Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1237
Peer-review started: July 24, 2020
First decision: September 17, 2020
Revised: September 27, 2020
Accepted: October 21, 2020
Article in press: October 21, 2020
Published online: November 15, 2020
Long non-coding RNAs (lncRNAs) have been shown to be associated with many tumors. However, the specific mechanism of lncRNAs in the occurrence and development of gastric cancer (GC) has not been fully elucidated.
To explore the expression level and molecular mechanism of HOXD-AS2 in GC tissues and cells, and analyze its significance in the prognosis of GC.
Real-time quantitative PCR was used to detect the expression of HOXD-AS2 in 79 pairs of GC tissues and five cell lines. The pcHOXD-AS2 plasmid vector was constructed and transfected into SGC-7901 and SNU-1 GC cells. Matrigel Transwell and wound healing assays were used to confirm the effect of HOXD-AS2 on invasion and migration of GC cells. Cell counting kit-8 assay and flow cytometry were used to verify the effect of HOXD-AS2 on the proliferation, cell cycle, and apoptosis of GC cells. The relevant regulatory mechanism between HOXD-AS2 and HOXD8 and PI3K/Akt signaling pathway was verified by Western blot analysis.
The low expression of lncRNA HOXD-AS2 was associated with lymph node metastasis and tumor-node-metastasis stage in GC. In vitro functional experiments demonstrated that overexpression of HOXD-AS2 inhibited GC cell progression. Mechanistic studies revealed that HOXD-AS2 regulated the expression of its nearby gene HOXD8 and inhibited the activity of the PI3K/Akt signaling pathway.
These results indicate that downregulation of HOXD-AS2 significantly promotes the progression of GC cells by regulating HOXD8 expression and activating the PI3K/Akt signaling pathway. HOXD-AS2 may be a novel diagnostic biomarker and effective therapeutic target for GC.
Core Tip: In this study we found that the expression of long non-coding RNA HOXD-AS2 was down-regulated in gastric cancer (GC) tissues and cells. The low expression of HOXD-AS2 was associated with lymph node metastasis and tumor-node-metastasis stage in GC. Overexpression of HOXD-AS2 inhibited the progression of GC cells. Decreased expression of HOXD-AS2 promotes GC cell progression by targeting HOXD8 and activating the PI3K/Akt signaling pathway. HOXD-AS2 may be a novel diagnostic biomarker and effective therapeutic target for GC.
- Citation: Yao L, Ye PC, Tan W, Luo YJ, Xiang WP, Liu ZL, Fu ZM, Lu F, Tang LH, Xiao JW. Decreased expression of the long non-coding RNA HOXD-AS2 promotes gastric cancer progression by targeting HOXD8 and activating PI3K/Akt signaling pathway. World J Gastrointest Oncol 2020; 12(11): 1237-1254
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1237.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1237
According to the latest statistics, in 2018 there were more than 1000000 new cases of gastric cancer (GC) and an estimated 783000 deaths from GC worldwide. The incidence rate of GC is fifth among all malignant tumors, and the mortality rate ranks third. GC seriously affects human health[1]. China is one of the countries with a high incidence of GC, and with new cases of GC in China accounting for more than 40% of the world’s GC cases, GC has become the second leading cause of cancer death in China[2]. Because most patients with GC are asymptomatic at the early stage[3], many are in advanced stage at the time of initial diagnosis, and the prognosis is often poor[4]. At present, the therapeutic effects on GC are still not satisfactory. Therefore, further understanding of the molecular mechanisms of gastric carcinogenesis, development, invasion, and migration and searching for new targeted drugs and methods are of great significance for the treatment of GC and improvement of the patient survival rate.
Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs that are more than 200 nucleotides in length and lack an open reading frame[5]. LncRNAs have been shown to regulate RNA transcription and mRNA splicing, and play an important role in regulating the stability of RNA in the cytoplasm and the activity of microRNAs[6,7]. An increasing number of studies have shown that abnormally expressed lncRNAs are involved in the process of tumor genesis and development and have a close relationship with tumor cell proliferation, apoptosis, invasion, and metastasis and poor prognosis[8-12]. In recent years, many studies have also found that abnormally expressed lncRNAs are involved in the occurrence and development of GC. For instance, lncRNA SLC7A11-AS1 is expressed at low levels in GC and significantly associated with tumor macroscopic type, distant migration, and tumor-node-metastasis (TNM) stage. SLC7A11-AS1 can be used as a biomarker for the diagnosis and prognosis of GC, and provides a potential target for GC treatment[13]. LncRNA LINC01606 is highly expressed in GC. LINC01606 can act as an endogenous competitive RNA (ceRNA) to adsorb miR-423-5p, attenuating the inhibitory effect of miR-423-5p on the Wnt3a gene, thereby activating the Wnt/β-catenin signaling pathway and promoting the invasion and migration of GC cells[14]. LncRNA LINC01133 is downregulated in GC tissues and cells. Low expression of LINC01133 is significantly associated with tumor size, depth of invasion, lymph node metastasis, and TNM stage and predicts poor prognosis. LINC01133 can act as a ceRNA to adsorb miR-106a-3p and thereby regulate the expression of the APC gene and affect the Wnt/β-catenin signaling pathway. The results suggest that LINC01133 can be used as a potential biomarker for poor prognosis of GC[15]. Although a major breakthrough has been achieved in the study of lncRNAs in the pathogenesis of GC, the specific mechanism of lncRNAs in the occurrence and development of GC has not been fully elucidated.
We screened for abnormally expressed lncRNA HOXD-AS2 in GC by gene chip analysis. HOXD-AS2, which is located at 2q31.1 and is encoded by three exons, was mapped to chromosome 2 region 176134841-176137098. In the present study, we used quantitative polymerase chain reaction (qPCR) to detect the expression of HOXD-AS2 in 79 pairs of GC tissues and 5 GC cell lines. Based on the clinical and pathological features of GC patients, we found that HOXD-AS2 may be involved in the progression of GC. Then, a HOXD-AS2 plasmid was constructed and transfected into SGC-7901 and SNU-1 cells. After transfection, the effect of HOXD-AS2 on the progression of GC cells was analyzed. Furthermore, we illustrated a potential mechanism by which HOXD-AS2 may modulate the expression of HOXD8 and activate the PI3K/Akt signaling pathway in SGC-7901 and SNU-1 GC cells. The objective of our study was to explore the role of HOXD-AS2 in the development and progression of GC and to elucidate its possible regulatory mechanisms.
A total of 79 human GC tissues and matched adjacent non-cancerous tissues (ANTs) (at a distance of 5 cm from the tumor margin) were obtained at the time of surgery from April 2015 to May 2017 at The Affiliated Hospital of North Sichuan Medical College (Sichuan, China). None of the patients received radiotherapy, chemotherapy, or other anti-tumor treatments before surgery. Following excision, the GC and non-cancerous tissues were immediately frozen in liquid nitrogen and preserved at -80 °C until use. The clinicopathological parameters of patients with GC were collected. All patients provided written informed consent, and the entire study protocol was approved by The Ethics Committee of the Affiliated Hospital of North Sichuan Medical College, Nanchong, China.
Five human GC cell lines, AGS, MGC-803, BGC-823, SNU-1, and SGC-7901, and the normal gastric mucosal cell line GES-1 were purchased from Shanghai Cell Bank (Shanghai, China). The results of short tandem repeat (STR) analysis showed that there was no tri-allelic phenomenon in any locus, and no cross-contamination of other human cells was found. STR analysis found a 100% match to the reference data in the ATCC cell bank. Cells were cultured in DMEM or RPMI-1640 (Gibco BRL, Gaithersburg, MD, United States) supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, United States) and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a humidified atmosphere with 5% CO2.
The pc-HOXD-AS2 plasmid vector was purchased from Beijing Syngentech (Beijing, China). The plasmid was transfected into SGC-7901 and SNU-1 cells using Lipofectamine 3000 (Invitrogen, United States). After 48 h, the efficiency of each transfection group was compared with regard to the expression of HOXD-AS2.
The PI3K inhibitor LY294002 was purchased from MedChemExpress (United States). LY294002 was dissolved in DMSO (100 mmol/L) and stored at -20 °C for 1 wk. Before use, LY294002 was quickly diluted into culture medium at a final concentration of 10 µmol/L.
Total RNA was extracted from GC tissues, ANTs, and GC cell lines using TRIzol reagent (Invitrogen, Carlsbad, CA, United States), and reverse transcription reactions were performed using the GoScript Reverse Transcription System (Promega, Madison, United States) according to the manufacturer’s instructions. Real-time PCR was performed according to the instructions using standard Roche fluorescence quantitative PCR reagents (Roche, United States) and Roche fluorescent PCR instruments. β-actin or GAPDH was used as an internal reference. Each sample was detected three times. After the end of PCR, the dissolution profile of each specimen was stringently checked to ensure that there was only a single amplification product. HOXD-AS2, HOXD8, GAPDH, β-actin, PI3K, Akt, MAM2, and p53 primers were purchased from Shanghai Biotech (Shanghai, China). The primer sequences are shown in Supplementary Table 1. Analysis of the relative quantification of gene expression was performed using the classical 2-ΔΔCt method.
The invasive ability of GC cells (SGC-7901 and SNU-1) was detected by Matrigel Transwell assay. A 24-well Transwell plate was used with membranes separated by 8 μm pores (Costar, Cambridge, MA, United States), and 50 mg/L Matrigel (BD Pharmingen, San Jose, CA, United States) was added to the upper chamber. Then, the 24-well plate was incubated at 37 °C in a humidified incubator containing 5% CO2 for 2 h. SGC-7901 and SNU-1 cells transfected for 48 h were inoculated into the upper chamber at 5 × 105 cells per well, and complete medium containing 10% FBS was added to the lower chamber. After 48 h of incubation, the cells that did not invade the Matrigel were wiped off with a cotton swab, and the invaded cells attached to the lower surface of the membrane were fixed with 4% paraformaldehyde (Sigma Aldrich, St. Louis, MO, United States), and stained with 1% crystal violet (Beyotime, Shanghai, China). The migration assay was similar to the invasion assay except that Matrigel was not added. Finally, three fields of view were randomly selected under a microscope for invasive and migratory cell counting. Three replicate wells were set in all experiments.
Cell migration ability was measured using a scratch wound healing assay. A straight line was marked with a marker in the middle on the back of a 3.5 cm dish. The SGC-7901 and SNU-1 GC cells transfected 24 h after each group were inoculated into a culture dish. After 24 h, a scratch was made on the bottom of the culture dish with a 200 μL tip, and the cell fragments were washed with sterile phosphate buffered saline (PBS). After incubation at 37 °C and 5% CO2 for 48 h, the distance between the two edges of the scratch was observed under a microscope.
Cell counting kit-8 assay (CCK-8, Beyotime Institute of Biotechnology, Shanghai, China) was used to detect the cell viability. Cells were seeded at 5000 cells per well in a 96-well plate. After the cells were transfected with plasmids for 24 h, 10 μL of CCK-8 was added to each well at 0 h, 24 h, 48 h, and 72 h. The absorbance at 450 nm of each well was measured with a spectrophotometer. All experiments were performed in triplicate.
Cell apoptosis was analyzed with Annexin V-APC/7-AAD Apoptosis Detection kit (KeyGEN BioTECH, China) and a NovoCyte flow cytometer (ACEA, China). Forty-eight hours after cell transfection, the cells were digested with trypsin without EDTA and washed twice with cold PBS. Then the digested cells were put into the flow sampling tube, and 500 μL of Binding Buffer was added to the tube according to the ratio of 100:1, followed by the addition of 5 µL Annexin V-APC and 5 µL 7-AAD staining solution. After 15 min of reaction at room temperature and protection from light, samples were taken on the machine for apoptosis analysis. Each experiment was repeated three times.
For cell cycle detection, the cells were collected into a brown EP tube. Add pre-cooled 70% alcohol was added, the tube was shaken gently, and put in a refrigerator at 4 °C for 4 h. After the cell membrane was broken, 0.5 mL of propidium iodide staining solution was added to the tube for staining at room temperature in the dark for 30 min, and then the sample was subject to cell cycle analysis. This experiment was performed in triplicate.
Total protein was extracted, and the concentration was determined using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, United States). Sample lysates (10 μg of protein) were separated by SDS-PAGE and transferred to a PVDF membrane. The membrane was incubated with specific antibodies against HOXD8 (1:1000), PI3K (1:1000), Akt (1:2000), MDM2 (1:500), or p53 (1:1000) (Abcam, Cambridge, MA, United States) at 4 °C overnight, followed by incubation with secondary antibody. Protein levels were normalized to those of total GAPDH, which were detected using a monoclonal anti-GAPDH antibody (1:10000; Sigma-Aldrich Corporation, St. Louis, MO, United States). Autoradiograms were quantified by densitometry (Quantity One software; Bio-Rad).
All experimental data were analyzed using SPSS20.0 (SPSS, Chicago, IL, United States) and GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, United States), and the measurement data are expressed as the mean ± SD. Two-tailed Student’s t-test was used to compare two groups of measurement data. The relationship between lncRNA expression levels and clinical indicators of patients was calculated by Chi-square test or Fisher's exact probability test (two-sided). Survival analysis for patients with GC was performed by the Kaplan–Meier method, and the differences between survival curves were estimated by the log-rank test. Spearman correlation analyses were performed to investigate correlations between gene expression levels. All tests were two-tailed, and P < 0.05 was considered statistically significant.
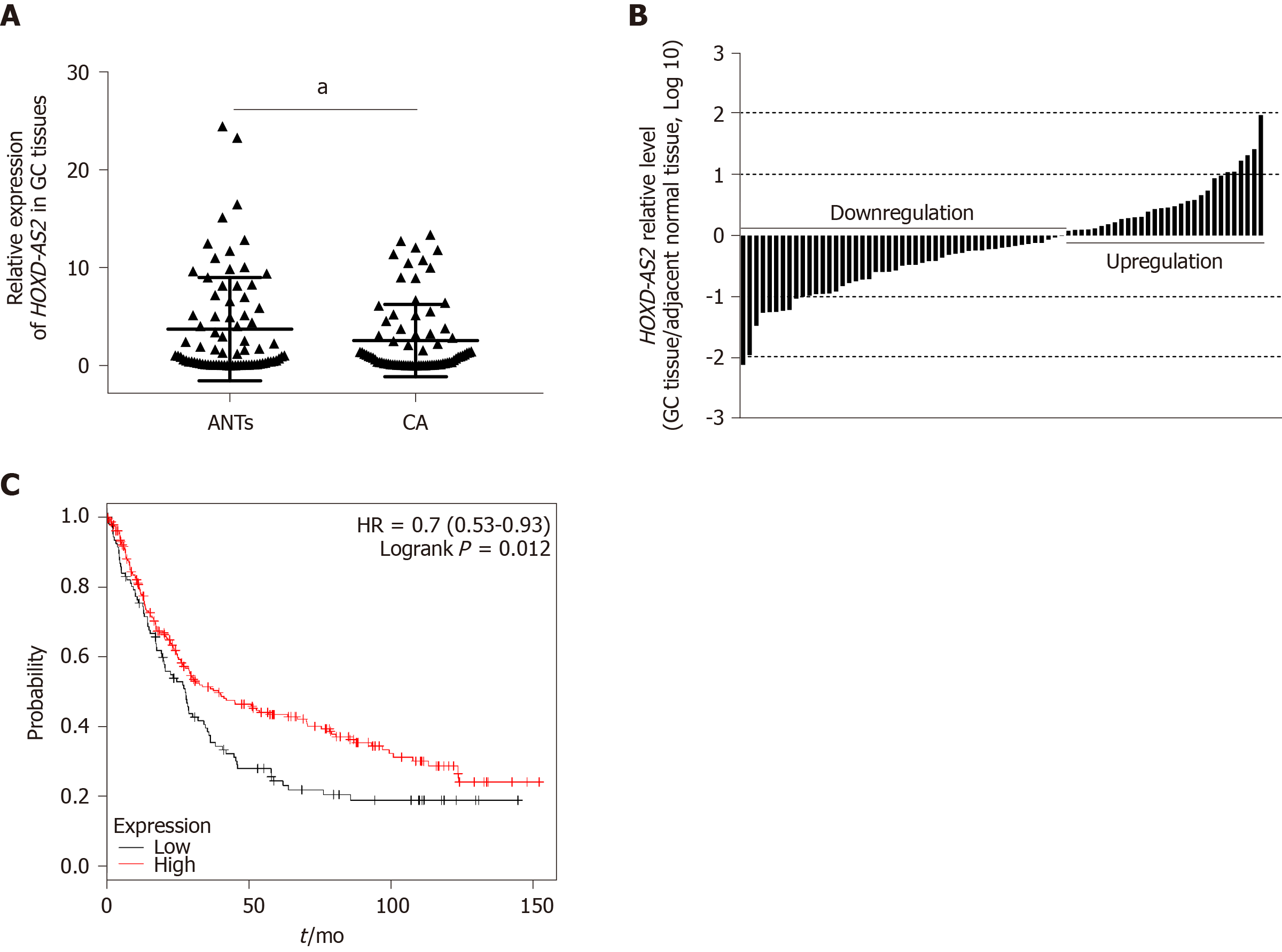
As shown in Figure 1A, the relative expression level of lncRNA HOXD-AS2 in 79 GC tissues was significantly decreased in comparison with that in the corresponding adjacent tissues (P = 0.030). As shown in Figure 1B, decreased expression of HOXD-AS2 was observed in 49 (62.03%) out of 79 cases, while high expression was observed in the 30 remaining cases (37.97%). The relationship between the expression level of HOXD-AS2 and the clinicopathological features of GC patients was analyzed. As shown in Table 1, low expression of HOXD-AS2 was associated with lymph node metastasis (P = 0.009) and TNM stage (P = 0.040). Data from the Kaplan-Meier Plotter database (http://kmplot.com/) was used to analyze the relationship between HOXD-AS2 expression and the overall survival rate of 348 patients with GC (109 patients with low HOXD-AS2 expression and 239 with high HOXD-AS2 expression). The prognosis of the HOXD-AS2 low expression group was worse than that of the HOXD-AS2 high expression group, and the difference was statistically significant (P = 0.012, Figure 1C).
| Characteristic | Each group (n) | HOXD-AS2 expression | P value | |
| High | Low | |||
| All cases | 79 | 30 | 49 | |
| Age (yr, mean ± SD) | 79 | 64.10 ± 9.323 | 62.04 ± 9.648 | 0.354 |
| BMI | 79 | 21.04 ± 2.797 | 21.90 ± 3.196 | 0.224 |
| Gender | 79 | 0.594 | ||
| Male | 63 | 23 | 40 | |
| Female | 16 | 7 | 9 | |
| Smoking | 79 | 0.179 | ||
| Yes | 32 | 15 | 17 | |
| No | 47 | 15 | 32 | |
| Drinking alcohol | 79 | 0.096 | ||
| Yes | 23 | 12 | 11 | |
| No | 56 | 18 | 38 | |
| Maximum tumor diameter (cm, mean ± SD) | 79 | 4.36 ± 1.856 | 4.87 ± 2.613 | 0.355 |
| Histology | 79 | 1.000 | ||
| Undifferentiated | 7 | 3 | 4 | |
| Differentiated | 72 | 27 | 45 | |
| Depth of invasion | 79 | 0.430 | ||
| pT 1-2 | 9 | 5 | 4 | |
| pT 3-4 | 70 | 25 | 45 | |
| Lymph node metastasis | 79 | 0.009 | ||
| pN0 | 19 | 12 | 7 | |
| pN1-3 | 60 | 18 | 42 | |
| Distant metastasis | 79 | 1.000 | ||
| pM0 | 75 | 28 | 47 | |
| pM1 | 4 | 2 | 2 | |
| Tumor TNM stage | 79 | 0.040 | ||
| I-II | 19 | 11 | 8 | |
| III-IV | 60 | 19 | 41 | |
| Venous/lymphatic invasion | 79 | 0.376 | ||
| Positive | 11 | 6 | 5 | |
| Negative | 68 | 24 | 44 | |
| Nervous invasion | 79 | 1.000 | ||
| Positive | 10 | 4 | 6 | |
| Negative | 69 | 26 | 43 | |
| Liver metastasis | 79 | 0.300 | ||
| Absent | 75 | 27 | 48 | |
| Present | 4 | 3 | 1 | |
| Ascitic fluid | 79 | 0.965 | ||
| Negative | 63 | 24 | 39 | |
| Positive | 16 | 6 | 10 | |
| Fatty nodules | 79 | 1.000 | ||
| Positive | 9 | 3 | 6 | |
| Negative | 70 | 27 | 43 | |
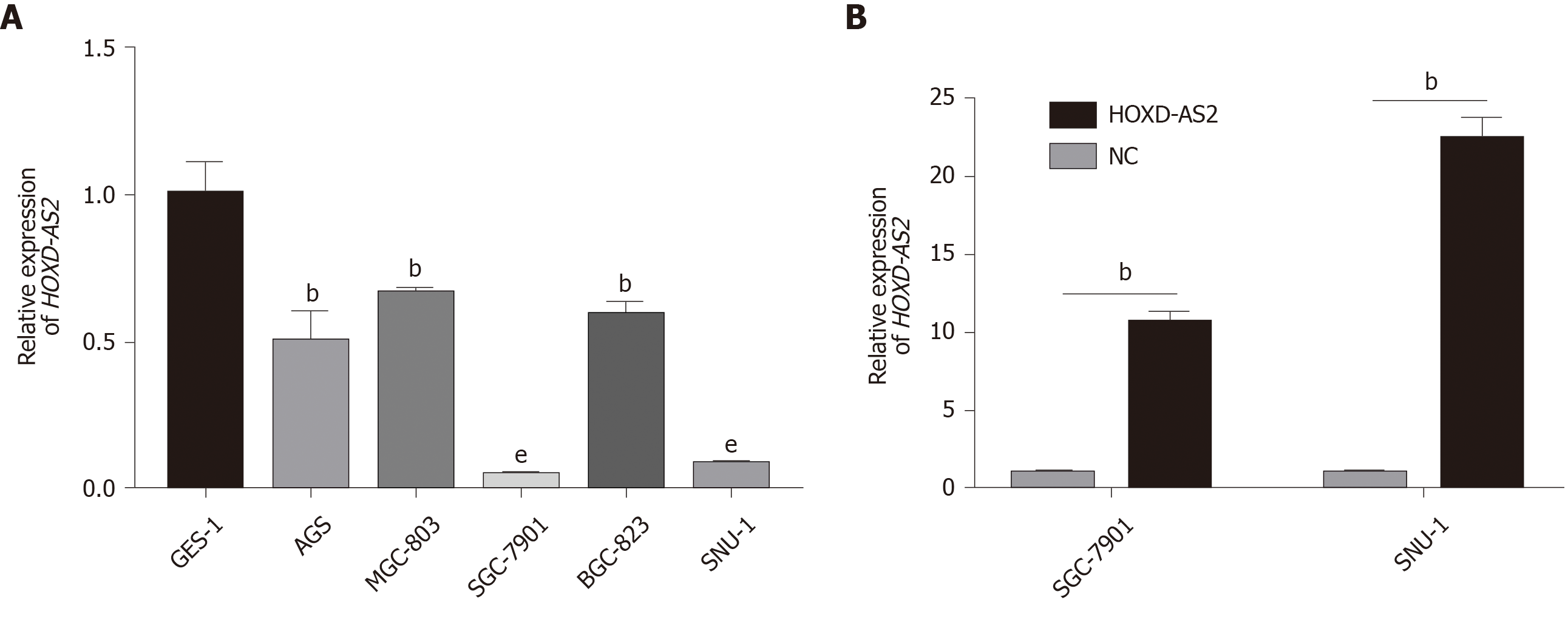
As shown in Figure 2A, the relative expression level of lncRNA HOXD-AS2 in the five GC cell lines SGC-7901, MGC-803, BGC-823, SNU-1, and AGS was lower than that in the normal gastric mucosal cell line GES-1 (P = 0.005, = 0.006, < 0.001, = 0.004, and < 0.001, respectively). The expression of HOXD-AS2 was the lowest in SGC-7901 and SNU-1 cells, so we selected these two cell lines for subsequent experimental studies. As shown in Figure 2B, after transfection of SGC-7901 and SNU-1 cells, the expression of HOXD-AS2 in the pcHOXD-AS2 group was significantly increased compared with that in the control group (P = 0.002 and 0.002, respectively).
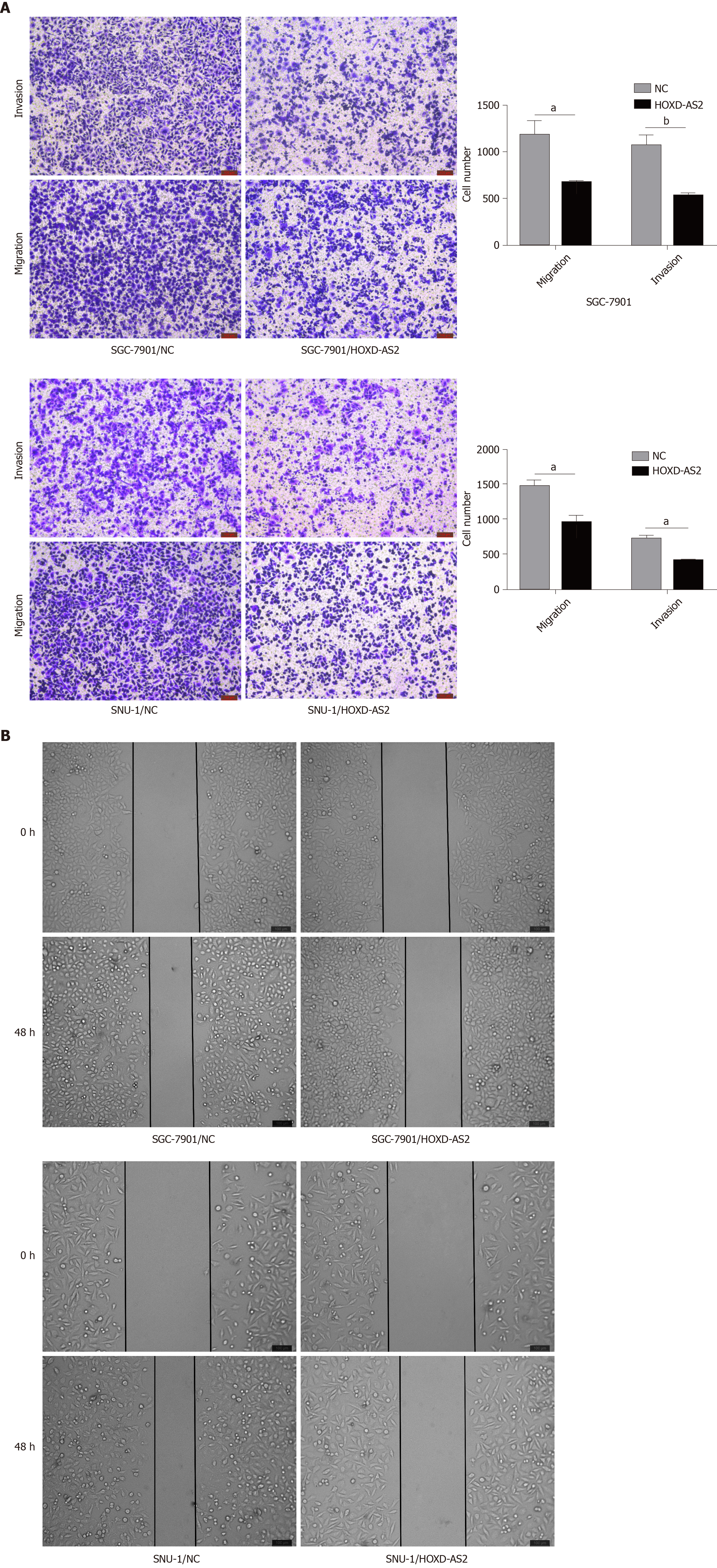
The effects of HOXD-AS2 on the invasion and migration of SGC-7901 and SNU-1 cells were detected by wound healing assay and Transwell assay. It was found that overexpression of HOXD-AS2 inhibited the invasion and migration of SGC-7901 and SNU-1 cells (Figure 3A). We calculated the number of invasive and migratory cells in the pc-HOXD-AS2 group and control group, and cell migration and invasion in the pc-HOXD-AS2 group were significantly decreased compared with those in the control group (Figure 3A). The wound healing assay also confirmed the ability to inhibit the migration of SGC-7901 and SNU-1 cells after overexpression of HOXD-AS2 (Figure 3B). These results show that HOXD-AS2 is involved in the regulation of invasion and migration of GC cells.
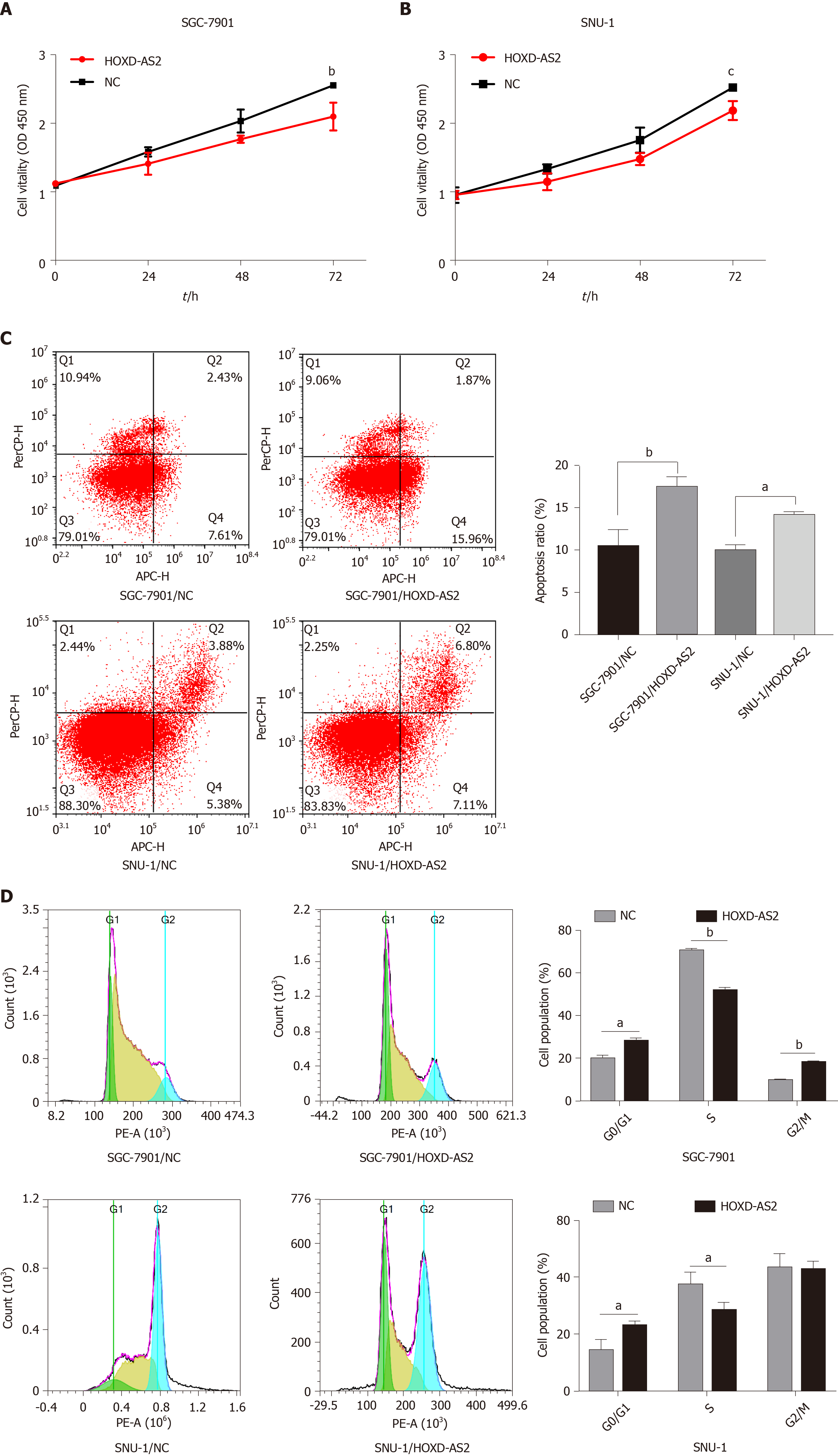
The cell proliferation activity was detected by the CCK8 experiment, and it was found that overexpression of HOXD-AS2 can inhibit the proliferation ability of SGC-7901 and SNU-1 cells (Figure 4A and B). We examined the effect of HOXD-AS2 on the apoptosis of SGC-7901 and SNU-1 cells by flow cytometry. The results showed that the apoptosis rate of the HOXD-AS2 overexpression group was higher than that of the control group (Figure 4C). Cell cycle detection results showed that in SGC-7901 and SNU-1 cells, overexpression of HOXD-AS2 can increase the number of G0/G1 phase cells, reduce the number of S phase cells, and block the cell cycle in G0/G1 phase (Figure 4D).
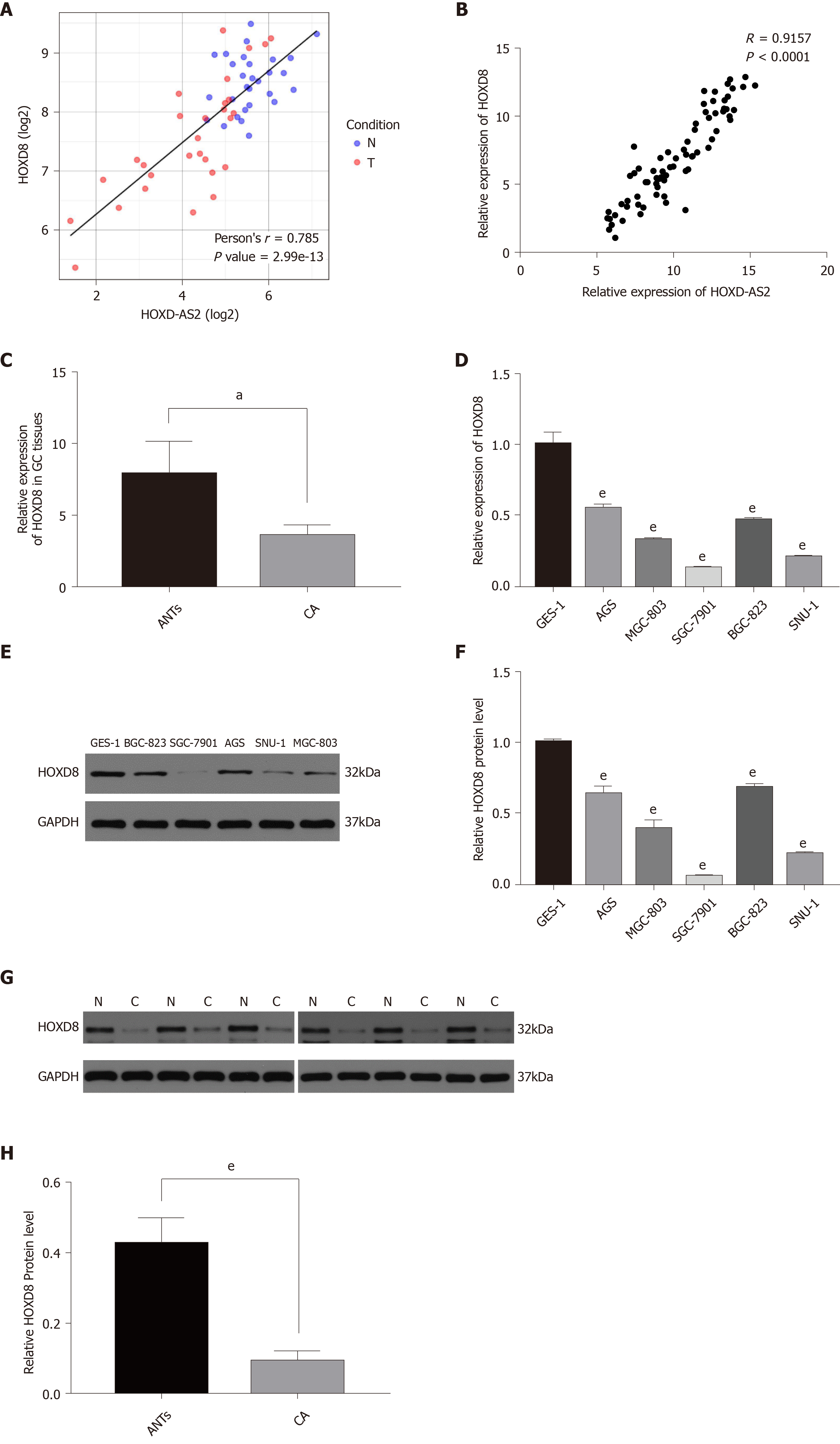
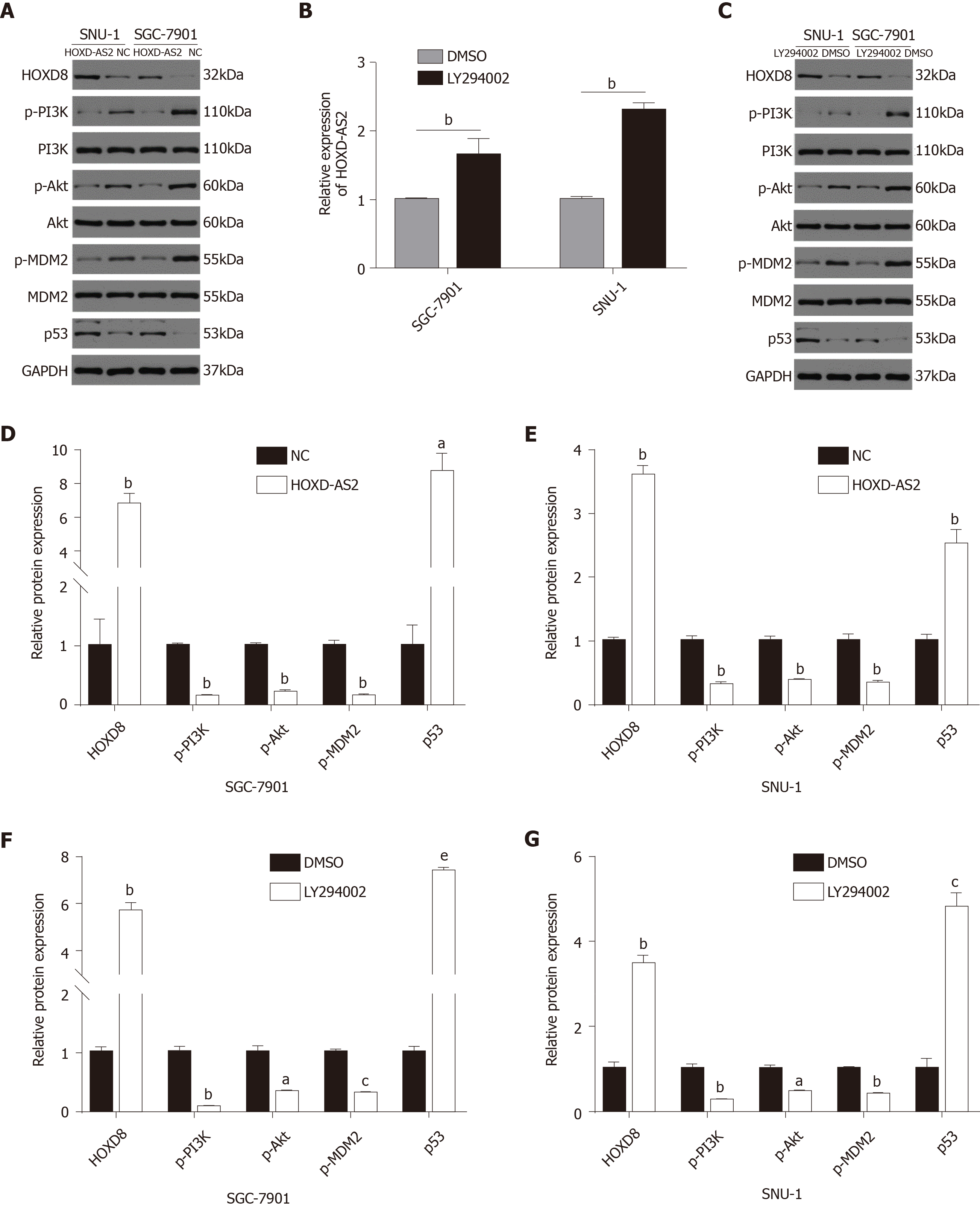
To explore the regulatory mechanism of HOXD-AS2 in GC, we predicted the co-expressed genes associated with lncRNA HOXD-AS2 via circlncRNAnet (http://app.cgu.edu.tw/circlnc/) and found that HOXD-AS2 was positively correlated with its nearby gene HOXD8 (r = 0.785, P < 0.05) (Figure 5A). Then, we included our qPCR data in the analysis and confirmed that HOXD-AS2 had a positive correlation with HOXD8 (r = 0.9157, P < 0.05) (Figure 5B). As shown in Figure 5C, G, and H, the relative mRNA expression level of HOXD8 in 79 GC tissues was significantly decreased in comparison with that in the corresponding adjacent tissues (P = 0.048), and the protein expression levels of HOXD8 in six GC tissues was significantly decreased in comparison with that in the corresponding adjacent tissues (P < 0.001). As shown in Figure 5D, E, and F, the relative mRNA and protein expression levels of HOXD8 in the five GC cell lines SGC-7901, MGC-803, BGC-823, SNU-1, and AGS were lower than those in the normal gastric mucosal cell line GES-1 (P < 0.001). In SGC-7901 and SNU-1 cells, we overexpressed HOXD-AS2 and found that HOXD8 protein levels also increased (Figure 6A, D, and E). According to these results, we speculate that HOXD-AS2 is likely to participate in the progression of GC cells via interaction with HOXD8.
In our previous research, through Gene Ontology (GO) analysis and KEGG signaling pathway enrichment analysis of GC-related signaling pathways, we identified the top 10 most enriched signal pathways in GC[14]. In this study, we found that lncRNA HOXD-AS2 may play an important role in regulating the progression of GC cells through the PI3K/Akt signaling pathway. The results showed that in SGC-7901 and SNU-1 cells, the protein levels of p-PI3K, p-Akt, and p-MDM2 in the pc-HOXD-AS2 group were lower than those in the control group, while the level of p53 was higher than that in the control group (Figure 6A, D, and E). To further demonstrate whether HOXD-AS2 regulates the progression of GC cells via the PI3K/Akt signaling pathway, we added 10 μmol/L PI3K inhibitor to SGC-7901 and SNU-1 cells. After the use of the PI3K-specific inhibitor LY294002, a similar effect was obtained as with transfection of the HOXD-AS2 plasmid. The results also showed that the expression of HOXD-AS2 increased and the expression of HOXD8 protein increased, which further demonstrated that HOXD-AS2 regulates the progression of GC cells by inhibiting the activation of the PI3K/Akt signaling pathway (Figure 6B, C, F, and G).
LncRNAs have diverse biological functions and mainly regulate gene expression at three levels: Epigenetic, transcriptional, and post-transcriptional. Regulation at the epigenetic level is mainly through DNA methylation and demethylation. Transcriptional regulation mainly affects the expression of genes through promoters and enhancers. Regulation at the post-transcriptional level is mainly through the regulation of post-transcriptional processing and modification processes such as mRNA splicing, editing, and degradation[16-18]. Many studies have found that lncRNAs are closely related to GC, and lncRNAs can act as tumor suppressor genes or oncogenes to play an important role in the regulation of malignant biological behaviors in GC such as proliferation, apoptosis, invasion, and metastasis[19]. However, the specific molecular mechanism of lncRNAs in GC has not been fully elucidated. In our previous study, we constructed an lncRNA expression chip to compare differentially expressed lncRNAs in GC tissues and matched paracancerous tissues[13,14,20]. HOXD-AS2 has great expression differences between GC tissues and matched paracancerous tissues, but its specific role in GC remains unclear. The purpose of this study was to detect the expression of HOXD-AS2 in GC tissues and cells, analyze its relationship with the clinicopathological features of GC patients, and explore its role and specific molecular regulatory mechanism in the occurrence and development of GC.
In the present study, we verified the expression of HOXD-AS2 in 79 GC tissues and 5 GC cell lines by qRT-PCR. The relationship between the expression of HOXD-AS2 and the clinicopathological features of GC patients was analyzed. It was found that low expression of HOXD-AS2 was significantly associated with lymph node metastasis and TNM stage. We analyzed data from the Kaplan-Meier plotter database and found that the HOXD-AS2 low expression group had a lower overall survival rate and worse prognosis than the HOXD-AS2 high expression group. Lymph node metastasis and TNM stage are closely related to the progression of tumors. We speculated that HOXD-AS2 may be involved in the biological behavior of GC cells. To confirm our hypothesis, we specifically upregulated the expression of HOXD-AS2 in GC cells in vitro and found that the invasion, migration, and proliferation ability of GC cells was significantly inhibited. All the results indicated that HOXD-AS2 may play an important regulatory role in the progression of GC.
Many studies have found that the natural antisense transcript types of lncRNAs can regulate the expression of their nearby genes. For example, lncRNA FOXP4-AS1 can act as a ceRNA to adsorb miR-3184-5p, attenuating the inhibitory effect of miR-3184-5p on its target gene FOXP4, thereby promoting prostate cancer cell proliferation[21]. LncRNA SLC7A11-AS1 is negatively regulated by its adjacent gene SLC7A11. Decreasing SLC7A11-AS1 can increase the expression of SLC7A11. SLC7A11-AS1 can also regulate the proliferation of GC cells by activating the ASK1-p38MAPK/JNK signaling pathway[13]. LncRNA MACC1-AS1 is highly expressed in GC tissues, and it can upregulate the expression of MACC1 by enhancing the stability of MACC1 mRNA, thereby enhancing the glycolysis process and antioxidant capacity of GC cells to promote their proliferation, invasion, and migration. MACC1-AS1 can be used as a biomarker for the diagnosis and prognosis of GC[22]. Similarly, when we predicted the co-expressed genes related to HOXD-AS2 through an online database, we found that the expression of the nearby gene HOXD8 was positively correlated with the expression of HOXD-AS2. Therefore, we speculated that HOXD-AS2 may be involved in the regulation of HOXD8 through a ceRNA mechanism and may play a role in regulating the process of GC cells. We used qRT-PCR assay to further verify that there was a significant positive correlation between the expression of the two genes in clinical GC tissues and GC cells. After overexpression of HOXD-AS2, we found that the protein level of HOXD8 was higher than that of the control group, further confirming that HOXD-AS2 can regulate the expression of HOXD8. However, little is known about the role of HOXD8 in tumorigenesis and development and the specific regulatory mechanisms. HOXD8, which is located at 2q31.1 and contains two exons, has been mapped to chromosome 2 region 176129705-176132695. The expression of HOXD8 is decreased in colorectal cancer. Overexpression of HOXD8 can inhibit the proliferation, invasion, and migration of colorectal cancer cells and promote their apoptosis[23]. HOXD8 is highly expressed in non-small cell lung cancer. Overexpression of HOXD8 can upregulate the expression of proliferation-related genes p53, PTEN, and p21 and promote the proliferation of non-small cell lung cancer cells. In addition, miR-520a-3p can inhibit the proliferation of non-small cell lung cancer cells by downregulating the expression of HOXD8[24]. The specific regulatory mechanism of HOXD8 in tumors still needs further exploration and confirmation.
The PI3K/Akt signaling pathway has been well documented in a number of studies as a key mechanism regulating tumor cell processes, including proliferation, apoptosis, invasion, and migration[25-27]. Many lncRNAs have also been found to be closely related to the PI3K/Akt signaling pathway in GC. Huang et al[28] found that lncRNA AK023391 promotes the development and progression of GC by activating the PI3K/Akt signaling pathway. Cheng et al[29] found that downregulation of lncRNA HOTAIR can upregulate the expression of miR-34a, inhibit the PI3K/Akt and Wnt/β-catenin signaling pathways, and attenuate the resistance of GC cells to cisplatin. In this study, we found a possible link between the PI3K/Akt signaling pathway and GC by GO and KEGG analysis. After overexpression of HOXD-AS2 in GC cells SGC-7901 and SNU-1, we found that the PI3K/Akt signaling pathway is inhibited. In vitro, after we specifically overexpressed HOXD-AS2 in GC cells SGC-7901 and SNU-1, we found that the expression levels of several key genes in the PI3K/Akt signaling pathway were downregulated and that the signaling pathway was inhibited. In the positive control group, we obtained similar results after adding the PI3K inhibitor LY294002. We found that the expression level of HOXD-AS2 increased, the protein level of HOXD8 also increased, and the PI3K/Akt signaling pathway was inhibited. The invasion, migration, and proliferation ability of GC cells also decreased. Based on the above findings, we speculate that the low expression of HOXD-AS2 may regulate the activation of the PI3K/Akt signaling pathway via HOXD8 through a ceRNA mechanism, which may promote the progression of GC.
In summary, our current results demonstrate that HOXD-AS2 might play an important role in suppressing the progression of GC cells by regulating HOXD8 gene expression and inhibiting the PI3K/Akt signaling pathway. These results suggest that HOXD-AS2 may be a key molecule in tumor development and a potential target for the treatment of GC.
Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs with a length of more than 200 nucleotides and lack an open reading frame. They mainly regulate RNA transcription and mRNA splicing and play an important role in regulating the stability of RNA in the cytoplasm and the activity of microRNAs. More and more studies have shown that abnormally expressed lncRNAs are involved in all aspects of tumor occurrence and development, and are closely related to tumor proliferation, apoptosis, invasion, metastasis, drug resistance, and poor prognosis. In recent years, more and more studies have found that abnormally expressed lncRNAs are involved in the occurrence and development of gastric cancer (GC), and they are expected to become new biomarkers for the diagnosis and treatment of GC.
GC is one of the most common malignant tumors in the world. The incidence and mortality of GC are in the forefront of all malignant tumors. Although a major breakthrough has been achieved in the study of lncRNAs in the pathogenesis of GC, the specific mechanism of lncRNAs in the occurrence and development of GC has not yet been fully elucidated. Exploring new lncRNAs can help to understand the molecular mechanism of GC more deeply.
The main purpose of this study was to explore the effect of downregulation of HOXD-AS2 on the biological behavior of GC cells SGC-7901 and SNU-1 and the underlying mechanism. Studies have found that the downregulation of HOXD-AS2 can regulate the expression of its neighboring gene HOXD8, and can also activate the PI3K/Akt signaling pathway, thereby promoting the progression of GC cells. The results of this study may provide a new idea for the treatment of GC.
The pcHOXD-AS2 plasmid vector was constructed and transfected into SGC-7901 and SNU-1 GC cells. Matrigel Transwell and wound healing assays were used to confirm the effect of HOXD-AS2 on invasion and migration of GC cells. Cell counting kit-8 assay and flow cytometry were used to verify the effect of HOXD-AS2 on proliferation, cell cycle, and apoptosis of GC cells. The relevant regulatory mechanism between HOXD-AS2 and HOXD8 and PI3K/Akt signaling pathway was verified by Western blot analysis.
In this study, we found that the low expression of lncRNA HOXD-AS2 was associated with lymph node metastasis and tumor-node-metastasis stage in GC. In vitro functional experiments demonstrated that overexpression of HOXD-AS2 inhibited GC cell progression. Mechanistic studies revealed that HOXD-AS2 regulated the expression of its nearby gene HOXD8 and inhibited the activity of the PI3K/Akt signaling pathway.
These results indicate that downregulation of HOXD-AS2 significantly promotes the progression of GC cells by regulating HOXD8 expression and activating the PI3K/Akt signaling pathway. HOXD-AS2 may be a novel diagnostic biomarker and effective therapeutic target for GC.
This study combines basic experimental research and bioinformatics results to reach a relatively novel conclusion. To further confirm the results of this study, siRNA and animal experiments may be better.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Matsuo Y, Nakajima N S-Editor: Huang P L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 50873] [Article Influence: 8478.8] [Reference Citation Analysis (44)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11444] [Cited by in F6Publishing: 12500] [Article Influence: 1562.5] [Reference Citation Analysis (0)] |
| 3. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1282] [Cited by in F6Publishing: 1367] [Article Influence: 170.9] [Reference Citation Analysis (0)] |
| 4. | Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, Tanabe K, Ohdan H. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1840] [Cited by in F6Publishing: 1999] [Article Influence: 181.7] [Reference Citation Analysis (0)] |
| 6. | Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 7. | Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435-1439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 925] [Cited by in F6Publishing: 951] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 8. | Zhang J, Li Z, Liu L, Wang Q, Li S, Chen D, Hu Z, Yu T, Ding J, Li J, Yao M, Huang S, Zhao Y, He X. Long noncoding RNA TSLNC8 is a tumor suppressor that inactivates the interleukin-6/STAT3 signaling pathway. Hepatology. 2018;67:171-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 9. | Xiao ZD, Han L, Lee H, Zhuang L, Zhang Y, Baddour J, Nagrath D, Wood CG, Gu J, Wu X, Liang H, Gan B. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat Commun. 2017;8:783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Tan DSW, Chong FT, Leong HS, Toh SY, Lau DP, Kwang XL, Zhang X, Sundaram GM, Tan GS, Chang MM, Chua BT, Lim WT, Tan EH, Ang MK, Lim TKH, Sampath P, Chowbay B, Skanderup AJ, DasGupta R, Iyer NG. Long noncoding RNA EGFR-AS1 mediates epidermal growth factor receptor addiction and modulates treatment response in squamous cell carcinoma. Nat Med. 2017;23:1167-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Kim J, Piao HL, Kim BJ, Yao F, Han Z, Wang Y, Xiao Z, Siverly AN, Lawhon SE, Ton BN, Lee H, Zhou Z, Gan B, Nakagawa S, Ellis MJ, Liang H, Hung MC, You MJ, Sun Y, Ma L. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50:1705-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 488] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 12. | Shang Z, Yu J, Sun L, Tian J, Zhu S, Zhang B, Dong Q, Jiang N, Flores-Morales A, Chang C, Niu Y. LncRNA PCAT1 activates AKT and NF-κB signaling in castration-resistant prostate cancer by regulating the PHLPP/FKBP51/IKKα complex. Nucleic Acids Res. 2019;47:4211-4225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | Luo Y, Wang C, Yong P, Ye P, Liu Z, Fu Z, Lu F, Xiang W, Tan W, Xiao J. Decreased expression of the long non-coding RNA SLC7A11-AS1 predicts poor prognosis and promotes tumor growth in gastric cancer. Oncotarget. 2017;8:112530-112549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Luo Y, Tan W, Jia W, Liu Z, Ye P, Fu Z, Lu F, Xiang W, Tang L, Yao L, Huang Q, Xiao J. The long non-coding RNA LINC01606 contributes to the metastasis and invasion of human gastric cancer and is associated with Wnt/β-catenin signaling. Int J Biochem Cell Biol. 2018;103:125-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, Liao QX, Zhang H, Zeng LS, Cui SZ. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer. 2018;17:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 16. | Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1625] [Cited by in F6Publishing: 1915] [Article Influence: 239.4] [Reference Citation Analysis (0)] |
| 17. | Li M, Izpisua Belmonte JC. Roles for noncoding RNAs in cell-fate determination and regeneration. Nat Struct Mol Biol. 2015;22:2-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3469] [Cited by in F6Publishing: 3789] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 19. | Fang XY, Pan HF, Leng RX, Ye DQ. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett. 2015;356:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 20. | Feng Y, Fu Z, Luo Y, Tan W, Liu Z, Ye P, Lu F, Xiang W, Tang L, Yao L, Song M, Huang Q, Liu Y, Xiao J. Long non-coding RNA RP11-6O2.4 indicates poor prognosis and suppresses cell cycle progression through the p38-MAPK signaling pathway in gastric cancer. Mol Cell Toxicol. 2019;15:335-344. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Wu X, Xiao Y, Zhou Y, Zhou Z, Yan W. LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of prostate cancer by sequestering miR-3184-5p to upregulate FOXP4. Cell Death Dis. 2019;10:472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Zhao Y, Liu Y, Lin L, Huang Q, He W, Zhang S, Dong S, Wen Z, Rao J, Liao W, Shi M. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer. 2018;17:69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 23. | Mansour MA, Senga T. HOXD8 exerts a tumor-suppressing role in colorectal cancer as an apoptotic inducer. Int J Biochem Cell Biol. 2017;88:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Miao L, Ni R, Zhang H, Li L, Wang X, Li X, Wang J. microRNA-520a-3p inhibits proliferation and cancer stem cell phenotype by targeting HOXD8 in non-small cell lung cancer. Oncol Rep. 2016;36:3529-3535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Xue G, Restuccia DF, Lan Q, Hynx D, Dirnhofer S, Hess D, Rüegg C, Hemmings BA. Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-β signaling axes. Cancer Discov. 2012;2:248-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Wang H, Yang X, Guo Y, Shui L, Li S, Bai Y, Liu Y, Zeng M, Xia J. HERG1 promotes esophageal squamous cell carcinoma growth and metastasis through TXNDC5 by activating the PI3K/AKT pathway. J Exp Clin Cancer Res. 2019;38:324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang Z, Li N, Chai N, Zhang S, Wu K, Nie Y, Lu N. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J Exp Clin Cancer Res. 2018;37:198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Huang Y, Zhang J, Hou L, Wang G, Liu H, Zhang R, Chen X, Zhu J. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36:194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 29. | Cheng C, Qin Y, Zhi Q, Wang J, Qin C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107:2620-2629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |