantly between the bad and ugly groups (96.0% vs 76.2%; P = 0.10); however, RFS was significantly poorer in the ugly group (38.5% vs 87.8% in bad group; P < 0.01).
We investigated the risk factors for LFS and RFS (Table 6). Multivariate analysis showed that no risk factors for LFS were detected, including previously described risk factors such as T4 stage, MFI, EMVI and LLN swelling. However, MFI and EMVI were associated with poor RFS for locally advanced rectal cancer (OR: 5.82, 95%CI: 1.68-20.2, P < 0.01; OR: 3.42, 95%CI: 1.02-11.5, P = 0.04).
| Factors | n | LFS | RFS | ||||||||||
| Univariate | Multivariate | Univariate | Multivariate | ||||||||||
| OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | ||
| Sex | |||||||||||||
| Female | 22 | ||||||||||||
| Male | 60 | 1.91 | 0.21-17.7 | 0.56 | 1.13 | 0.36-3.60 | 0.83 | ||||||
| Location from anal verge (cm) | |||||||||||||
| ≥ 5.0 | 29 | ||||||||||||
| < 5.0 | 53 | 1.10 | 0.19-6.41 | 0.91 | 1.89 | 0.61-5.89 | 0.26 | ||||||
| Tumor diameter (cm) | |||||||||||||
| < 4.5 | 42 | ||||||||||||
| ≥ 4.5 | 40 | 2.00 | 0.86-2.89 | 0.43 | 0.63 | 0.22-1.74 | 0.37 | ||||||
| cT | |||||||||||||
| 3 | 72 | ||||||||||||
| 4 | 10 | 4.25 | 0.67-27.0 | 0.10 | 1.97 | 0.23-17.1 | 0.54 | 3.80 | 0.97-14.9 | 0.06 | 2.05 | 0.39-10.7 | 0.39 |
| cN | |||||||||||||
| − | 36 | ||||||||||||
| + | 46 | 1.62 | 0.28-9.38 | 0.59 | 1.25 | 0.34-2.60 | 0.90 | ||||||
| Mesorectal fascia invasion | |||||||||||||
| − | 53 | ||||||||||||
| + | 29 | 4.08 | 0.70-23.8 | 0.10 | 2.88 | 0.39-21.5 | 0.30 | 7.31 | 2.39-22.4 | < 0.01 | 5.82 | 1.68-20.2 | < 0.01 |
| Extramural vascular invasion | |||||||||||||
| − | 46 | ||||||||||||
| + | 36 | 2.75 | 0.47-15.9 | 0.24 | 3.15 | 1.10-9.03 | 0.03 | 3.42 | 1.02-11.5 | 0.04 | |||
| Lateral lymph node (> 7.0 mm) | |||||||||||||
| − | 74 | ||||||||||||
| + | 8 | 5.83 | 0.88-38.7 | 0.09 | 4.71 | 0.65-34.2 | 0.12 | 2.01 | 0.44-9.29 | 0.36 | |||
| Histology | |||||||||||||
| Well/moderately differentiated | 66 | ||||||||||||
| Poorly differentiated/mucinous/signet | 16 | 0.81 | 0.09-7.49 | 0.86 | 1.55 | 0.46-7.58 | 5.15 | ||||||
We reported the safety, effectiveness and long-term outcomes of concomitant use of CPT-11 with 5-FU-based CRT for locally advanced rectal cancer. S-1 is an oral anticancer agent containing tegafur (a prodrug of 5-FU) with two modulators, gimeracil and oteracil potassium, which markedly increase the radiosensitivity of cancer cells[33]. CPT-11 augments inhibition of thymidylate synthase – the target enzyme of 5-FU[34]. In addition, 5-FU induces topoisomerase I, and cancer cells overexpressing topoisomerase I show increased chemosensitivity to CPT-11[35]. Such in vitro mechanisms are effective in combination with 5-FU as a radiosensitizer for preoperative CRT[7]. Furthermore, UGT1A1 polymorphisms that can predict the probability of developing potentially severe toxicity during treatment with CPT-11-based regimens could be clinical factors in the proper management of treatment[26]. The purpose of this study was to investigate the clinical outcomes of patients with locally advanced rectal cancer treated with preoperative CRT using S-1 plus CPT-11.
Current standard CRT regimens include only 5-FU. However, several clinical trials incorporating a second active systemic agent into conventional CRT regimens have been performed to examine the ability of the regimens to increase pCR rate and improve resectability and locoregional control[6,10]. Two such second drugs, oxaliplatin and CPT-11, have been investigated in clinical trials.
With regard to oxaliplatin, six randomized Phase III studies have compared oxaliplatin-based with 5-FU-based regimens[12-17]. Among these, the STAR-01 (16% both groups), ACCORD 12/0405 (19% vs 14%), NSABP R-04 (21% vs 19%) and PETACC-6 (15% vs 13%) studies reported that there were no substantial improvements in pCR rates, and significantly increased intolerable Grade 3 or 4 toxicity. For this reason, the concomitant use of oxaliplatin in 5-FU-based CRT has not been permitted (Supplementary Table 1). No Phase III studies using CPT-11 have been documented; however, nine Phase II studies (2 randomized controlled trials and 7 single-arm studies) have assessed the usefulness of CPT-11 as a radiosensitizer[7,18-25]. These studies indicated that this CPT-based regimen was promising in terms of pCR rate (range 13.7%-37%). Grade 3 or 4 toxicity was mild and led to good relative dose intensity with on-schedule treatment without dose reduction (Supplementary Table 2).
The most frequent severe toxicity was neutropenia (2.1%-12%) and diarrhea (2.1%-22%). Generally, toxicity was correlated with the dose of chemotherapy. Jung et al[25], who used 40 mg/m2 CPT-11, demonstrated that the rate of Grade 3 or 4 hematological toxicity was 1.4% and the rate of Grade 3 or 4 nonhematological toxicity was 5.7%. Sato et al[7], who used 80 mg/m2 CPT-11, demonstrated that the rate of Grade 3 or 4 hematological toxicity was 6% and the rate of Grade 3 or 4 nonhematological toxicity was 4.5%. These results suggest that concurrent use of second drugs, such as CPT-11 as a radiosensitizer, is well tolerated in terms of toxicity.
UGT1A1 polymorphisms have been confirmed as predictive markers of severe toxicity of CPT-11 in a metastatic setting[26]. Our previous study demonstrated the effectiveness of UGT1A1 polymorphism in predicting the toxicity of preoperative CRT using CPT-11, although it was only a small retrospective study[36]. Thus, to provide patients with the full benefit of CRT, good tolerance of CPT-11-based regimens for patients with UGT1A1 mutant type, as well as the prevention and early treatment of severe toxicity, is important. This suggests that drawing definitive conclusions about the role of UGT1A1 polymorphisms requires a randomized trial, to assess whether genotype-adjusted dose of CPT-11 would help establish a well-tolerated, effective dose for tumor response in patients with wild-type and mutant UGT1A1.
The present study included patients with highly advanced rectal cancer: 29 (35.4%) with T4 or T3 with MFI, 36 (43.9%) with EMVI, 24 (29.8%) with N2, and 32 (39.0%) with ugly rectal cancer. Even such highly advanced rectal cancer demonstrated favorable local control. With respect to systemic recurrence, highly advanced rectal cancer has a high recurrence rate, with poor prognosis; therefore, combined use of systemic treatment, mainly including chemotherapy, is important for prolonging survival benefit[37]. Further studies are warranted to examine the additional effect of CPT-11 on those tumors.
Our study had several limitations. First, it was a small retrospective study performed in a single institution. Second, we excluded atypical rectal cancer, such as mucinous carcinoma caused by anal fistula, which is associated with a poorer response to CRT, because we chose surgery without radiation. Third, we excluded patients with performance status 3/4 or those aged > 80 years who cannot tolerate this regimen owing to comorbidity and old age. Such patients (n = 3) were treated with stoma creation alone. Fourth, the follow-up time was not sufficient to evaluate OS, LFS and RFS. Fifth, UGT1A1 polymorphism analysis was not performed for all patients receiving preoperative CRT. Finally, we did not study toxicity-based dose-finding methods for S-1 plus CPT-11 preoperative CRT in a Phase I study. Nevertheless, this study demonstrated the safety, effectiveness and long-term oncological outcomes of locally advanced rectal cancer treated with concomitant CPT-11 and 5-FU-based CRT.
In conclusion, our single-center retrospective study confirmed good compliance, favorable tumor regression and feasible oncological outcomes of preoperative CRT using S-1 plus CPT-11, and favorable local control of highly advanced rectal cancer by this regimen.
Prospective studies have investigated the optimal treatment strategies for management of locally advanced rectal cancer, and have concluded that preoperative 5-fluorouracil-based chemoradiotherapy (CRT) at 45–50.4 Gy is a standard treatment. However, local recurrence rate remains about 10%; mainly for highly advanced cases.
Multidisciplinary treatments were planned to overcome highly advanced rectal cancer, such as extended surgery, higher radiation doses, and concurrent use of second drugs, such as oxaliplatin or CPT-11.
The aim of this study was to investigate the safety, therapeutic effect, and outcome of preoperative CRT using S-1 plus irinotecan for locally advanced lower rectal cancer.
Between 2009 and 2016, 82 patients underwent total mesorectal excision after preoperative CRT. Preoperative CRT consisted of S-1 (80 mg/m2/d), CPT-11 (60 mg/m2/d), and radiation (total 45 Gy). The median follow-up was 51 months (range: 17-116 mo).
This regimen was well tolerated in terms of toxicity. Associations between toxicity/feasibility and UGT1A1 polymorphisms were investigated. Compared with patients with wild-type UGT1A1, those with mutant type had more Grade 3 or 4 hematological toxicity (P < 0.05). With regard to oncological outcome, mesorectal fascia invasion and extramural vascular invasion were associated with poor relapse-free survival for locally advanced rectal cancer. However, Cox regression analysis did not detect any risk factors for local recurrence-free survival.
This regimen had favorable oncological outcomes for highly advanced rectal cancer.
This was a small retrospective study performed in a single institution. A randomized multicenter study is needed to investigate the influence of dose setting by UGT1A1 polymorphism for preoperative CRT using irinotecan.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu GL, Pan ZZ S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-1933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1352] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 2. | Park JH, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer. 2011;117:3703-3712. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Glimelius B, Holm T, Blomqvist L. Chemotherapy in addition to preoperative radiotherapy in locally advanced rectal cancer - a systematic overview. Rev Recent Clin Trials. 2008;3:204-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G; Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 5. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Kusters M, Slater A, Muirhead R, Hompes R, Guy RJ, Jones OM, George BD, Lindsey I, Mortensen NJ, Cunningham C. What To Do With Lateral Nodal Disease in Low Locally Advanced Rectal Cancer? A Call for Further Reflection and Research. Dis Colon Rectum. 2017;60:577-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Sato T, Ozawa H, Hatate K, Onosato W, Naito M, Nakamura T, Ihara A, Koizumi W, Hayakawa K, Okayasu I, Yamashita K, Watanabe M. A Phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: clinical feasibility and response rate. Int J Radiat Oncol Biol Phys. 2011;79:677-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y; Colorectal Cancer Study Group of Japan Clinical Oncology Group. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg. 2017;266:201-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 9. | Kusters M, Beets GL, van de Velde CJ, Beets-Tan RG, Marijnen CA, Rutten HJ, Putter H, Moriya Y. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg. 2009;249:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Mohiuddin M, Regine WF, John WJ, Hagihara PF, McGrath PC, Kenady DE, Marks G. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys. 2000;46:883-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D, Yang JL, Zhou ZR, Liu SX. Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: An updated systematic review and meta-analysis. Oncotarget. 2016;7:45513-45524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O, Mirabel X, Denis B, Mineur L, Berdah JF, Mahé MA, Bécouarn Y, Dupuis O, Lledo G, Montoto-Grillot C, Conroy T. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 547] [Cited by in F6Publishing: 548] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 13. | O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC, Ryan DP, Parda DS, Mohiuddin M, Arora A, Evans LS, Bahary N, Soori GS, Eakle J, Robertson JM, Moore DF, Mullane MR, Marchello BT, Ward PJ, Wozniak TF, Roh MS, Yothers G, Wolmark N. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927-1934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, Bonetti A, Negru ME, Tronconi MC, Luppi G, Silvano G, Corsi DC, Bochicchio AM, Chiaulon G, Gallo M, Boni L. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 544] [Cited by in F6Publishing: 551] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 15. | Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA, Lang-Welzenbach M, Raab HR, Wittekind C, Ströbel P, Staib L, Wilhelm M, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, Liersch T; German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 471] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 16. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 17. | Schmoll HJ, Haustermans K, Price T, Stein A, Nordlinger B, Hofheinz R, Jean-Francois D, Brenner B, Schmidt P, Reinel H, Hollerbach S, Caca K, Fauth F, J, Hannig C, Zalcberg J-R, Tebbutt N, Mauer M, Messina C, Lutz M. Van Cutsem E. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: Disease-free survival results at interim analysis. J Clin Oncol. 2014;32:3501. [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Mehta VK, Cho C, Ford JM, Jambalos C, Poen J, Koong A, Lin A, Bastidas JA, Young H, Dunphy EP, Fisher G. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int J Radiat Oncol Biol Phys. 2003;55:132-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Navarro M, Dotor E, Rivera F, Sánchez-Rovira P, Vega-Villegas ME, Cervantes A, García JL, Gallén M, Aranda E. A Phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil, for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Willeke F, Horisberger K, Kraus-Tiefenbacher U, Wenz F, Leitner A, Hochhaus A, Grobholz R, Willer A, Kähler G, Post S, Hofheinz RD. A phase II study of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (CapIri-RT) as neoadjuvant treatment of locally advanced rectal cancer. Br J Cancer. 2007;96:912-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Shin SJ, Kim NK, Keum KC, Kim HG, Im JS, Choi HJ, Baik SH, Choen JH, Jeung HC, Rha SY, Roh JK, Chung HC, Ahn JB. Phase II study of preoperative chemoradiotherapy (CRT) with irinotecan plus S-1 in locally advanced rectal cancer. Radiother Oncol. 2010;95:303-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Hong YS, Kim DY, Lim SB, Choi HS, Jeong SY, Jeong JY, Sohn DK, Kim DH, Chang HJ, Park JG, Jung KH. Preoperative chemoradiation with irinotecan and capecitabine in patients with locally advanced resectable rectal cancer: long-term results of a Phase II study. Int J Radiat Oncol Biol Phys. 2011;79:1171-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Gollins S, Sun Myint A, Haylock B, Wise M, Saunders M, Neupane R, Essapen S, Samuel L, Dougal M, Lloyd A, Morris J, Topham C, Susnerwala S. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcomes. J Clin Oncol. 2011;29:1042-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Wong SJ, Winter K, Meropol NJ, Anne PR, Kachnic L, Rashid A, Watson JC, Mitchell E, Pollock J, Lee RJ, Haddock M, Erickson BA, Willett CG. Radiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1367-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Jung M, Shin SJ, Koom WS, Jung I, Keum KC, Hur H, Min BS, Baik SH, Kim NK, Kim H, Lim JS, Hong SP, Kim TI, Roh JK, Park YS, Ahn JB. A Randomized Phase 2 Study of Neoadjuvant Chemoradiaton Therapy With 5-Fluorouracil/Leucovorin or Irinotecan/S-1 in Patients With Locally Advanced Rectal Cancer. Int J Radiat Oncol Biol Phys. 2015;93:1015-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Miyata Y, Touyama T, Kusumi T, Morita Y, Mizunuma N, Taniguchi F, Manabe M. UDP-glucuronosyltransferase 1A1*6 and *28 polymorphisms as indicators of initial dose level of irinotecan to reduce risk of neutropenia in patients receiving FOLFIRI for colorectal cancer. Int J Clin Oncol. 2016;21:696-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187-4191. [PubMed] [Cited in This Article: ] |
| 28. | Sato T, Hayakawa K, Tomita N, Noda M, Kamikonya N, Watanabe T, Kato D, Sakai Y, Hiraoka M, Shimada M, Ikushima H, Baba H, Oya N, Oya M, Nemoto-Murofushi K, Takeuchi M, Watanabe M. A multicenter phase I study of preoperative chemoradiotherapy with S-1 and irinotecan for locally advanced lower rectal cancer (SAMRAI-1). Radiother Oncol. 2016;120:222-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Blomqvist L, Glimelius B. The 'good', the 'bad', and the 'ugly' rectal cancers. Acta Oncol. 2008;47:5-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18532] [Cited by in F6Publishing: 21909] [Article Influence: 1095.5] [Reference Citation Analysis (0)] |
| 31. | Japanese Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum, and anus. 8th ed. Tokyo: Kanehara & Co., 2013. [Cited in This Article: ] |
| 32. | U.S. Department of Health and Human Sciences. National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Cited in This Article: ] |
| 33. | Nakata E, Fukushima M, Takai Y, Nemoto K, Ogawa Y, Nomiya T, Nakamura Y, Milas L, Yamada S. S-1, an oral fluoropyrimidine, enhances radiation response of DLD-1/FU human colon cancer xenografts resistant to 5-FU. Oncol Rep. 2006;16:465-471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Guichard S, Hennebelle I, Bugat R, Canal P. Cellular interactions of 5-fluorouracil and the camptothecin analogue CPT-11 (irinotecan) in a human colorectal carcinoma cell line. Biochem Pharmacol. 1998;55:667-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2273] [Cited by in F6Publishing: 2190] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 36. | Kimura K, Yamano T, Igeta M, Imada A, Jihyung S, Babaya A, Hamanaka M, Kobayashi M, Tsukamoto K, Noda M, Ikeda M, Tomita N. UGT1A1 polymorphisms in rectal cancer associated with the efficacy and toxicity of preoperative chemoradiotherapy using irinotecan. Cancer Sci. 2018;109:3934-3942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Brændengen M, Glimelius B. Preoperative radiotherapy or chemoradiotherapy in rectal cancer - Is survival improved? An update of the "Nordic" LARC study in non-resectable cancers. Radiother Oncol. 2018;127:392-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |